Nuclear Chemistry Radiation and Radioactivity What do you









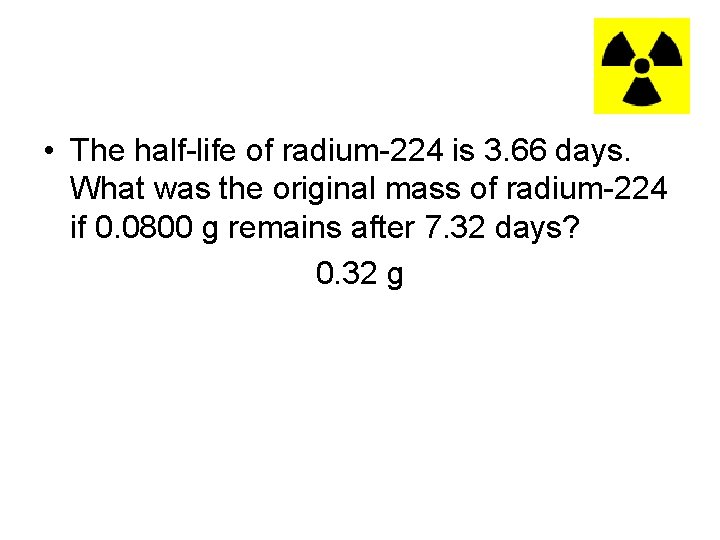
- Slides: 31

Nuclear Chemistry Radiation and Radioactivity

What do you think? T/F • Radioactivity first appeared during WWII. • Atoms cannot be changed from one element to another. • Fission and fusion are the same thing. • Radioactivity lasts forever. • Exposure to radiation makes something radioactive. • Nuclear power plants can explode like bombs. • Radioactivity is man-made. • When radioactive substances decay, they disappear.

What is radiation? • Electromagnetic Radiation – Electric Fields – Magnet Fields – Fields oscillate and travel in waves

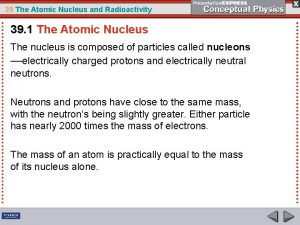
Rules of Radiation • An atom will release energy as electromagnetic radiation in order to become stable. • A stable nucleus has at least as many or more neutrons as protons. • Atoms with a mass #209 or greater are never stable.

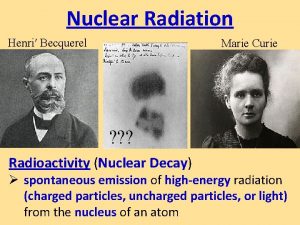
Decay doesn’t mean disappear! • Radioactive decay is the process by which the nucleus of an unstable atom loses energy by emitting ionizing radiation. • The emission is spontaneous, in that the atom decays without any interaction with another particle from outside the atom

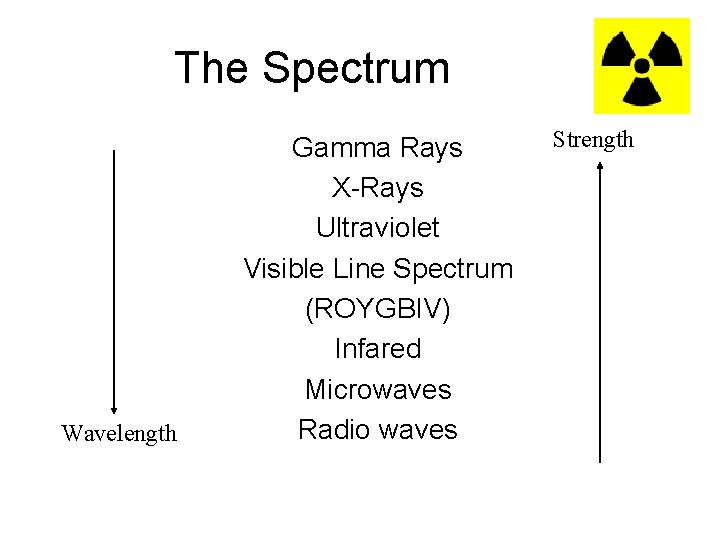
The Spectrum Wavelength Gamma Rays X-Rays Ultraviolet Visible Line Spectrum (ROYGBIV) Infared Microwaves Radio waves Strength

Fission or Fusion? • Fission: one atom splits into two – Uranium-235 to Barium and Krypton – Used in medical radiology • Fusion: two atoms join to form one new – The Sun – H + H = He + energy

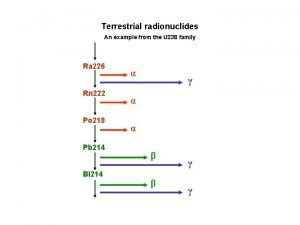
Radiation • There are three main types of radiation: • • • * Alpha radiation * Beta radiation * Gamma radiation

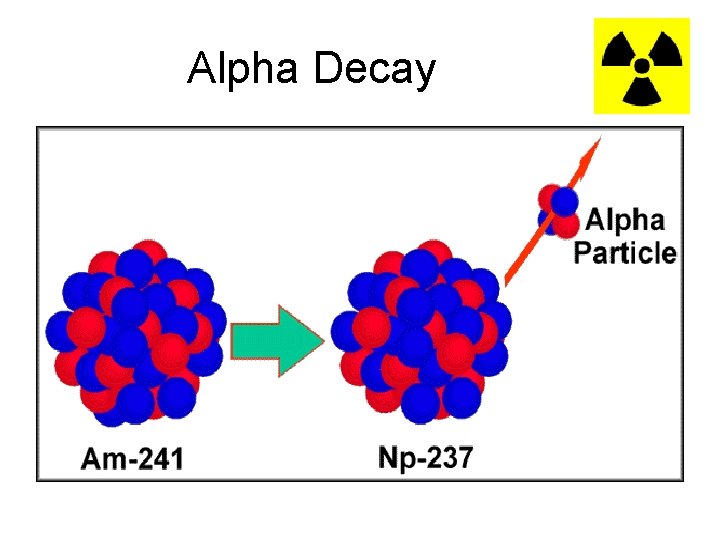
Alpha Decay • Alpha (α) decay occurs is because the nucleus has too many protons which cause excessive repulsion. • Alpha particles can be stopped by a thin sheet of paper • In an attempt to reduce the repulsion, a Helium nucleus is emitted. 4 He 2

Alpha Decay

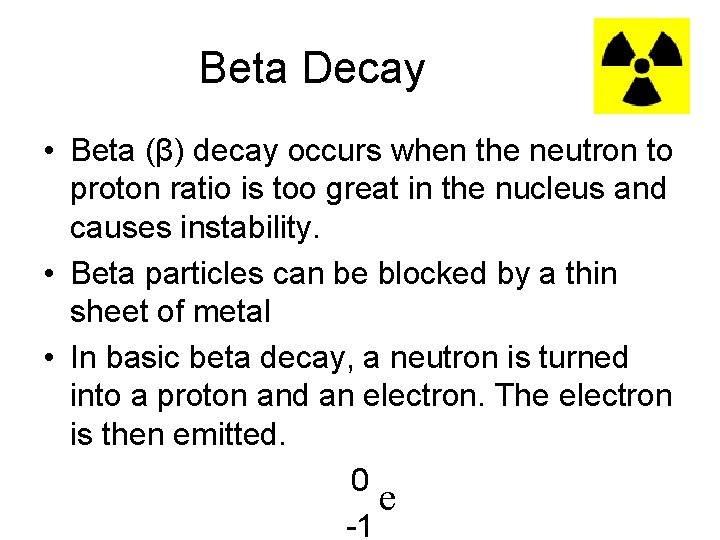
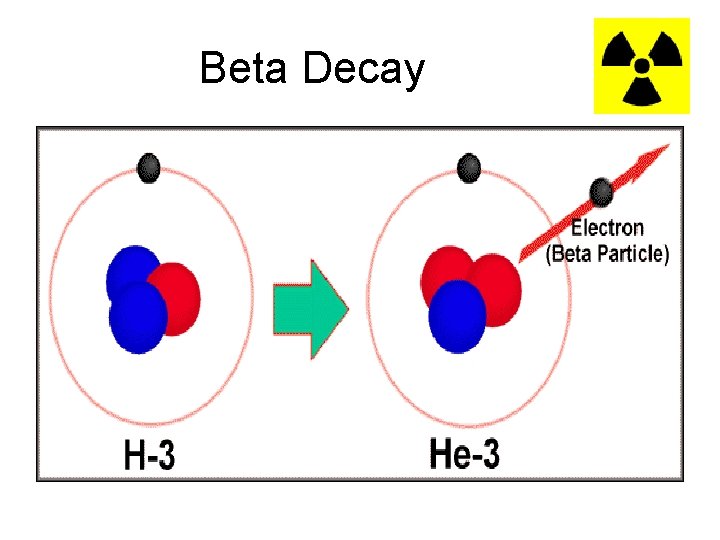
Beta Decay • Beta (β) decay occurs when the neutron to proton ratio is too great in the nucleus and causes instability. • Beta particles can be blocked by a thin sheet of metal • In basic beta decay, a neutron is turned into a proton and an electron. The electron is then emitted. 0 e -1

Beta Decay

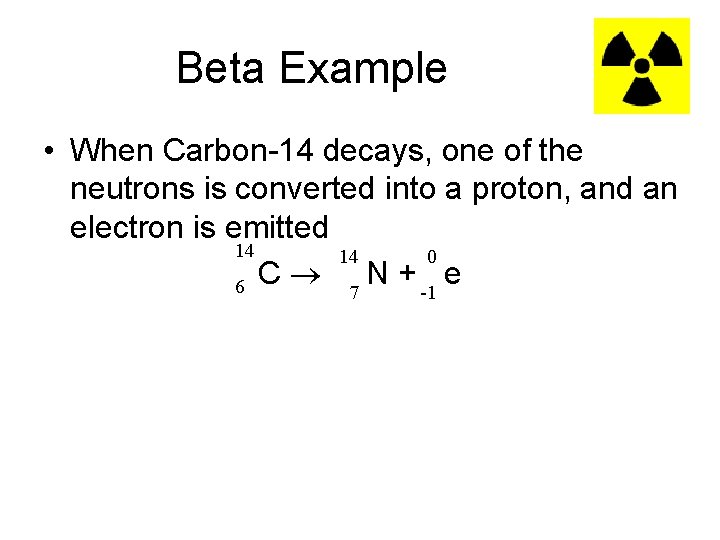
Beta Example • When Carbon-14 decays, one of the neutrons is converted into a proton, and an electron is emitted 14 14 0 6 C 7 N + -1 e

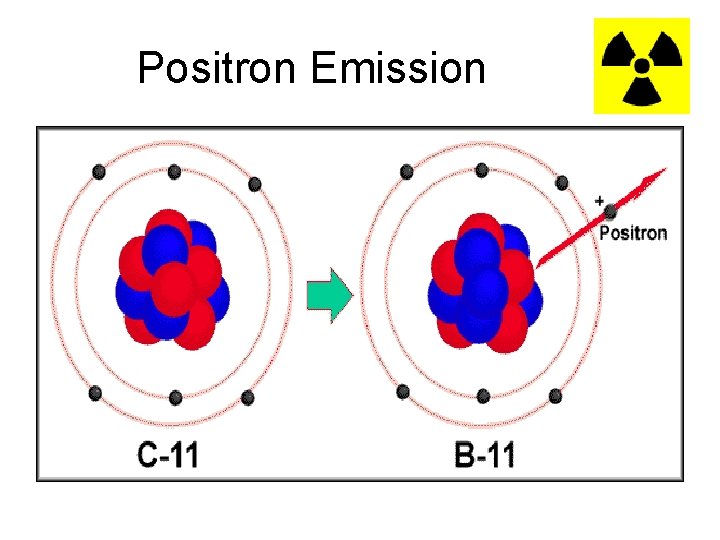
Positron • There is also positron emission when the neutron to proton ratio is too small. • A proton turns into a neutron and a positron and the positron is emitted. • A positron is basically a positively charged electron. • This is another form of Beta decay

Positron Emission

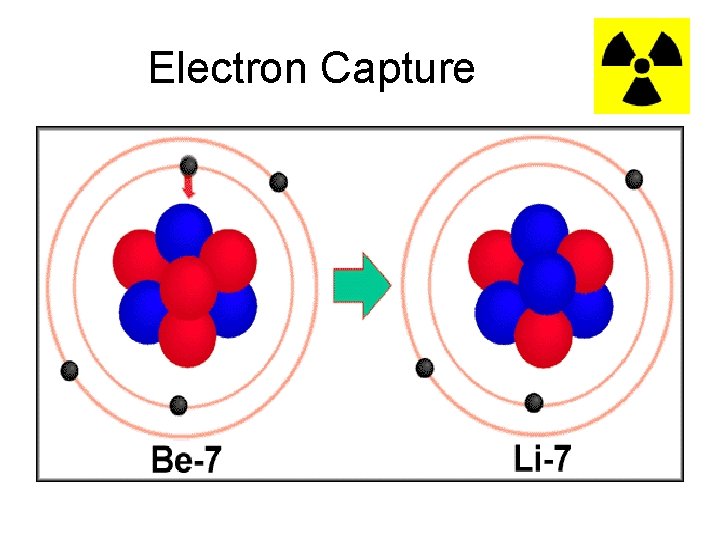
Electron Capture • The final type of beta decay is known as electron capture and also occurs when the neutron to proton ratio in the nucleus is too small. • The nucleus captures an electron which basically turns a proton into a neutron.

Electron Capture

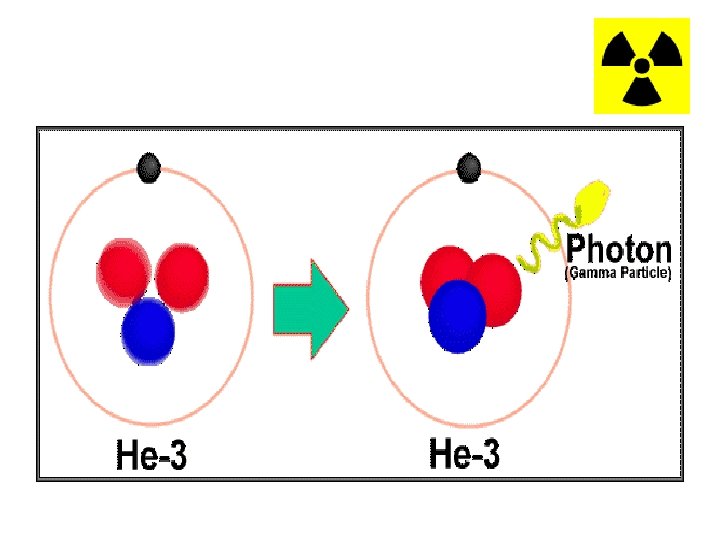
Gamma Decay • Gamma (γ) decay occurs because the nucleus is at too high an energy. • The nucleus falls down to a lower energy state and, in the process, emits a high energy photon known as a gamma particle. • Gamma radiation can only be blocked with very thick metal, usually Lead


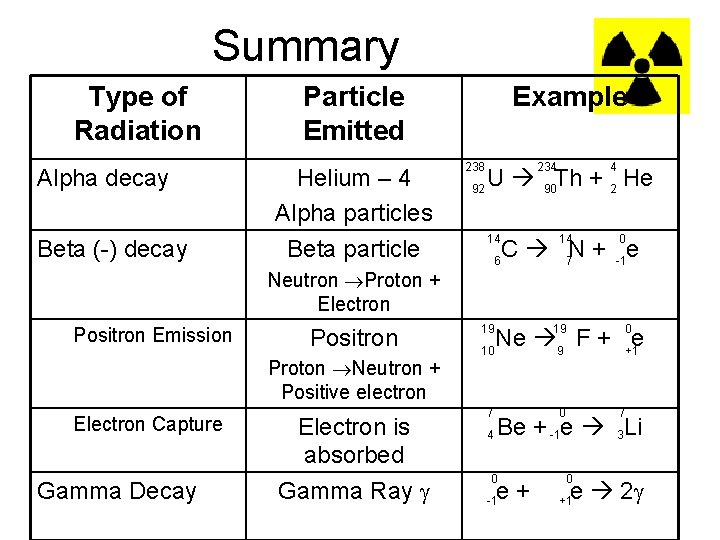
Summary Type of Radiation Alpha decay Beta (-) decay Particle Emitted Helium – 4 Alpha particles Beta particle Neutron Proton + Electron Positron Emission Positron Proton Neutron + Positive electron Electron Capture Gamma Decay Electron is absorbed Gamma Ray Example 238 92 234 4 U 90 Th + 2 He 14 6 14 0 C 7 N + -1 e 19 19 7 0 0 Ne 9 F + +1 e 10 7 4 Be + -1 e 3 Li 0 0 -1 +1 e+ e 2

Half Life • The rate of radioactive decay is related to the energy change that accompanies the transformation, but it is not a direct relationship. • The rate of radioactive emissions of a radioactive nuclide is directly proportional to the amount of radioactive material present. • The rate of decay of a radioactive nuclide is measured by its half-life.

• The half-life of a radioactive substance is the time it takes for half of an initial amount of the substance to decay. • The half-live is independent of chemical activity, external pressure, and temperature.

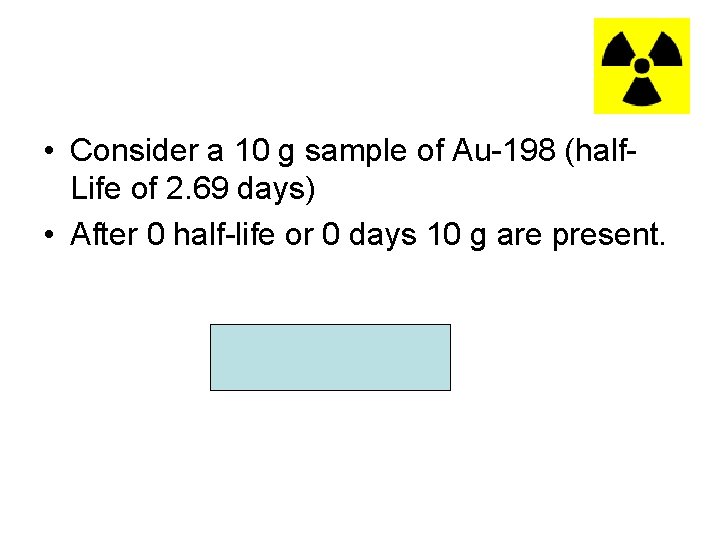
• Consider a 10 g sample of Au-198 (half. Life of 2. 69 days) • After 0 half-life or 0 days 10 g are present.

• After 1 half-life or 2. 69 days, 5 g remains.

• After 2 half-life or 5. 38 days (2 x 2. 69 days) 2. 5 g remains.

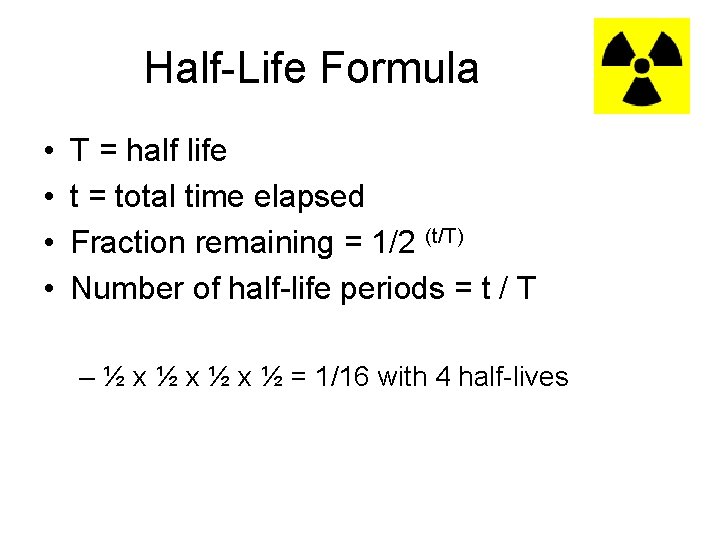
Half-Life Formula • • T = half life t = total time elapsed Fraction remaining = 1/2 (t/T) Number of half-life periods = t / T – ½ x ½ x ½ = 1/16 with 4 half-lives

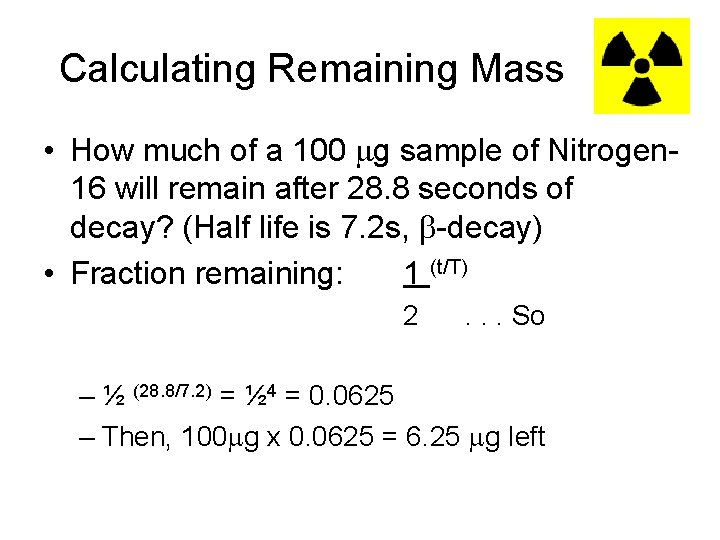
Calculating Remaining Mass • How much of a 100 g sample of Nitrogen 16 will remain after 28. 8 seconds of decay? (Half life is 7. 2 s, -decay) • Fraction remaining: 1 (t/T) 2 . . . So – ½ (28. 8/7. 2) = ½ 4 = 0. 0625 – Then, 100 g x 0. 0625 = 6. 25 g left

Calculating Half life periods • How many half-lives are required for a radioisotope to decay to 1/32 of its initial value? – Fraction remaining = ½(t/T) ; and – Number of periods = t / T ; so – 1/32 = ½ t/T – t/T = 5

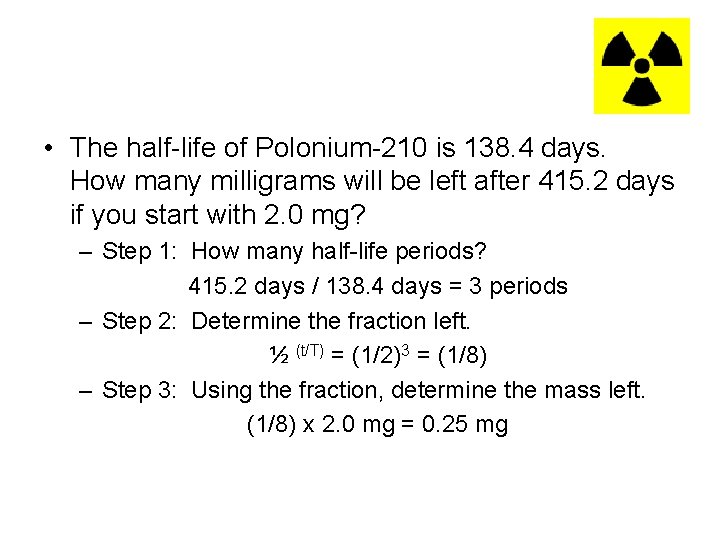
• The half-life of Polonium-210 is 138. 4 days. How many milligrams will be left after 415. 2 days if you start with 2. 0 mg? – Step 1: How many half-life periods? 415. 2 days / 138. 4 days = 3 periods – Step 2: Determine the fraction left. ½ (t/T) = (1/2)3 = (1/8) – Step 3: Using the fraction, determine the mass left. (1/8) x 2. 0 mg = 0. 25 mg

• After 4797 years, how much of a 0. 250 g sample of Radium-226 is left? (half-life is 1599 years) • 0. 0312 G
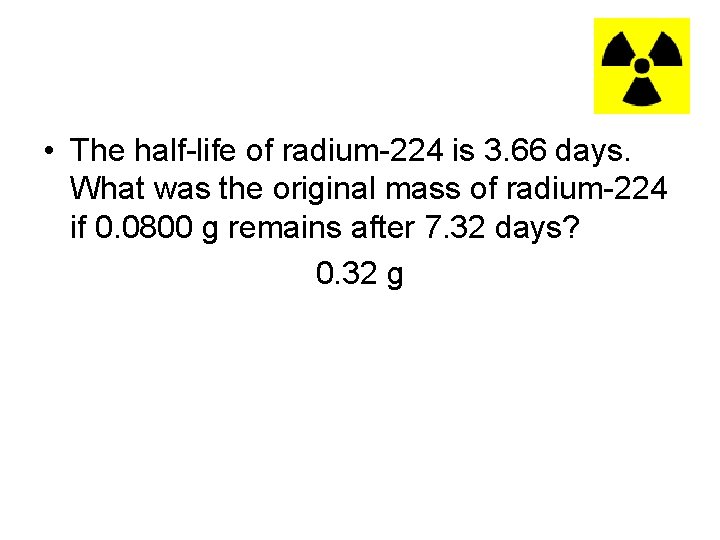
• The half-life of radium-224 is 3. 66 days. What was the original mass of radium-224 if 0. 0800 g remains after 7. 32 days? 0. 32 g
 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear fission radiation
Nuclear fission radiation 25.1 nuclear radiation
25.1 nuclear radiation What is nuclear radiation
What is nuclear radiation Nuclear radiation
Nuclear radiation Natural and artificial radioactivity
Natural and artificial radioactivity Fission and fusion similarities
Fission and fusion similarities Natural and artificial radioactivity
Natural and artificial radioactivity Atoms and radioactivity
Atoms and radioactivity Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Law of radioactive decay
Law of radioactive decay Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Radioactivity
Radioactivity Radioactivity
Radioactivity The great unconformity
The great unconformity Natural radioactivity
Natural radioactivity Environmental radioactivity
Environmental radioactivity Natural radioactivity
Natural radioactivity Radioactivity phenomenon
Radioactivity phenomenon Units of radioactivity
Units of radioactivity Decay equation
Decay equation Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Uranium 238 alpha decay equation
Uranium 238 alpha decay equation Nuclear chemistry webquest answer key
Nuclear chemistry webquest answer key Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry