Nuclear Chemistry Nuclear Nucluar Nuclear Reactions vs Normal









- Slides: 21

Nuclear Chemistry Nuclear ≠ Nucluar

Nuclear Reactions vs. Normal Chemical Changes • • • Nuclear reactions involve the nucleus Protons and neutrons are rearranged Releases a tremendous amount of energy – called binding energy • “Normal” Chemical Reactions involve electrons, not protons and neutrons

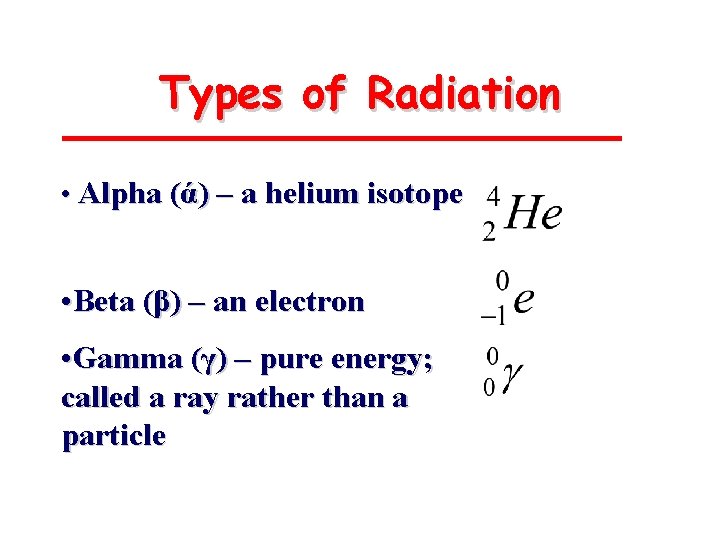
Types of Radiation • Alpha (ά) – a helium isotope • Beta (β) – an electron • Gamma (γ) – pure energy; called a ray rather than a particle

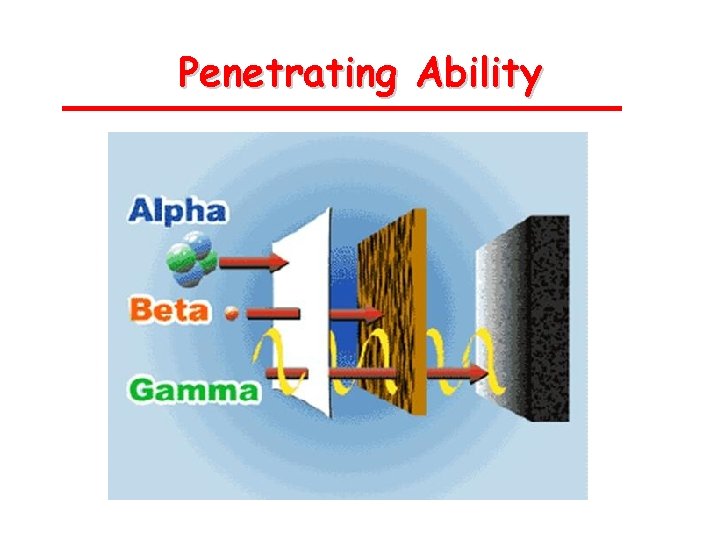
Penetrating Ability

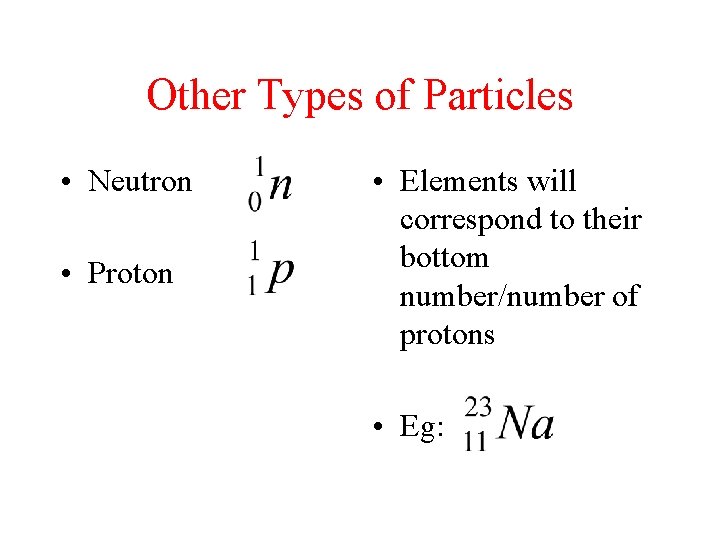
Other Types of Particles • Neutron • Proton • Elements will correspond to their bottom number/number of protons • Eg:

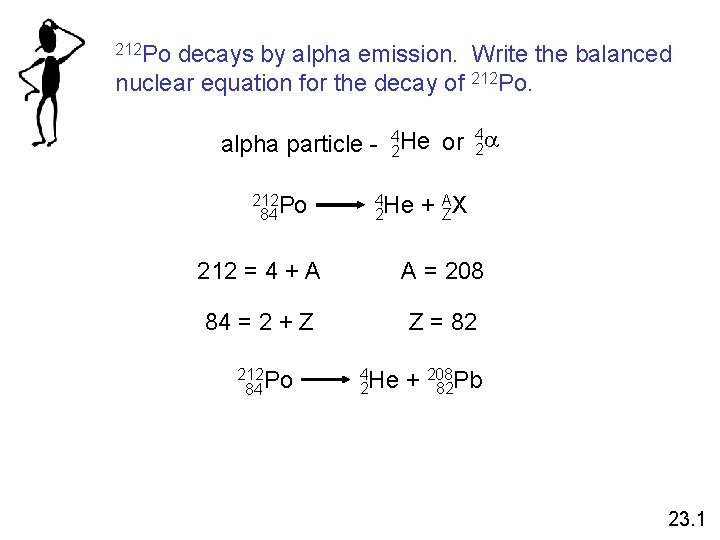
212 Po decays by alpha emission. Write the balanced nuclear equation for the decay of 212 Po. 4 alpha particle - 42 He or 2 a 212 Po 84 4 He 2 + AZX 212 = 4 + A A = 208 84 = 2 + Z Z = 82 212 Po 84 4 He 2 + 208 82 Pb 23. 1

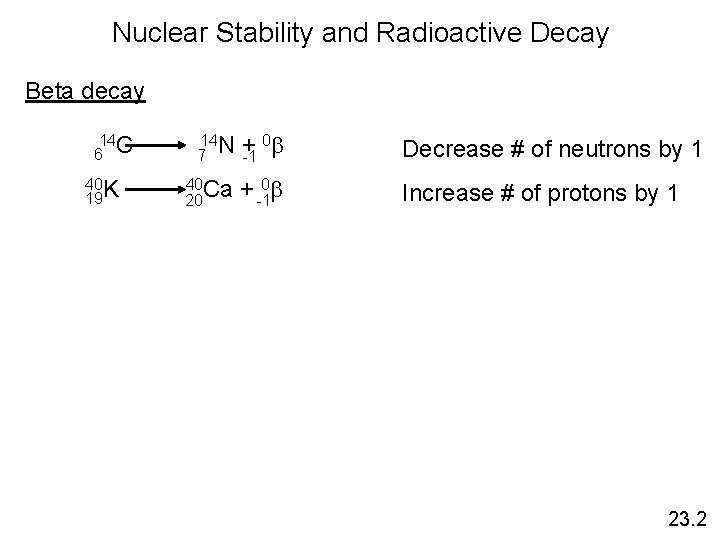
Nuclear Stability and Radioactive Decay Beta decay 14 6 C 40 K 19 14 N + 0 b 7 -1 40 Ca 20 + -10 b Decrease # of neutrons by 1 Increase # of protons by 1 23. 2

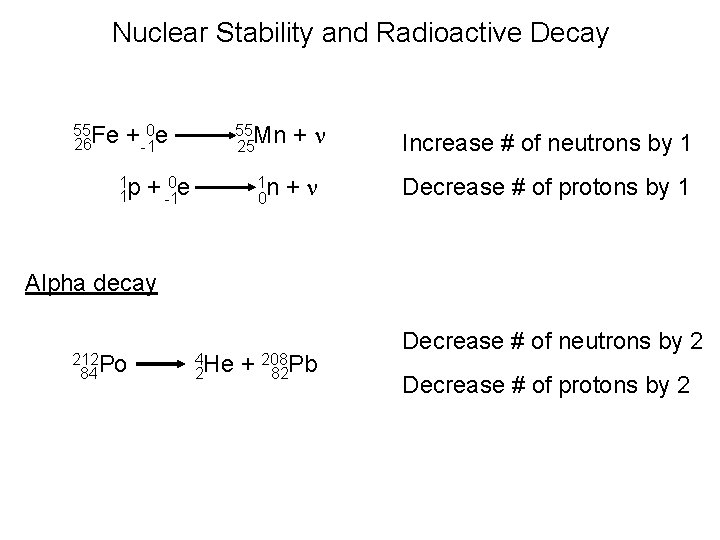
Nuclear Stability and Radioactive Decay 55 Fe 26 + -10 e 1 p 1 55 Mn 25 + -10 e 1 n 0 +n +n Increase # of neutrons by 1 Decrease # of protons by 1 Alpha decay 212 Po 84 4 He 2 + 208 82 Pb Decrease # of neutrons by 2 Decrease # of protons by 2

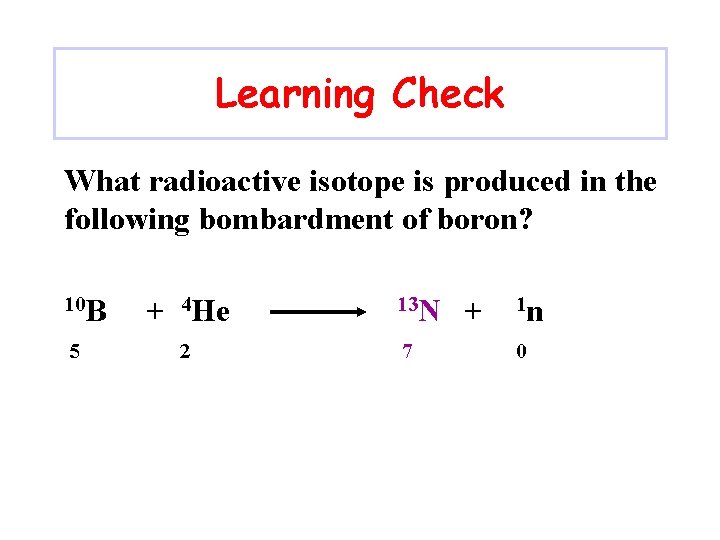
Learning Check What radioactive isotope is produced in the following bombardment of boron? 10 B 5 + 4 He 2 ? + 1 n 0

Learning Check What radioactive isotope is produced in the following bombardment of boron? 10 B 5 + 4 He 2 13 N 7 + 1 n 0

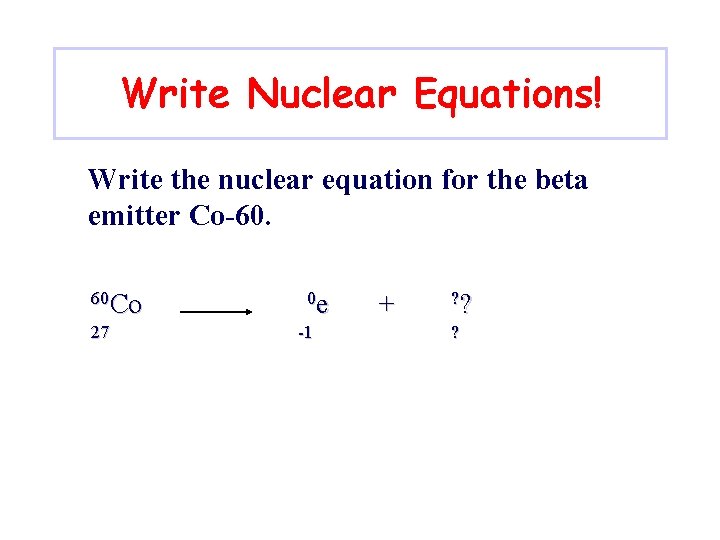
Write Nuclear Equations! Write the nuclear equation for the beta emitter Co-60. 60 Co 27 0 e -1 + ? ? ?

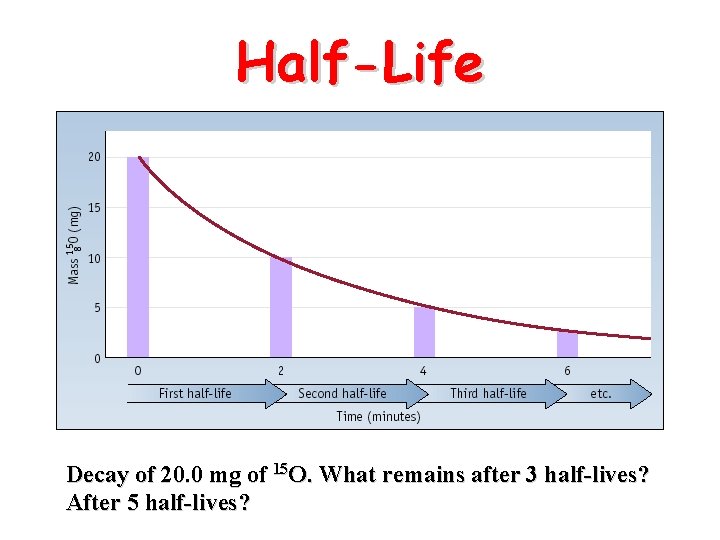
Half-Life • HALF-LIFE is the time that it takes for 1/2 a sample to decompose.

Half-Life Decay of 20. 0 mg of 15 O. What remains after 3 half-lives? After 5 half-lives?

Sample question • How much U-238 is left over if a 100 gram sample undergoes 8 half lives?

Learning Check! The half life of I-123 is 13 hr. How much of a 64 mg sample of I-123 is left after 39 hours?

Last learning check • What fraction of an unknown mass of Iodine, which has a half life of 8 hours, would you expect to be left after 72 hours?

Nuclear Fission • Larger and unstable nuclei being bombarded with particles and breaking into smaller nuclei and releasing energy at the same time.

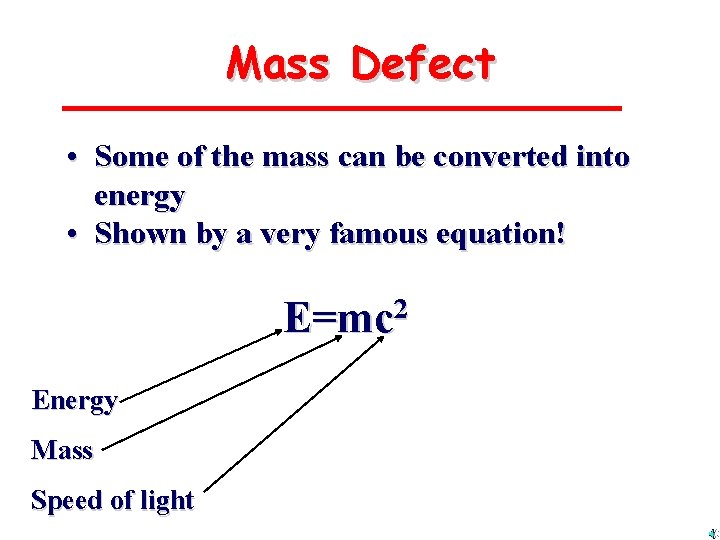
Mass Defect • Some of the mass can be converted into energy • Shown by a very famous equation! E=mc 2 Energy Mass Speed of light

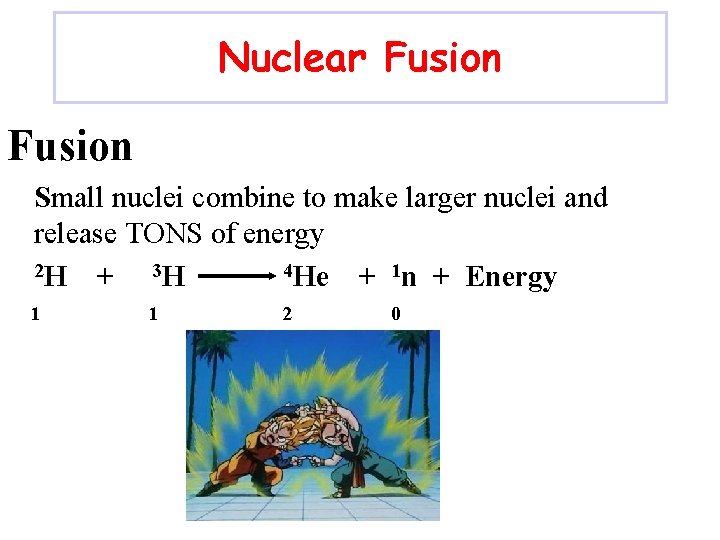
Nuclear Fusion Small nuclei combine to make larger nuclei and release TONS of energy 2 H 4 He + 3 H + 1 n + Energy 1 1 2 0

Nuclear Fusion • Excessive heat can not be contained • Attempts at “cold” fusion have FAILED. • “Hot” fusion is difficult to contain

Summarize what you’ve learned