Nuclear Chemistry Lesson 15 and 16 Nuclear Quest














- Slides: 50

Nuclear Chemistry Lesson 15 and 16 Nuclear Quest and Old Gold

Nuclear Energy and Radiation: A Discussion What do you think? Pros: Crash Course Cons: There Will Be Soft Rains Pros and Cons Defined: Video What do you think now?

Radioactivity An _____atomic nucleus emits a form of radiation (_____, _______ or _______) to become stable. In other words, the nucleus decays into a different _______

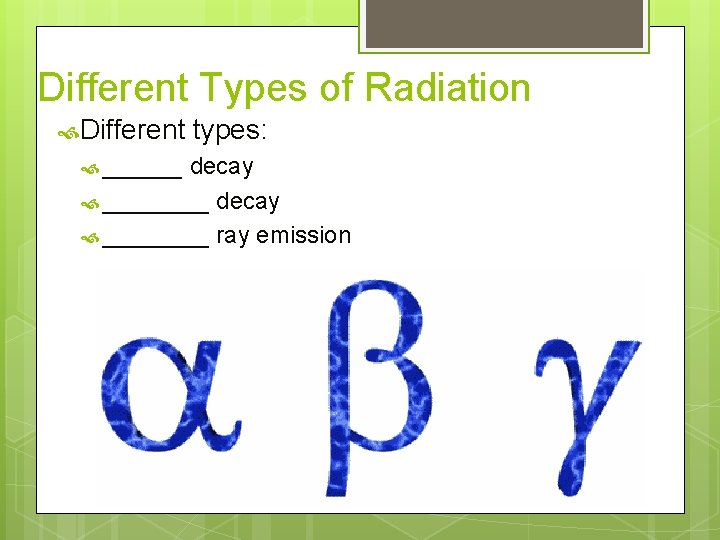
Different Types of Radiation Different ______ types: decay ________ ray emission

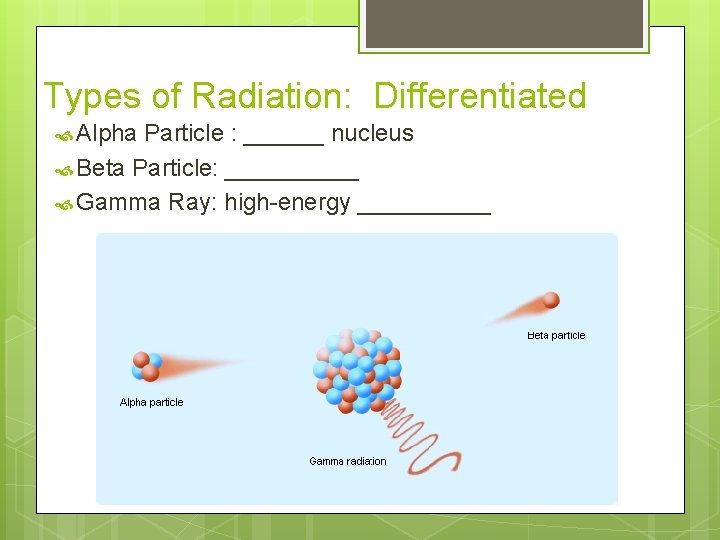
Types of Radiation: Differentiated Alpha Particle : ______ nucleus Beta Particle: _____ Gamma Ray: high-energy _____

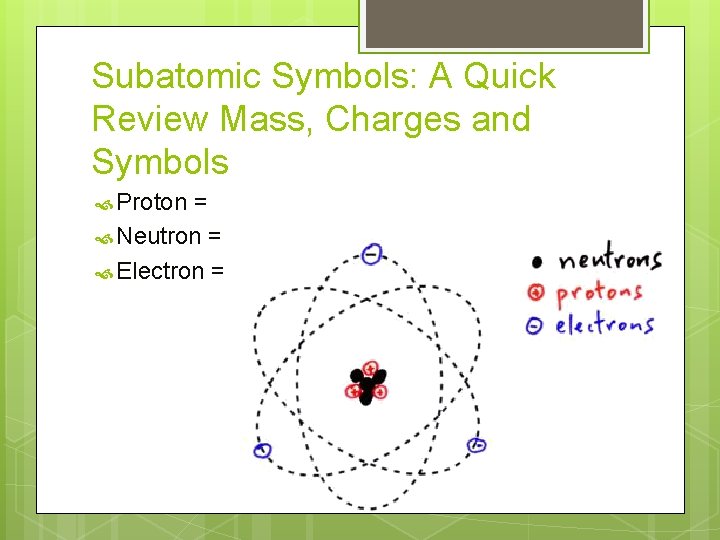
Subatomic Symbols: A Quick Review Mass, Charges and Symbols Proton = Neutron = Electron =

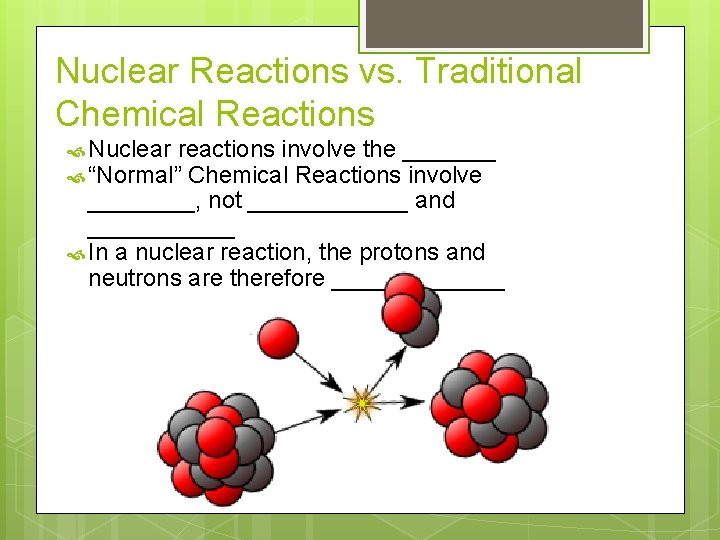
Nuclear Reactions vs. Traditional Chemical Reactions Nuclear reactions involve the _______ “Normal” Chemical Reactions involve ____, not ______ and ______ In a nuclear reaction, the protons and neutrons are therefore _______

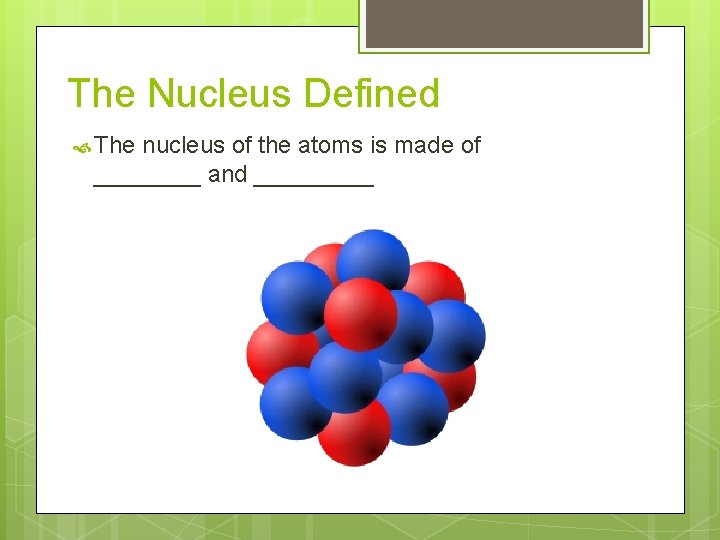
The Nucleus Defined The nucleus of the atoms is made of ____ and _____

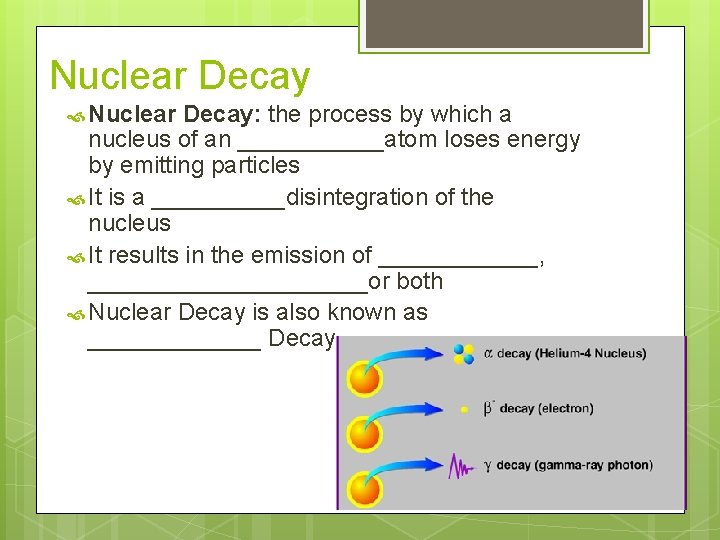
Nuclear Decay Nuclear Decay: the process by which a nucleus of an ______atom loses energy by emitting particles It is a _____disintegration of the nucleus It results in the emission of ______, ___________or both Nuclear Decay is also known as _______ Decay

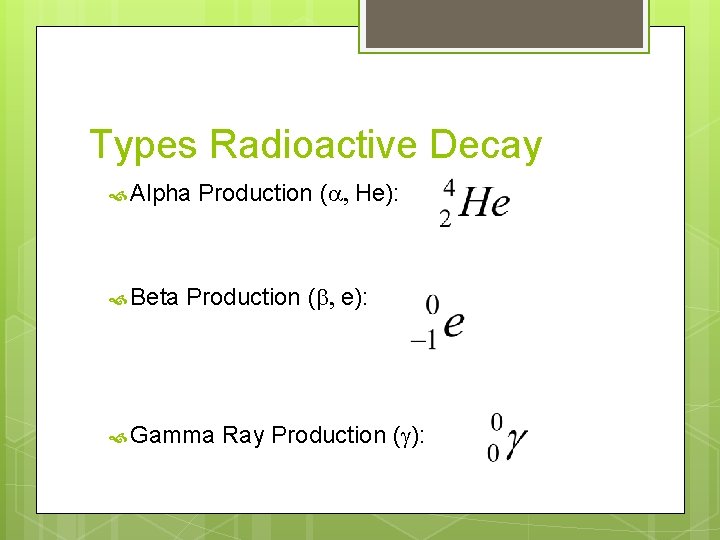
Types Radioactive Decay Alpha Beta Production (a, He): Production (b, e): Gamma Ray Production (g):

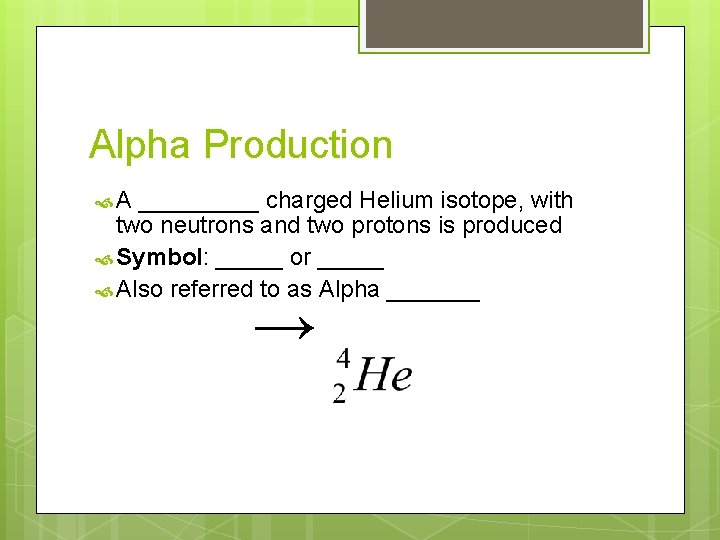
Alpha Production A _____ charged Helium isotope, with two neutrons and two protons is produced Symbol: _____ or _____ Also referred to as Alpha _______ →

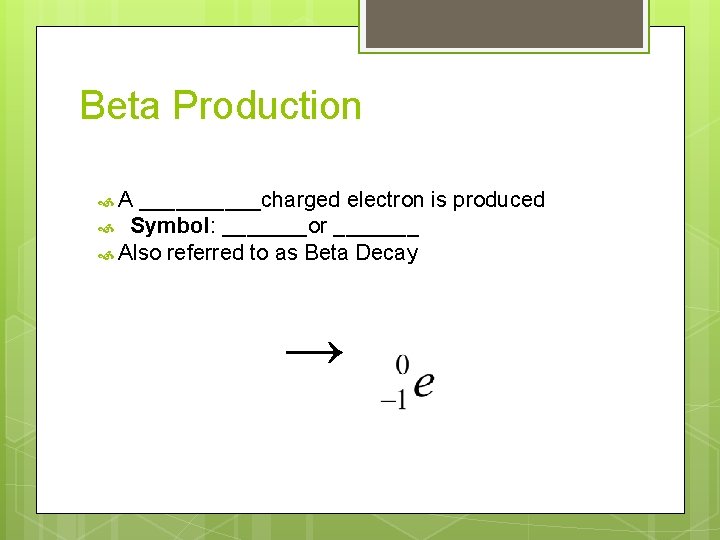
Beta Production A _____charged electron is produced Symbol: _______or _______ Also referred to as Beta Decay →

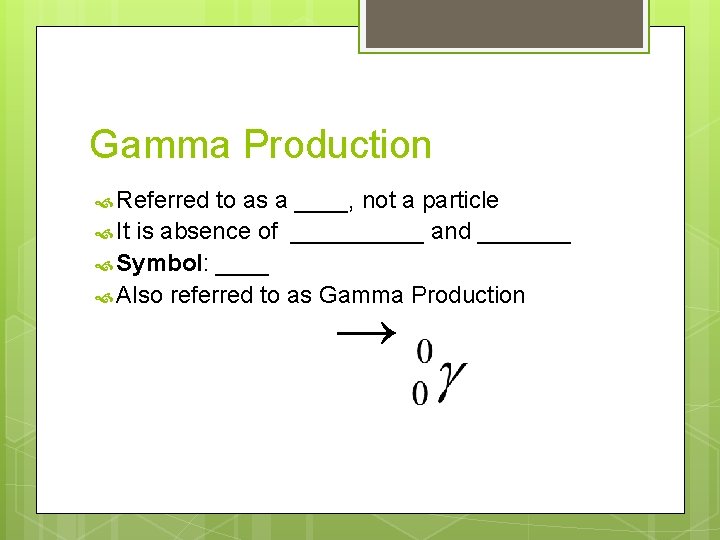
Gamma Production Referred to as a ____, not a particle It is absence of _____ and _______ Symbol: ____ Also referred to as Gamma Production →

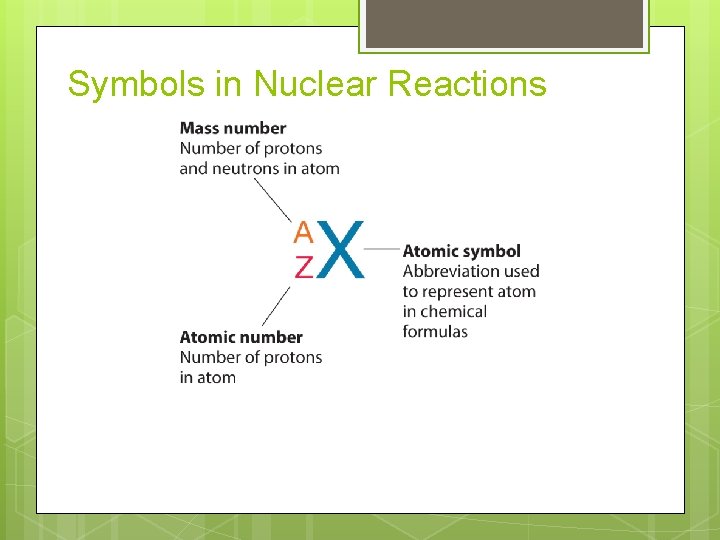
Symbols in Nuclear Reactions

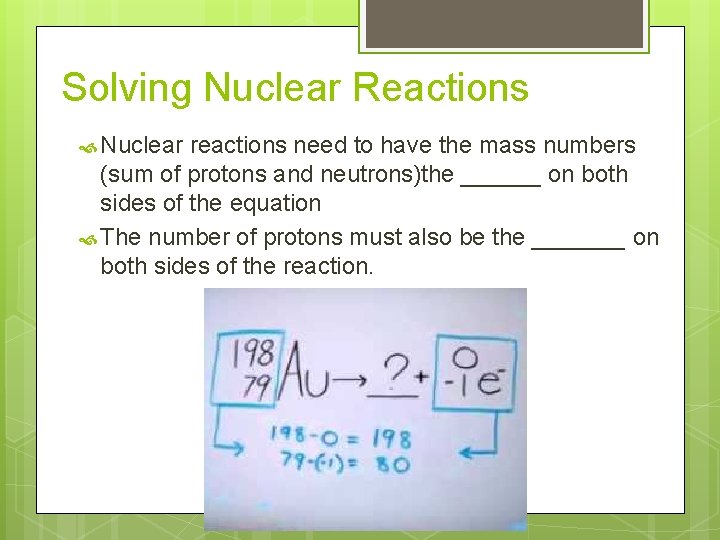
Solving Nuclear Reactions Nuclear reactions need to have the mass numbers (sum of protons and neutrons)the ______ on both sides of the equation The number of protons must also be the _______ on both sides of the reaction.

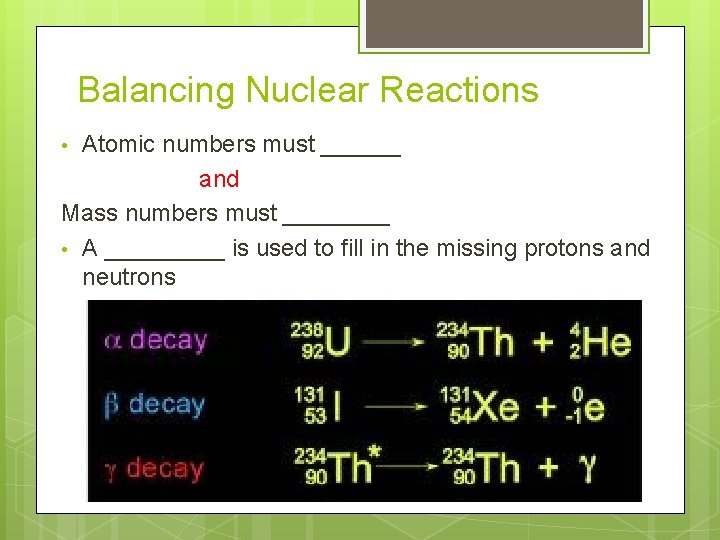
Balancing Nuclear Reactions Atomic numbers must ______ and Mass numbers must ____ • A _____ is used to fill in the missing protons and neutrons •

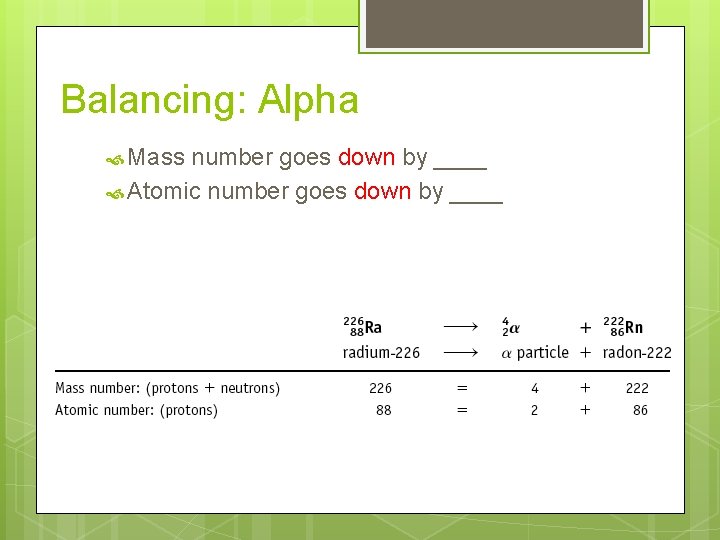
Balancing: Alpha Mass number goes down by ____ Atomic number goes down by ____

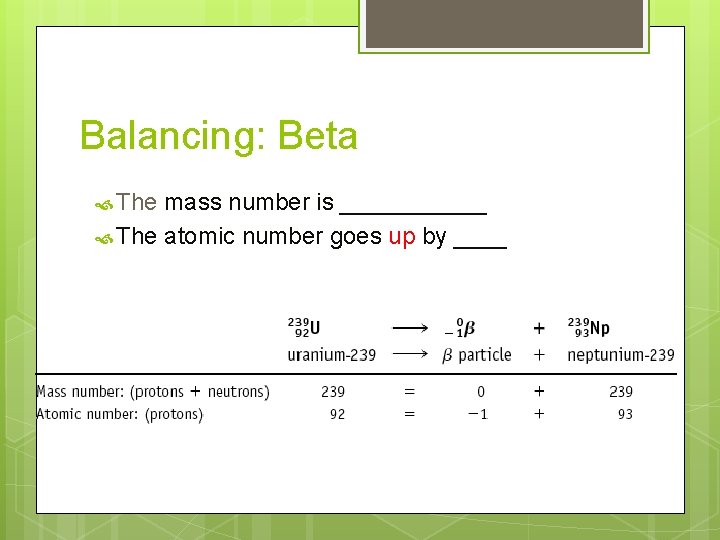
Balancing: Beta The mass number is ______ The atomic number goes up by ____

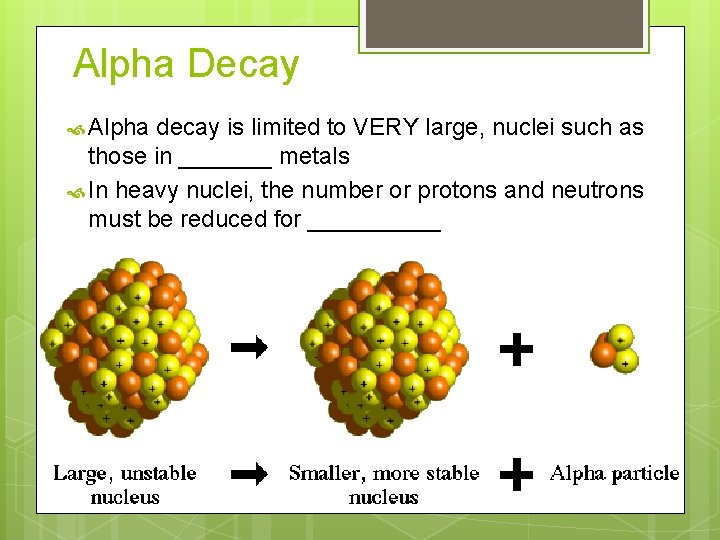
Alpha Decay Alpha decay is limited to VERY large, nuclei such as those in _______ metals In heavy nuclei, the number or protons and neutrons must be reduced for _____

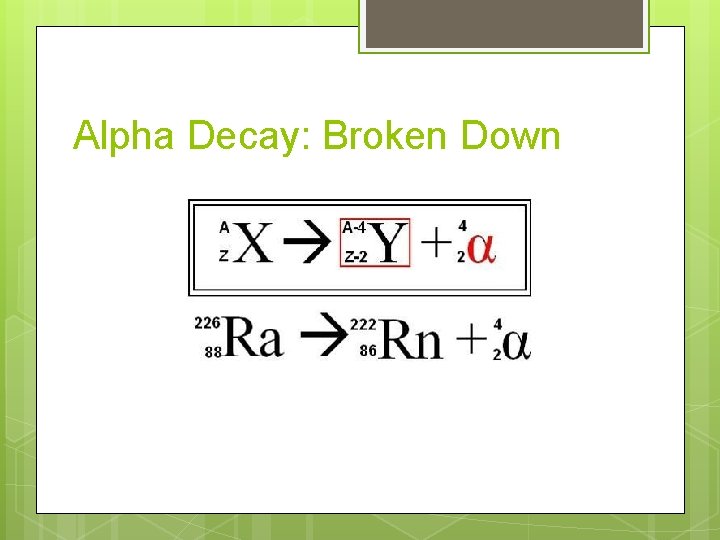
Alpha Decay: Broken Down

An Example Solution: Alpha Decay If you know that A-241, undergoes alpha decay, what will the nuclear reaction look like? 241 95 Am→ ZAX + 42 He 2 A = number of protons = _____ X = the element with atomic number = _____ Z = Mass number =_____ Therefore 241 95 Am→ X + 42 He 2, where X represents______

Alpha Decay Problem Write the alpha decay of Uranium-238

Beta Decay Elements that undergo beta decay have too many _____ Therefore they convert a neutron to a proton and an _____ or beta particle

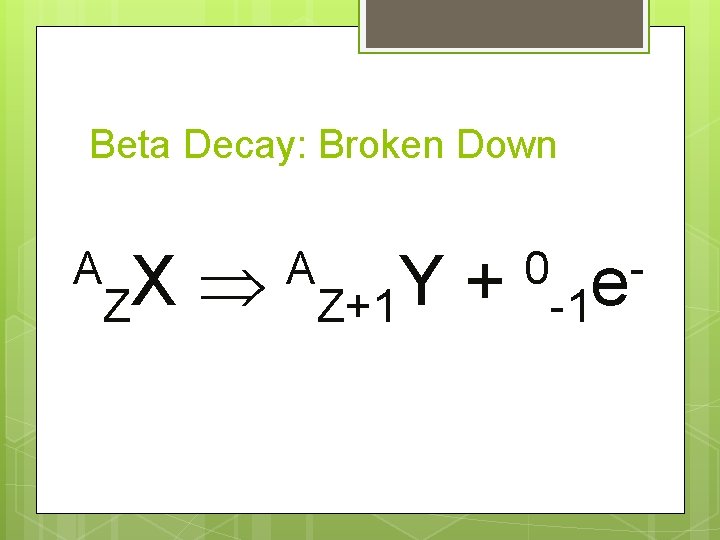
Beta Decay: Broken Down A X Z A Y + Z+1 0 -1 e

Beta Decay Problem Write the beta decay of Cs-137 55 Cs

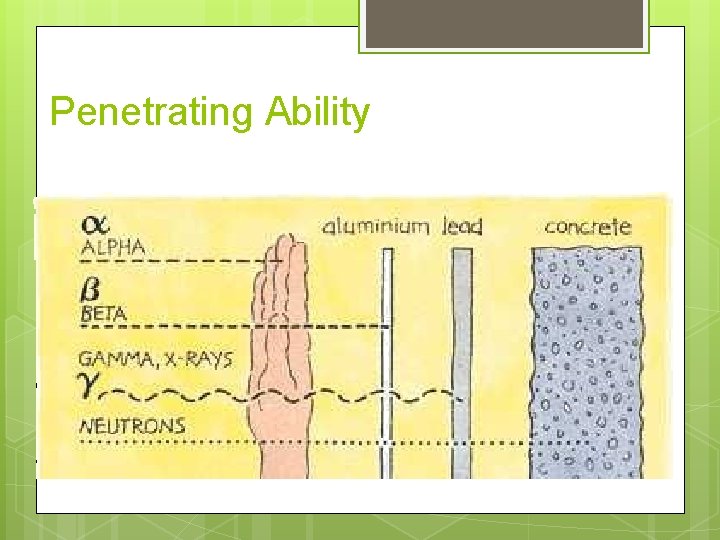
Gamma Radiation Symbol: ______ No _______, no _______ Usually emitted in _____ with other radiation types Highest _____ power requires several inches lead shielding

Gamma Radiation: an Example

Nuclear Decay Practice

Penetrating Ability

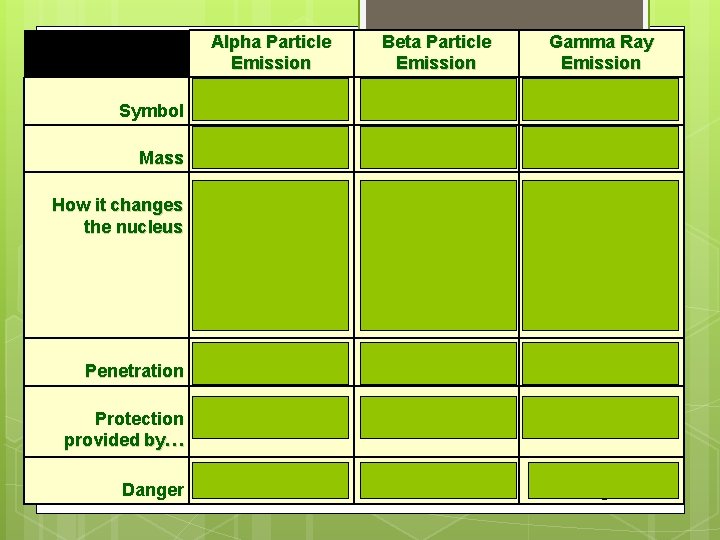
Symbol Mass How it changes the nucleus Alpha Particle Emission Beta Particle Emission or or Heavy Light Decreases the mass number by 4 Decreases the atomic number by 2 Converts a neutron into a proton Increases atomic number by 1 Gamma Ray Emission No Mass No change to the nucleus Penetration Low Medium High Protection provided by… Skin Paper, clothing Lead Danger Low Medium High

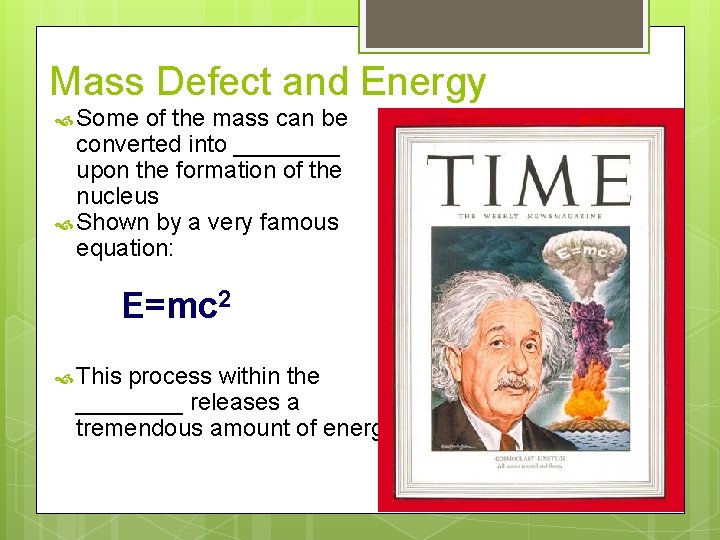
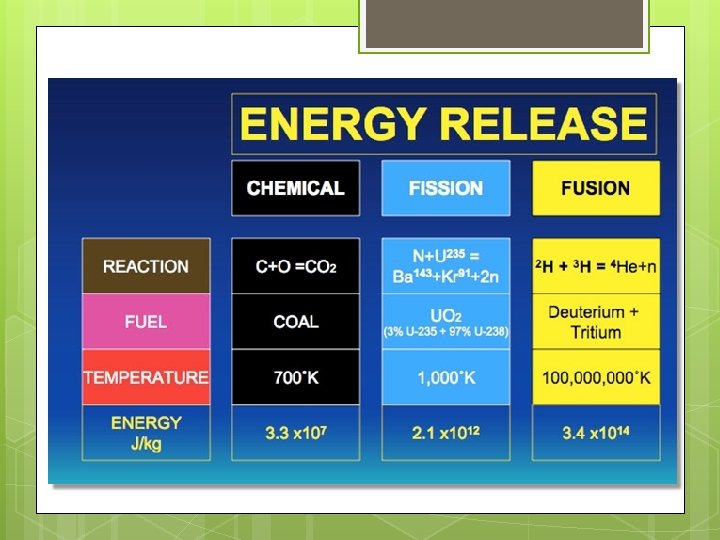
Mass Defect and Energy Some of the mass can be converted into ____ upon the formation of the nucleus Shown by a very famous equation: E=mc 2 This process within the ____ releases a tremendous amount of energy

Stability The stability of the nucleus determines the nuclear _______ of an element Stable nucleus ____decay spontaneously Unstable nucleus _______decay spontaneously AND would lead to the emission of different forms of ______

Nuclear Energy Nuclear Energy: Energy associated with and contained in the _______ Nuclear _________ Ex: Sun (fusion) Nuclear Reactors Bombs

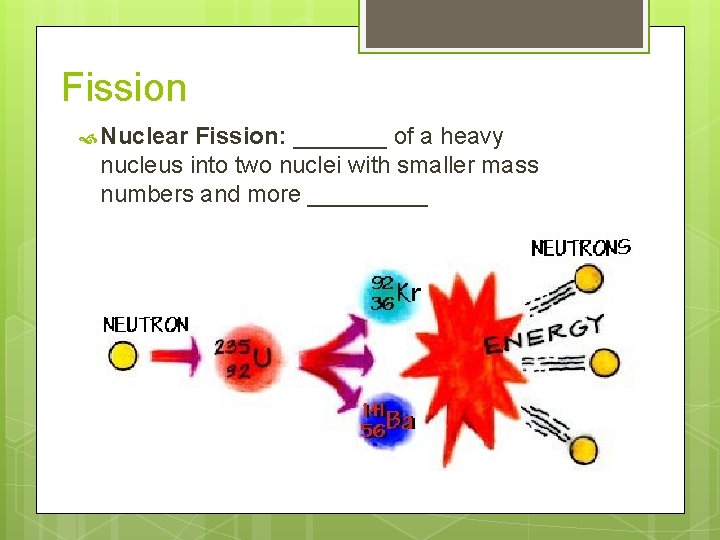
Fission Nuclear Fission: _______ of a heavy nucleus into two nuclei with smaller mass numbers and more _____

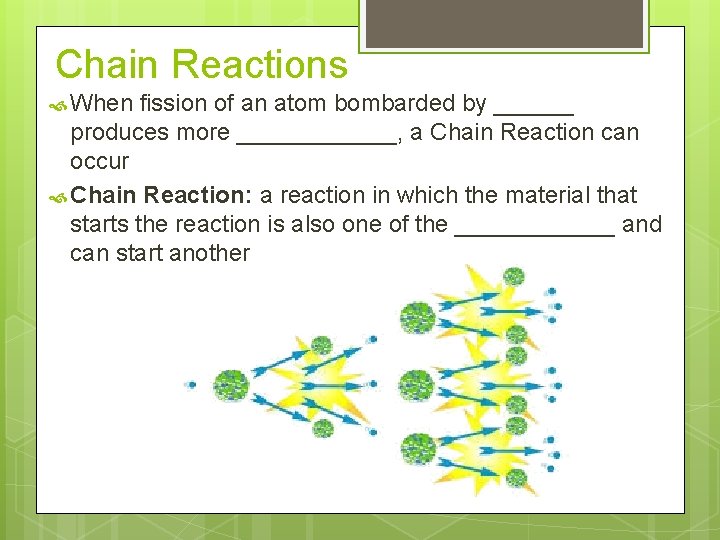
Chain Reactions When fission of an atom bombarded by ______ produces more ______, a Chain Reaction can occur Chain Reaction: a reaction in which the material that starts the reaction is also one of the ______ and can start another

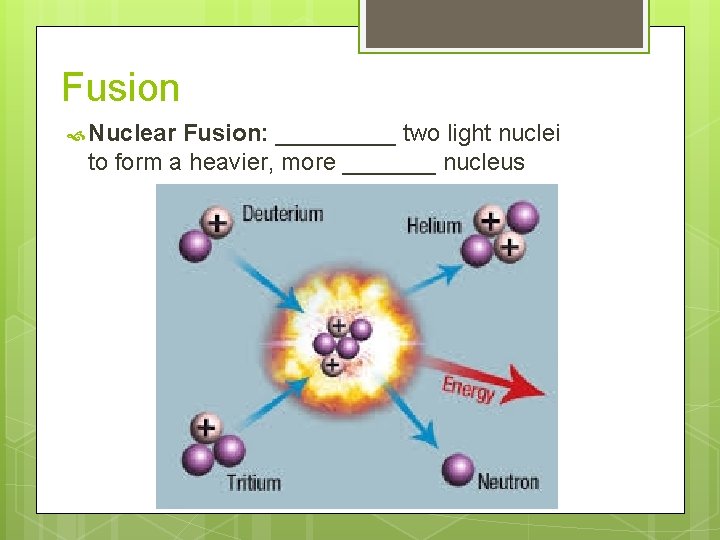
Fusion Nuclear Fusion: _____ two light nuclei to form a heavier, more _______ nucleus

Nuclear Power, A Quick Perspective In America, about ____% electricity generated by nuclear _______ Imagine: Nuclear-powered car Fuel = pencil-sized U-cylinder Energy = 1000, 20 -gallon tanks of gasoline Refuel every _______ weeks (about ____years)

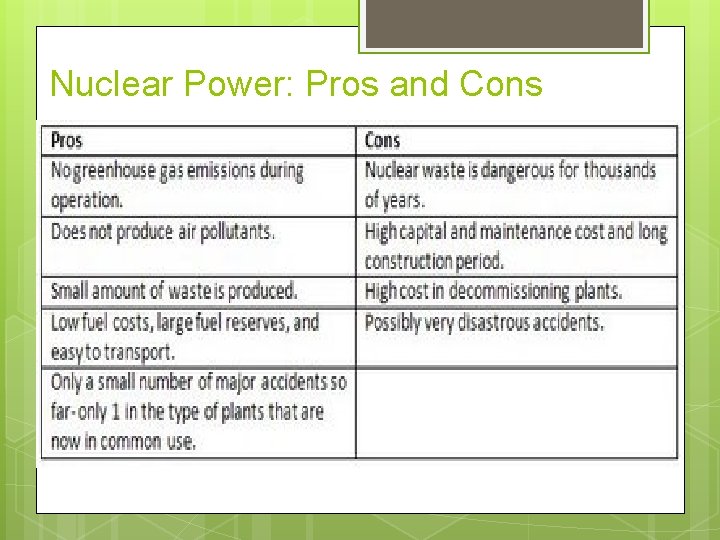
Nuclear Power: Pros and Cons Can you think of some?

Nuclear Power: Pros and Cons


Effects of Radiation Effects 1. of radiation on life: Acute Radiation Can Effect: Intestinal lining, Immune response Cells, Likelihood of ______ This depends on _____ and _____ 2. Increased _______ risk 3. _____ effects

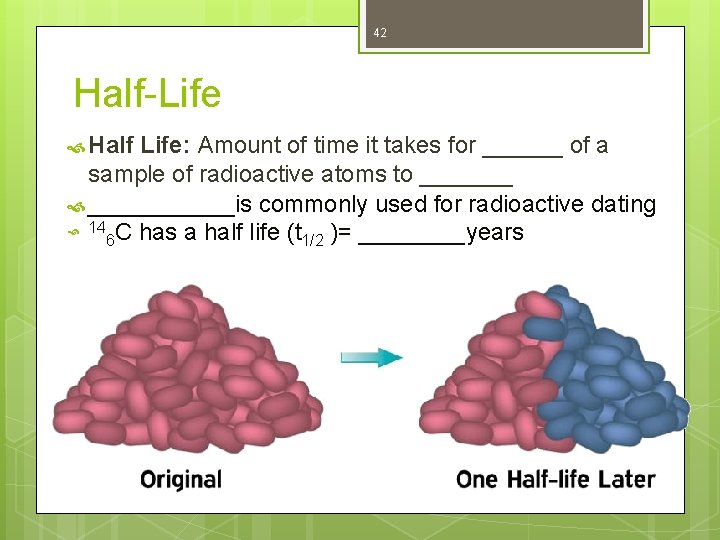
42 Half-Life Half Life: Amount of time it takes for ______ of a sample of radioactive atoms to _______ ______is commonly used for radioactive dating 14 C has a half life (t 6 1/2 )= ____years

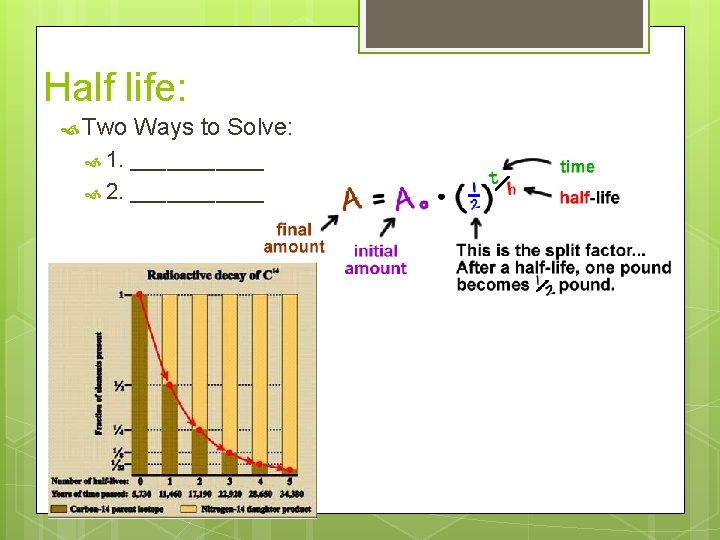
Half life: Two 1. Ways to Solve: _______ 2. _______

44 Half-Life Calculation #1 You have 400 mg of a radioisotope with a half -life of 5 minutes. How much will be left after 30 minutes?

Using a table to solve

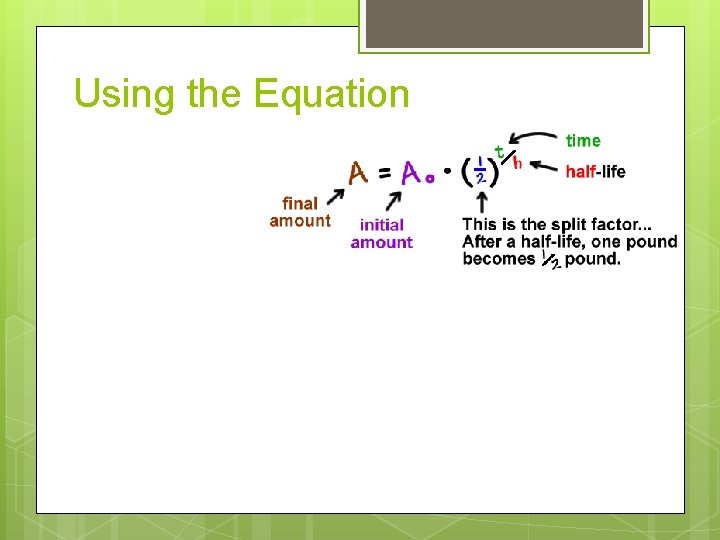
Using the Equation

47 Half-Life Calculation #2 Suppose you have a 100 mg sample of Au 191, which has a half-life of 3. 4 hours. How much will remain after 10. 2 hours?

Using a table to solve

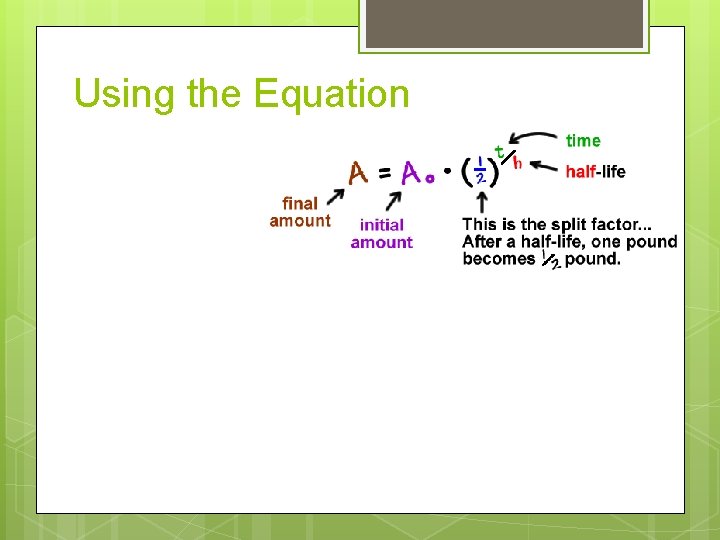
Using the Equation

50 Half Life Practice