Nuclear Chemistry What is nuclear chemistry Most chemical





































































- Slides: 80

Nuclear Chemistry

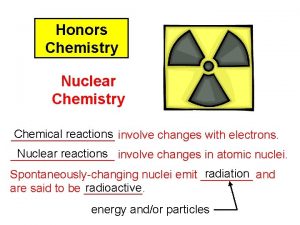
What is nuclear chemistry? Most chemical reactions involve exchange or sharing of electrons

What is nuclear chemistry? Nuclear chemistry is quite different because it involves changes in the nucleus.



The Three Mile Island nuclear plant on Thursday, March 11, 2004 in Pennsylvania's Londonderry Twp. It was 25 years ago on March 28, 1979 that an accident in the Unit Two nuclear reactor at the plant caused a near meltdown of the unit's core.

PRISCILLA was a 37 kiloton balloon shot fired June 24, 1957 at the Nevada Test Site.

Transmutation When the nucleus of one element is changed into the nucleus of a different element the reaction is called a transmutation.

Why do transmutations occur? Because of the stability of the nucleus

Most nuclei are stable The ratio of protons to neutrons determines the stability The ratio in all nuclei with atomic numbers greater than 83 makes them unstable

Radioactive Because of their instability, all nuclei with atomic numbers >83 are said to be radioactive.

Radioisotopes For any element, an isotope that is unstable (and thus radioactive) is called a radioisotope.

An unstable nucleus spontaneously decays, forming products that are more stable

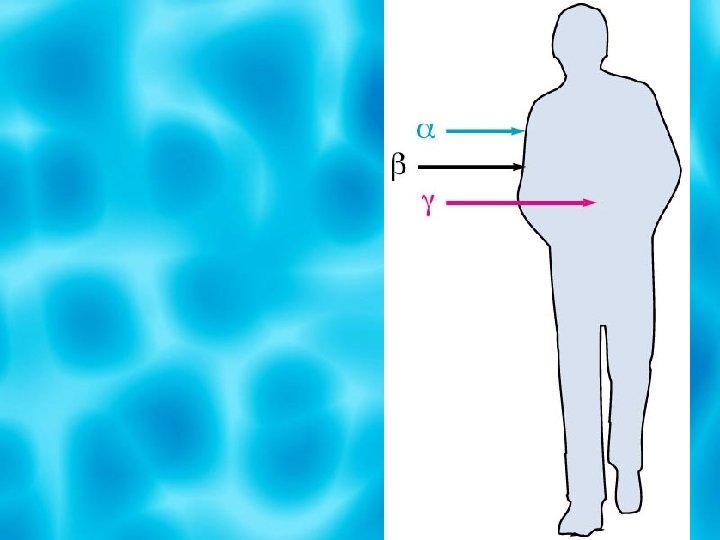
When a nucleus decays, it emits radiation in the form of Alpha particles Beta particles Gamma Particles

Alpha Particle Is a helium nucleus composed of two protons and two neutrons It is represented by the 4 He, symbol 2 or the symbol , which is the greek letter alpha

Beta Particle Is an electron whose source is an atomic nucleus It is represented by the symbol , which is the Greek letter beta.

What’s a positron? A positron is identical to an electron, except that it has a positive charge

Gamma Rays Almost all nuclear decay also releases some energy in the form of gamma rays Gamma rays similar to X-Rays, but have more energy.

Demo Geiger Counter


Right now I'm having amnesia and deja vu at the same time. I think I've forgotten this before. Steven Wright

Challenge Question is unstable and emits an alpha particle. 226 88 Ra Is the radium atom still a radium atom? If not, what is it?

Nuclear Equations Nuclear reactions can be represented by equations As in chemical equations, mass and charge must balance on both sides

Alpha Decay Example on overhead Alpha decay summary atomic number decreases by two number of protons decreases by two number of neutrons decreases by two mass number decreases by four

Beta Decay Summary atomic number increases by 1 number of protons increases by 1 number of neutrons decreases by 1 mass number remains the same

Sample Problem Sample of Beta Decay Sample of Positron Emmission

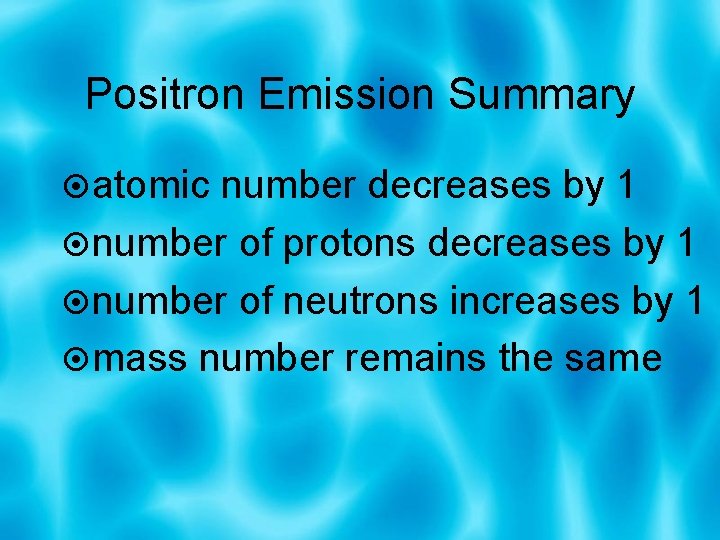
Positron Emission Summary atomic number decreases by 1 number of protons decreases by 1 number of neutrons increases by 1 mass number remains the same

Half-Life

Radioactive substances decay at a constant rate The rate of decay does not depend on temperature, pressure, or concentration

Decay of an individual nucleus is a random event – we can’t predict when its going to occur

However, the number of unstable nuclei that will decay in a given time in a sample of the element can be predicted.

Half-Life The time it takes for half of the atoms in a given sample to decay is called the half-life of the element

Half-Life Each isotope has its own half- life (See Reference Tables Table N) The shorter the half-life of an isotope, the less stable it is.


Chemistry Lab Half-Life of Red Licorice

Half-Life Equation The fraction remaining after a given number of half-lives is calculated using the relationship fraction remaining = (1/2)t/T where: t = total elapsed time T = half-life

Half-Life Equation (Con’t) The quantity t/T is the number of half-life periods = t/T (Reference Tables Table T)

Sample Problem Most chromium atoms are stable, but Cr-51 is an unstable isotope with a half-life of 28 days. ØWhat fraction of a sample of Cr-51 will remain after 168 days? ØIf a sample of Cr-51 has an original mass of 52. 0 g, what mass will remain after 168 days?

Sample Problem How much was present originally in a sample of Cr-51 if 0. 75 mg remains after 168 days?

Determining Half-Life Graphically Illustrate on overhead Reference Licorice Lab

Nuclear Energy


Challenge Question Why do nuclear reactions produce so much energy?

Artificial Radioactivity Elements can be made radioactive by bombarding their nuclei with high energy particles.

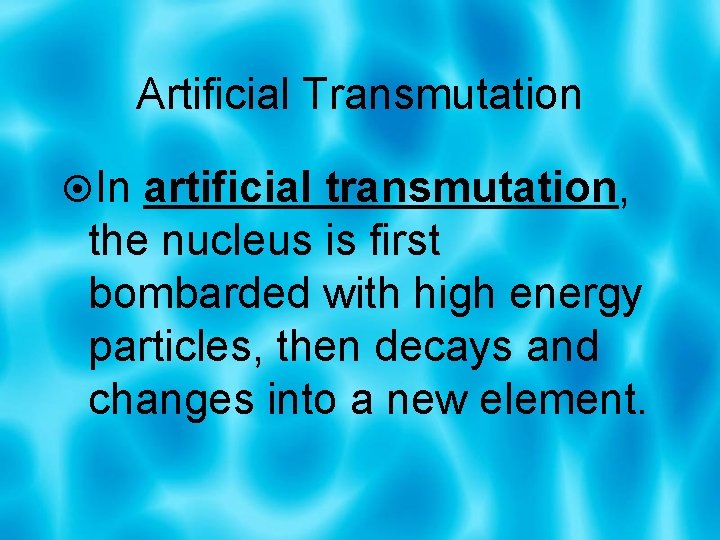
Artificial Transmutation In artificial transmutation, the nucleus is first bombarded with high energy particles, then decays and changes into a new element.

What “High energy particles”? Protons 1 Neutrons 1 n 0 1 H Alpha particles 2 4 He (Reference Tables Table O)

Example - Artificial Transmutation 13 27 Al + 24 He -------> 1530 P + 01 n

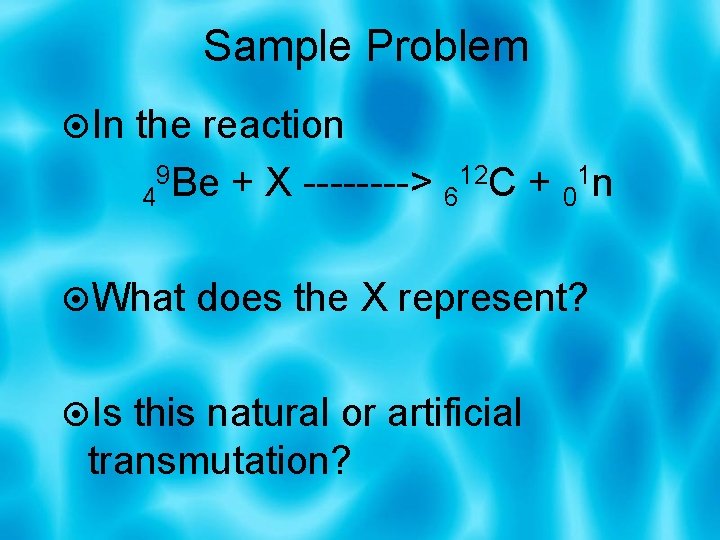
Sample Problem In the reaction 4 9 Be + X ----> 612 C + 01 n What does the X represent? Is this natural or artificial transmutation?

Nuclear Reactions and Energy Turns out (in the unusual case of a nuclear reaction) mass can be converted to energy E = mc 2

Nuclear Reactions and Energy Converting a little mass makes a lot of energy

Nuclear Reactions and Energy The total mass of a nucleus is less than the sum of the masses of the individual protons and neutrons. The matter that has been converted into energy is known as the mass defect.

Fission Reactions In a fission reaction, a high energy particle (usually a neutron) is captured by a nucleus, causing it to go unstable, and split. When the nucleus splits, a lot of energy is released.



Chemistry Lab Chain Reactions

Challenge Question Why are neutrons usually used to initiate a fission reaction? Answer: They’re neutral, and therefore aren’t repelled by the positive charge of the nucleus.

Fusion Reactions Involve the combining of light nuclei to form heavier ones. Best example - on the sun, hydrogen nuclei react in a series to form helium and release lots of energy.

Fusion Reactions Require extremely high temperatures and pressures Major advantage - products are not highly radioactive, like the products of fission reactions.



Activity With a partner, research the pro’s and cons of nuclear energy. In your opinion, should we use nuclear energy?

Chain Reaction Lab Page 805 in your textbook. Write out lab on separate sheet of paper Be sure to include your name, title of the lab, procedure (out of your text), summary of your findings, and your answers to the analysis questions

Uses and Dangers of Radioisotopes

Animals and plants have a known proportion of Carbon-14 (a radioisotope of Carbon) in their tissues. When they die they stop taking Carbon in, then the amount of Carbon-14 goes down at a known rate (Carbon-14 has a halflife of 5700 years). The age of the ancient organic materials can be found by measuring the amount of Carbon-14 that is left. Dating

Radioactive Tracers Radioisotopes can be used for medical purposes, such as checking for a blocked kidney. To do this a small amount of Iodine-123 is injected into the patient, after 5 minutes 2 Geiger counters are placed over the kidneys.

Radioactive Tracers The most common tracer is called Technetium-99 and is very safe because it only emits gamma rays and doesn't cause much ionization.

Radioactive Tracers Also radioisotopes are used in industry, to detect leaking pipes. To do this, a small amount is injected into the pipe. It is then detected with a GM counter above ground.

Smoke alarms contain a dustrial Applications weak source made of Americium-241. Alpha particles are emitted from here, which ionize the air, so that the air conducts electricity and a small current flows. If smoke enters the alarm, this absorbs the alpha particles, the current reduces, and the alarm sounds. Am-241 has a half-life of 460 years.

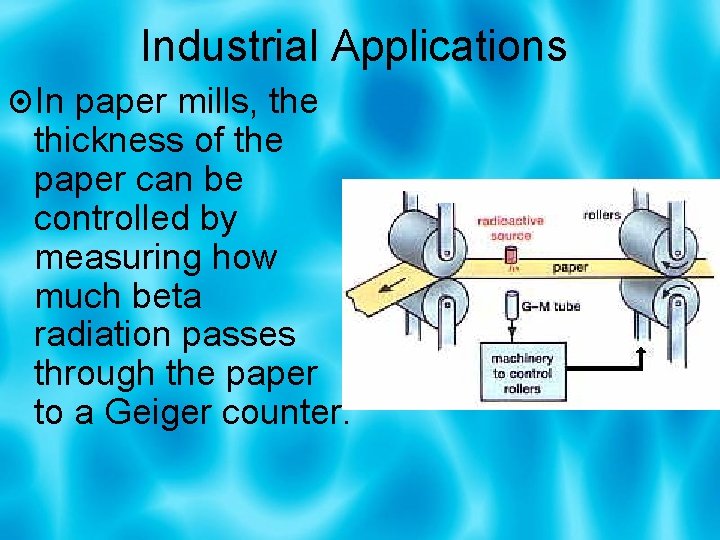
Industrial Applications In paper mills, the thickness of the paper can be controlled by measuring how much beta radiation passes through the paper to a Geiger counter.

Industrial Applications Gamma rays are also used to sterilize hospital equipment, especially plastic syringes that would be damaged if heated.

Industrial Applications Even after it has been packaged, gamma rays can be used to kill bacteria, mould and insects in food. This process prolongs the shelf-life of the food, but sometimes changes the taste.

Industrial Applications Checking Welds If a gamma source is placed on one side of the welded metal, and a photographic film on the other side, weak points or air bubbles will show up on the film, like an Xray.

Medical Applications Because Gamma rays can kill living cells, they are used to kill cancer cells without having to resort to difficult surgery. This is called "Radiotherapy", and works because cancer cells can't repair themselves when damaged by gamma rays, as healthy cells can.

Medical Applications I-131 is used in both detection and treatment of thyroid conditions, because I-131 accumulates in the thyroid gland. Cobalt-60 emits large amounts of gamma radiation that can be aimed at cancerous tumors.

Dangers of Radioisotopes

Alpha Particles are slow, have a short range in air, and can be stopped by a sheet of paper.

Alpha Particles However, they can ionize the atoms in the cells in your body (such as a DNA molecule), scrambling the DNA’s instructions to the body.

Beta Particles Have a longer range than alpha’s, but ionize much less strongly. However, they do have more penetrating power, which means that they can get through your skin and affect cells inside you.

Gamma Rays Hardly ionize atoms at all However, they are very difficult to stop (requires thick lead and/or concrete).

Gamma Rays When they are absorbed by an atom, that atom gains quite a bit of energy, and may then emit other particles. If that atom is in one of your cells, this is not good!
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Nuclear chemistry webquest
Nuclear chemistry webquest Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave Applications of nuclear chemistry
Applications of nuclear chemistry Nuclear chemistry
Nuclear chemistry Rectangle real life examples
Rectangle real life examples Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Why do most atoms form chemical bonds
Why do most atoms form chemical bonds Molecularity
Molecularity Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions A chemist shorthand way of representing chemical reaction.
A chemist shorthand way of representing chemical reaction. Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Equilibrium
Equilibrium Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Most general to most specific classification
Most general to most specific classification Most general to most specific classification
Most general to most specific classification In the name of allah the most
In the name of allah the most In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful سsh
سsh In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful Crayfish taxonomy
Crayfish taxonomy Most general to most specific classification
Most general to most specific classification In the name of god most gracious prayer
In the name of god most gracious prayer In the name of allah the most gracious the most merciful
In the name of allah the most gracious the most merciful In the name of allah the beneficent the merciful
In the name of allah the beneficent the merciful In the name of god most gracious most merciful
In the name of god most gracious most merciful The most gracious
The most gracious In the name of god most gracious prayer
In the name of god most gracious prayer In the name of allah the most beneficent
In the name of allah the most beneficent Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Nucleoplasma
Nucleoplasma Intranet
Intranet Artificial transmutation worksheet
Artificial transmutation worksheet Nuclear concepto
Nuclear concepto Physics topic 12
Physics topic 12 What are fuels used for
What are fuels used for Spindle fibers
Spindle fibers The dyad family adalah
The dyad family adalah Pros of natural gas
Pros of natural gas Gamma emission equation
Gamma emission equation Creencia nuclear
Creencia nuclear Fases de la terapia cognitiva de beck
Fases de la terapia cognitiva de beck Teoria de las panspermia
Teoria de las panspermia Magic number in nuclear physics
Magic number in nuclear physics Difference between nuclear family and joint family
Difference between nuclear family and joint family Skobeltsyn institute of nuclear physics
Skobeltsyn institute of nuclear physics Electron charge
Electron charge Nuclear charge periodic trend
Nuclear charge periodic trend Nuclear charge trend
Nuclear charge trend Fission v fusion
Fission v fusion Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Nuclear membrane job
Nuclear membrane job Q value of nuclear reaction
Q value of nuclear reaction Zeff of chlorine
Zeff of chlorine Agham na pag-aaral ng makabuluhang tunog ng isang wika
Agham na pag-aaral ng makabuluhang tunog ng isang wika