Nuclear Chemistry Nuclear Chemistry The study of reactions



























- Slides: 40

Nuclear Chemistry

Nuclear Chemistry • The study of reactions that take place in the nucleii of atoms

Chemical Reactions • In normal chemical reactions, only the electrons are involved

Radioactive Nucleii • A nucleus that spontaneously decomposes

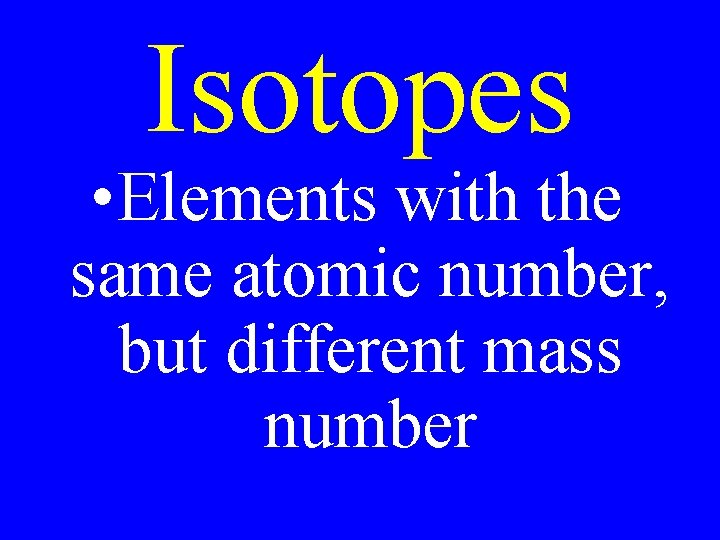
Isotopes • Elements with the same atomic number, but different mass number

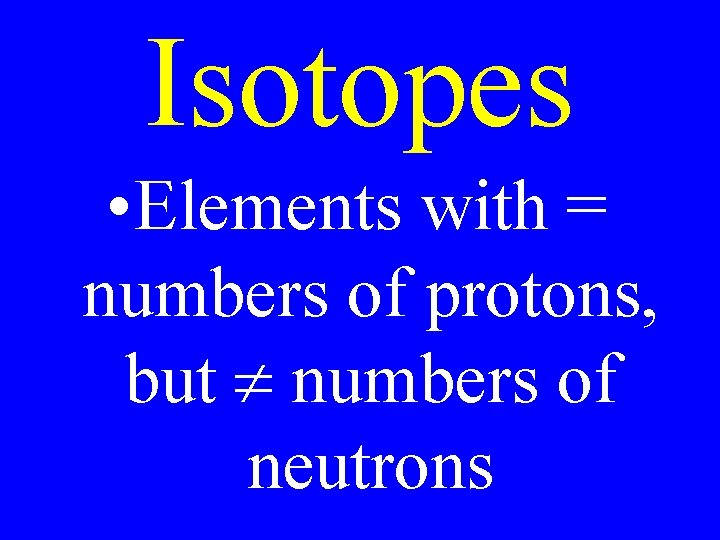
Isotopes • Elements with = numbers of protons, but numbers of neutrons

Isotopes • All elements have at least one radioactive isotope

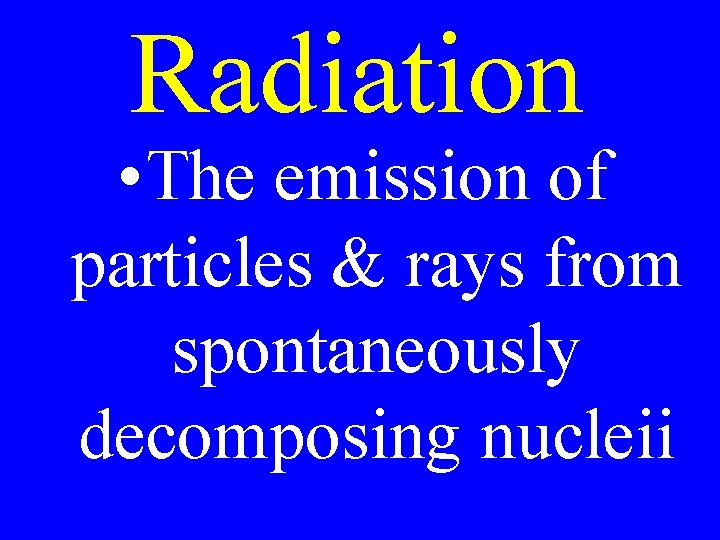
Radiation • The emission of particles & rays from spontaneously decomposing nucleii

Modes of Decay • Alpha emission • Beta emission • Gamma emission • Positron emission • K-electron capture

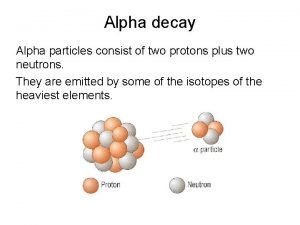
Alpha Particle (a) • Helium nucleus • 2 protons & 2 neutrons • mass = 4 amu • charge = +2 • Penetration power: small

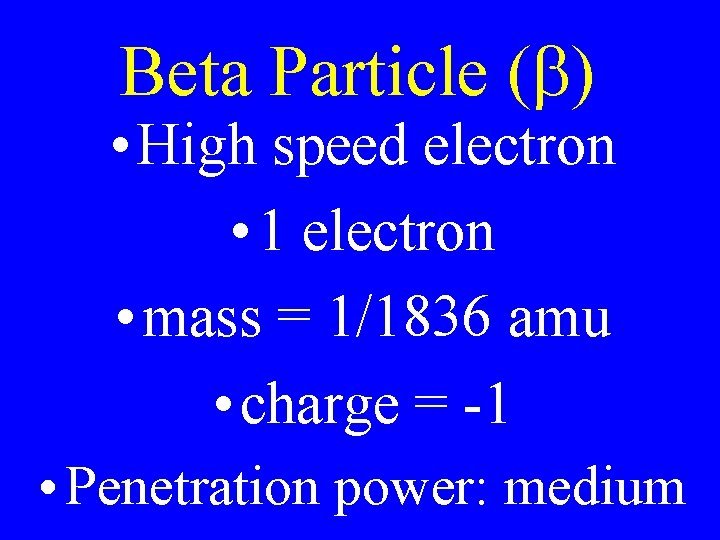
Beta Particle (b) • High speed electron • 1 electron • mass = 1/1836 amu • charge = -1 • Penetration power: medium

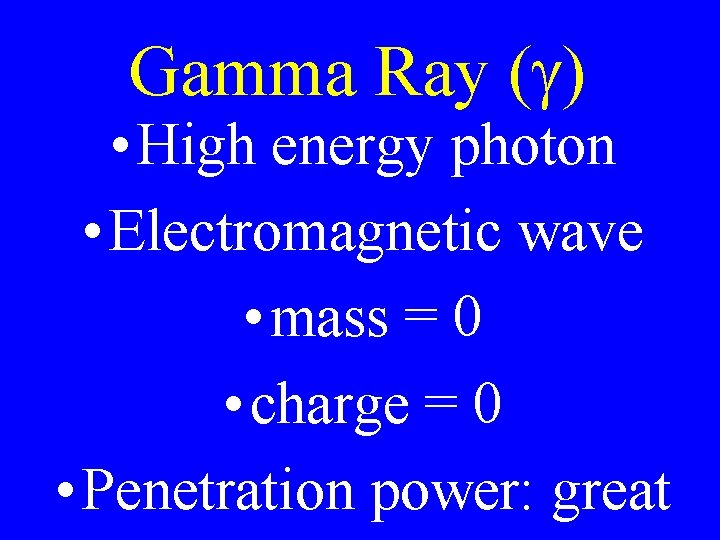
Gamma Ray ( ) • High energy photon • Electromagnetic wave • mass = 0 • charge = 0 • Penetration power: great

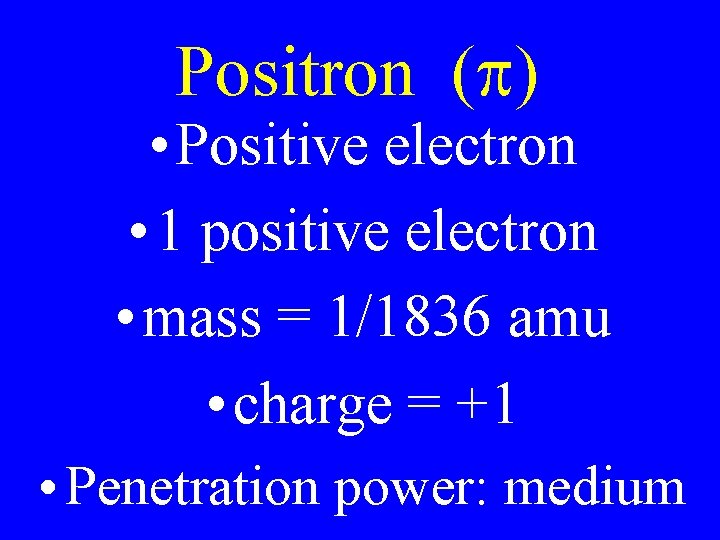
Positron (p) • Positive electron • 1 positive electron • mass = 1/1836 amu • charge = +1 • Penetration power: medium

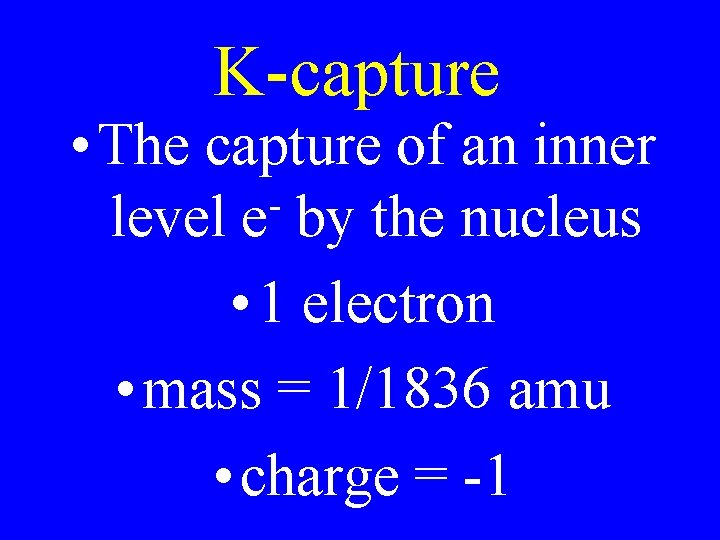
K-capture • The capture of an inner level e by the nucleus • 1 electron • mass = 1/1836 amu • charge = -1

Nuclear Symbol 4 4 • Alpha: 2 He or 2 a 0 0 • Beta: -1 e or – 1 b 0 • Gamma: 0 0 • Positron: +1 e 0 • K-electron: -1 e

Fission • The splitting of a nucleus into smaller nucleii involving the release of energy

Fusion • The combining of smaller nuclei into a larger one involving the release of energy

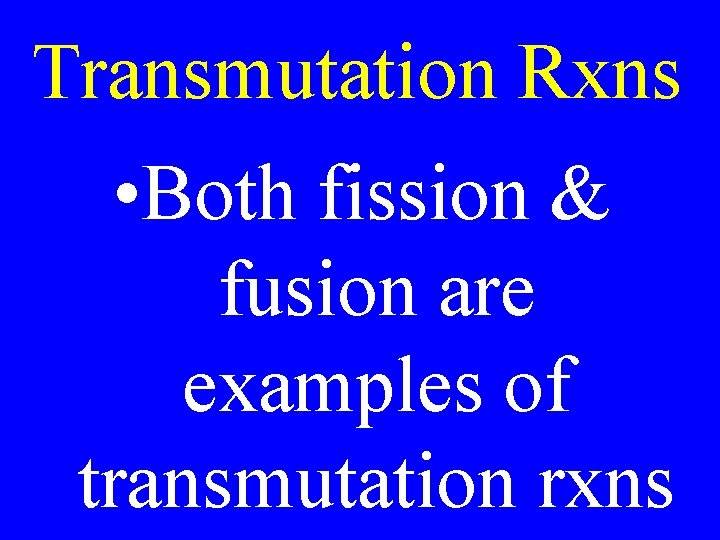
Transmutation Rxns • Nuclear reactions in which one element is changed into another

Transmutation Rxns • Reactions in which the nucleus of an atom is changed

Transmutation Rxns • Both fission & fusion are examples of transmutation rxns

Transmutation Rxns • Can occur through emission of or bombardment by radioactive particles

Transmutation Rxns b emission of Pm-142 a emission of U-238 K-capture by O-15 p addition of O-18

Transmutation Rxns a emission of U 238 followed by two separate b emissions:

Transmutation Rxns a bombardment of Th-234 followed by two separate b emission:

Predict Products a Neutron absorption by U 238 followed by two separate b emission:

Predict Products a emission of O-18 followed by a b emission:

Predict Products K-capture by V-45 followed by neutron emission then a emission

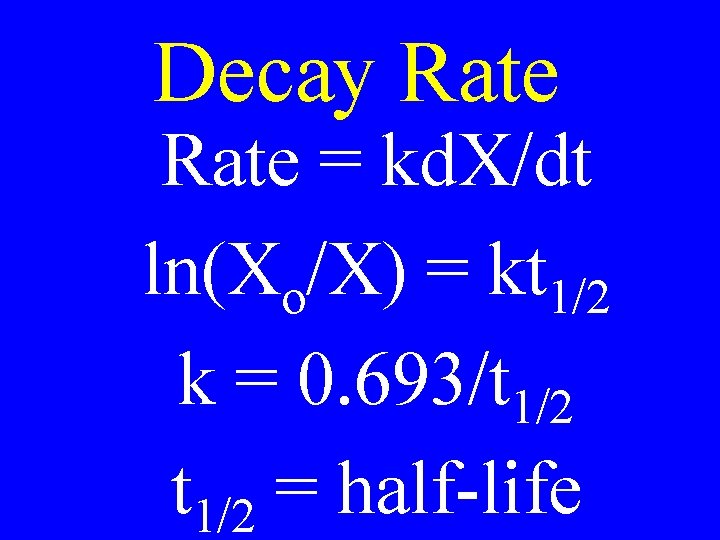
Decay Rate The rate at which a radioactive nucleus breaks down

Half-Life The time it takes for 50 % of the radioactive nucleii to decompose

Decay Rate = kd. X/dt ln(Xo/X) = kt 1/2 k = 0. 693/t 1/2 = half-life

1 st Order Age Dating Formula t = ln(X /X )t i f 1/2 0. 693

Calculate the age of a skeleton found with 0. 125 % C-14 when atmospheric C-14 = 1. 00 %. t 1/2 C-14 = 5720 yr

Calculate the age of a tooth found with 0. 00132 % C-14 when atmospheric C-14 = 1. 00 %. t 1/2 C-14 = 5720

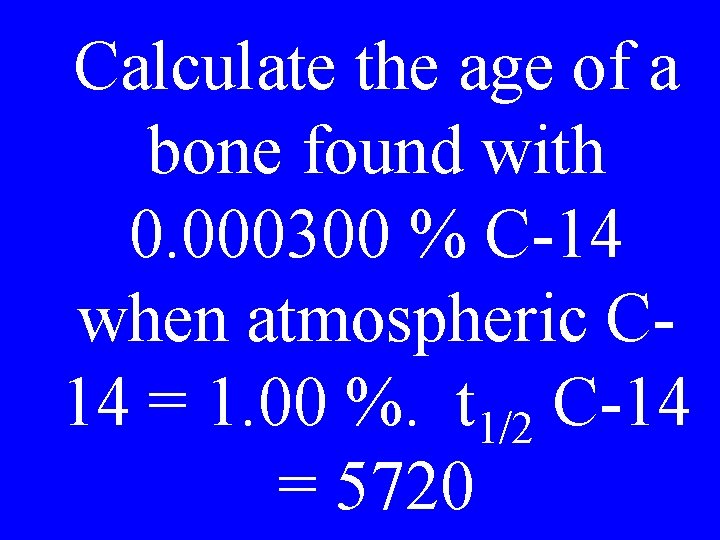
Calculate the age of a bone found with 0. 000300 % C-14 when atmospheric C 14 = 1. 00 %. t 1/2 C-14 = 5720

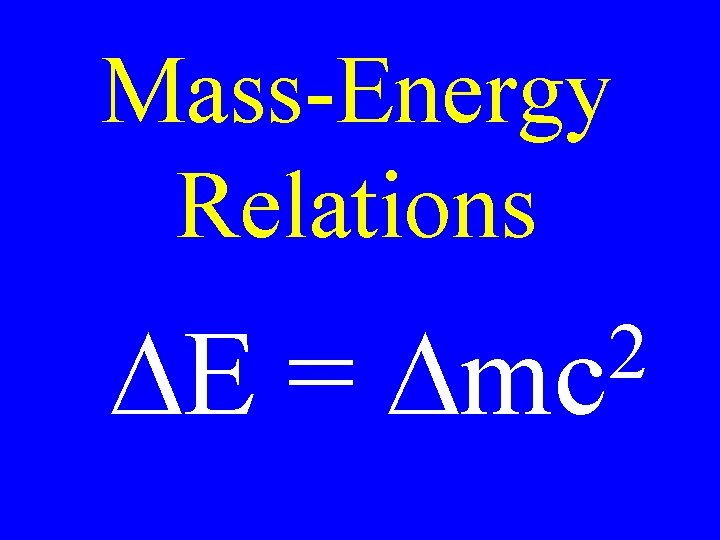
Mass-Energy Relations DE = 2 Dmc

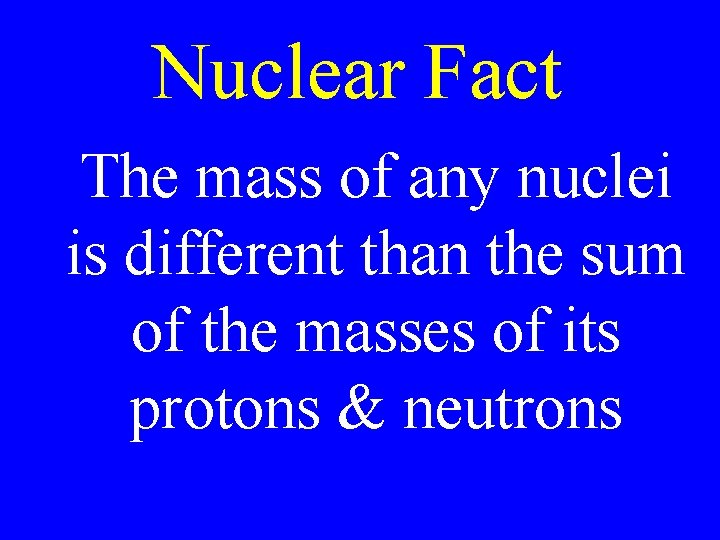
Nuclear Fact The mass of any nuclei is different than the sum of the masses of its protons & neutrons

Nuclear Fact The energy corresponding to the mass difference can be solved using: DE = 2 Dmc

Binding Energy The energy that holds a nucleus together which corresponds to Dm of nucleus

In an atomic bomb, 40. 00 kg of U-235 (235. 401) is split into Ba-144 (14 3. 223) + Kr-89 (89. 335) + 2 neutrons (1. 014). A) Calculate the energy released. B) Calculate the wavelength of the ray

Show neutron bombardment of Ra 223 followed by 3 alpha emissions
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Redox reaction example
Redox reaction example Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Alpha beta gamma neutron
Alpha beta gamma neutron Artificial transmutation equation
Artificial transmutation equation Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Carbon 14 decay equation
Carbon 14 decay equation Two types of nuclear reactions
Two types of nuclear reactions Nuclear bombardment
Nuclear bombardment Nuclear reactions are at
Nuclear reactions are at Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear 4 types of chemical reactions
4 types of chemical reactions 5 types of reactions chemistry
5 types of reactions chemistry Type of reactions chemistry
Type of reactions chemistry Symbols in chemical equations
Symbols in chemical equations Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Slidetodoc.com
Slidetodoc.com Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Solubility rules
Solubility rules Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chernobyl webquest answer key
Chernobyl webquest answer key Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave 25 m/s
25 m/s Nuclear chemistry
Nuclear chemistry Real life examples of fusion
Real life examples of fusion