Radioactivity Chapters 4 4 25 Nuclear Radiation Nuclear















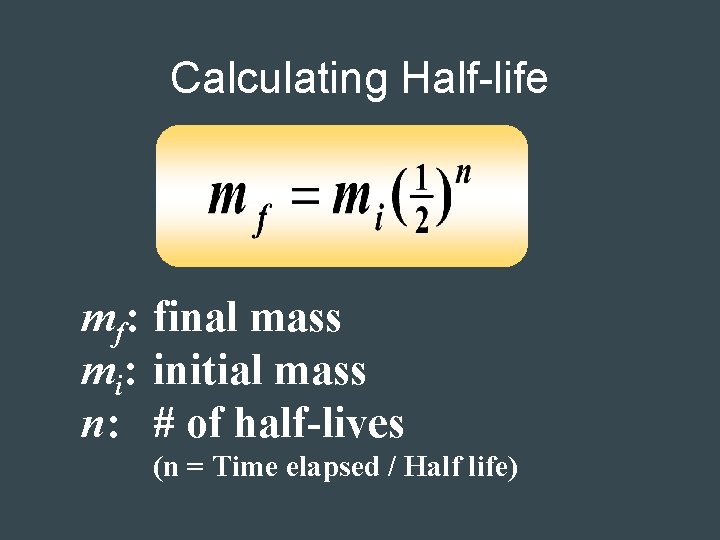












- Slides: 52

Radioactivity Chapters 4. 4 & 25

Nuclear Radiation • Nuclear chemistry • study of the structure of atomic nuclei & the changes they undergo

The Discovery of Radioactivity • Wilhelm Roentgen (1845– 1923) • 1895 -invisible rays were emitted when electrons bombarded the surface of certain materials. • caused photographic plates to darken. • named the invisible high-energy emissions X rays.

The Discovery of Radioactivity • Henri Becquerel (1852– 1908) was studying phosphorescence • minerals that emit light after being exposed to sunlight • phosphorescent uranium salts produced spontaneous emissions that darkened photographic plates.

The Discovery of Radioactivity • Marie Curie (1867– 1934) and her husband Pierre (1859– 1906) took Becquerel’s mineral sample (called pitchblende) and isolated the components emitting the rays. • darkening of the photographic plates was due to rays emitted specifically from the uranium atoms present in the mineral sample.

The Discovery of Radioactivity • Marie Curie named the process by which materials give off such rays radioactivity • the rays and particles emitted by a radioactive source are called radiation.

Types of Radiation • isotopes are atoms of the same element that have different numbers of neutrons. • Isotopes of atoms with unstable nuclei are called radioisotopes • emit radiation to attain more stable atomic configurations in a process called radioactive decay • lose energy by emitting one of several types of radiation.

Why do some atoms decay? • The nucleus contains tightly packed protons and neutrons (nucleons) • The strong nuclear force keeps the nucleons packed together even though protons want to push each other away • Stable atoms have a neutron to proton ratio of about 1: 1

• As atomic number increases, more neutrons are required to have enough of a strong force to keep the protons pushed together • The neutron to proton ratio for stable atoms increases to 1. 5: 1

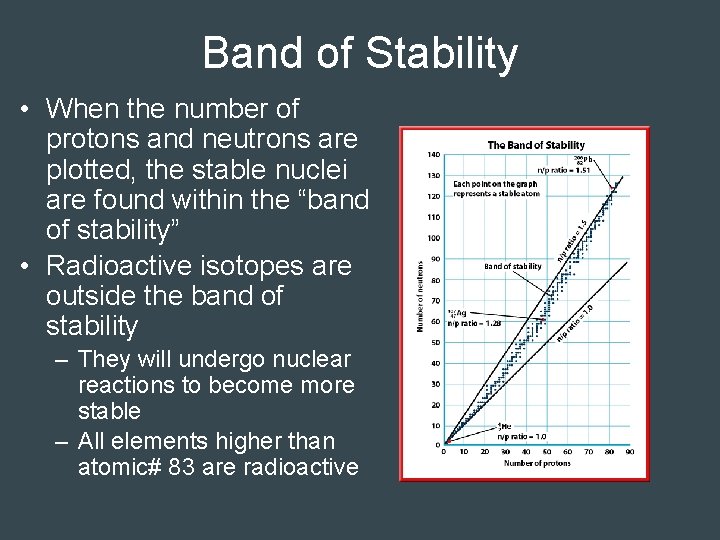
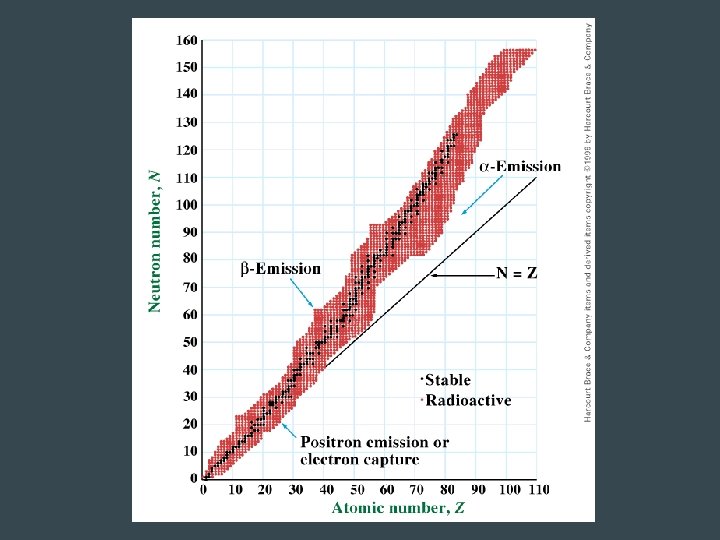
Band of Stability • When the number of protons and neutrons are plotted, the stable nuclei are found within the “band of stability” • Radioactive isotopes are outside the band of stability – They will undergo nuclear reactions to become more stable – All elements higher than atomic# 83 are radioactive


Topic 26 Basic Assessment Questions Question 3 Calculate the neutron-to-proton ratio for .

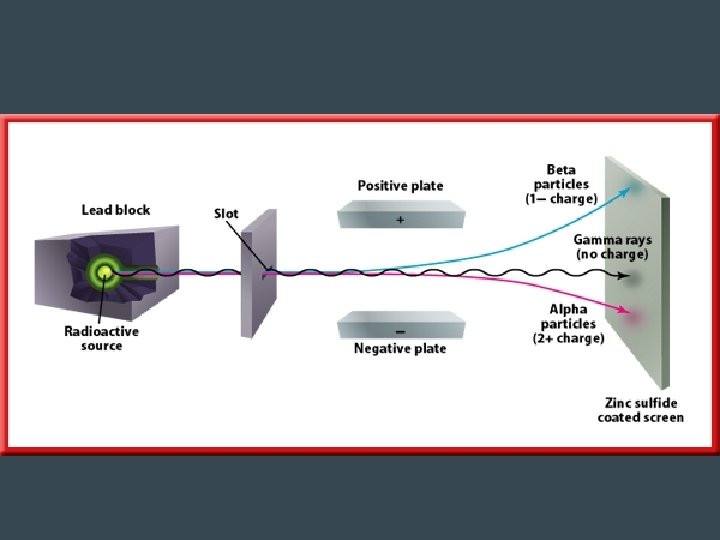
Types of Nuclear Radiation • • • Alpha Beta Gamma Positron Emission Electron Capture

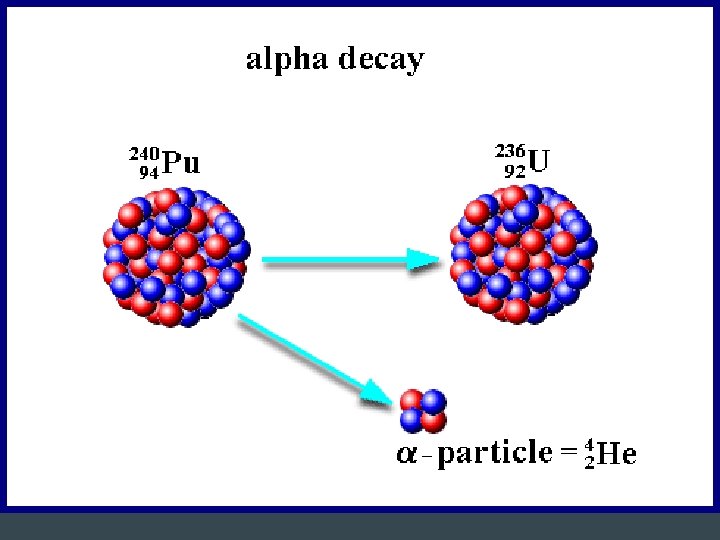
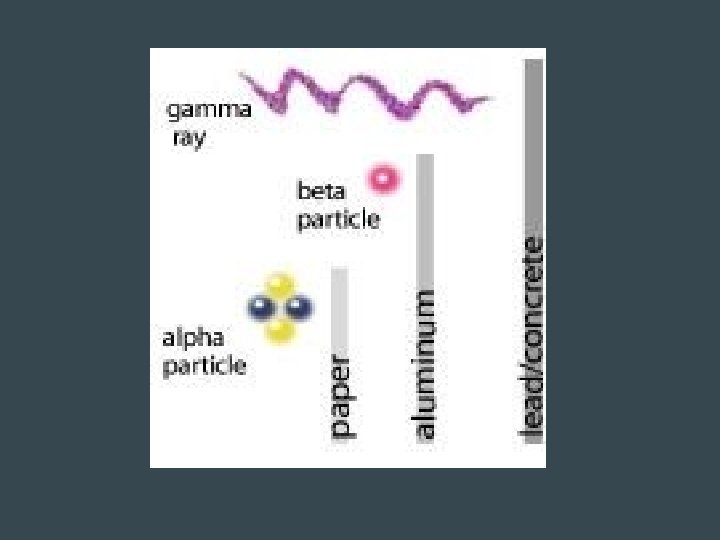
Alpha Radiation • Release of 2 protons and 2 neutrons – Equivalent to a He nucleus – Charge of 2+ – Mass = 4 amu • Largest and slowest – Least penetrating → can be stopped by paper • Changes to a different element with a lower atomic mass and lower atomic number • Example: Polonium-212 (atomic# 84) is converted to Lead-208 (atomic# 82)


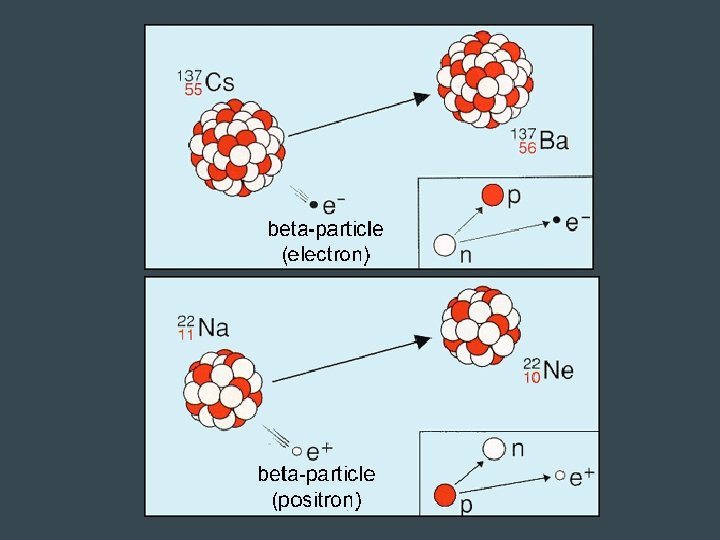
Beta Radiation • Decay of a neutron into a proton and electron – Electron is emitted, proton stays – Forms a new element b/c of addition of proton • Decay of the proton into a neutron and positron (like a positive electron) – The positron is emitted as a beta particle • Faster than alpha particles → can be stopped by aluminum foil


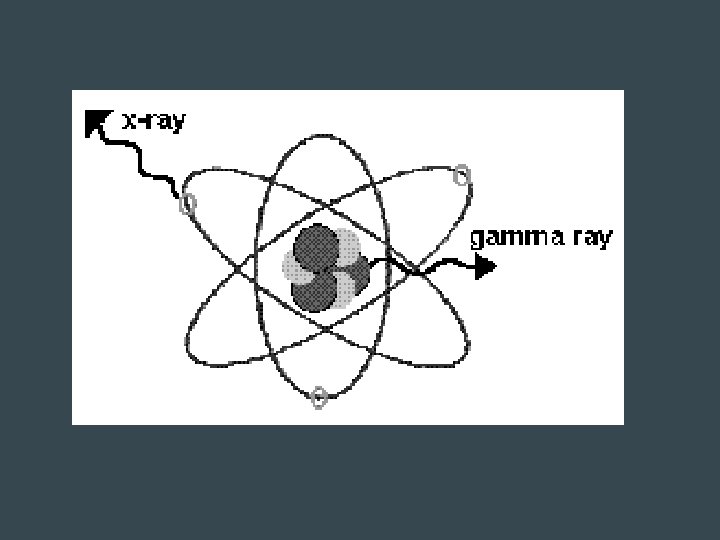
Gamma Radiation • Not a particle • Electromagnetic wave with short wavelength and high frequency & energy • No mass, no charge • Very fast → speed of light • Stronger than X-ray • Stopped by several centimeters of lead



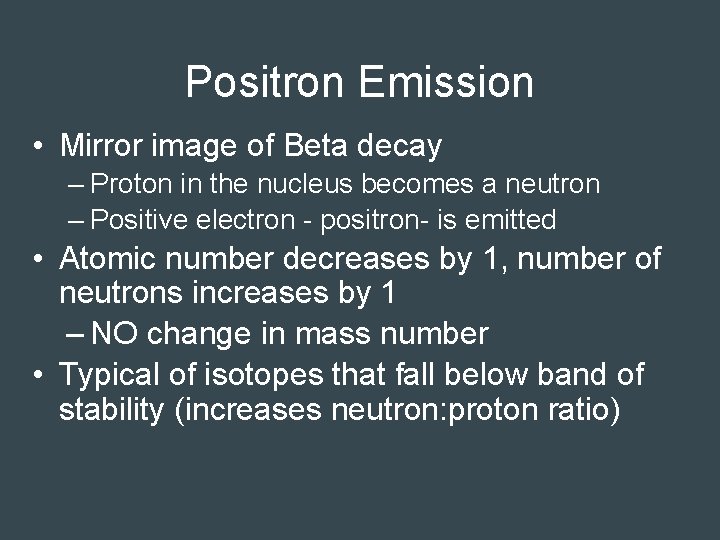
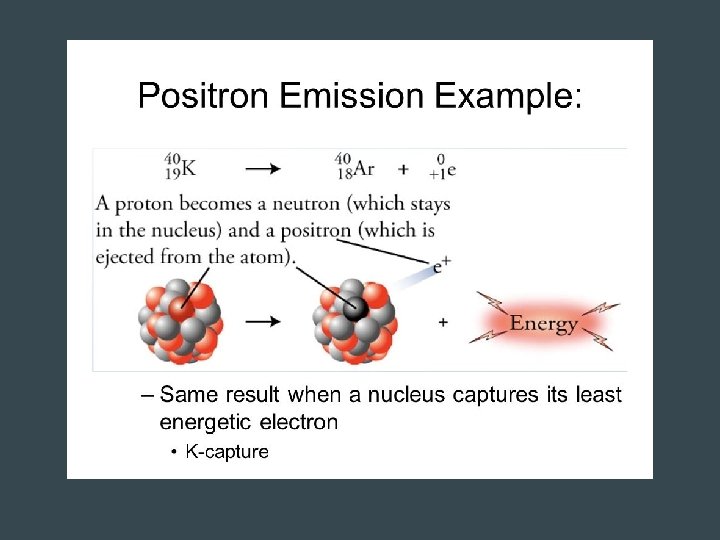
Positron Emission • Mirror image of Beta decay – Proton in the nucleus becomes a neutron – Positive electron - positron- is emitted • Atomic number decreases by 1, number of neutrons increases by 1 – NO change in mass number • Typical of isotopes that fall below band of stability (increases neutron: proton ratio)


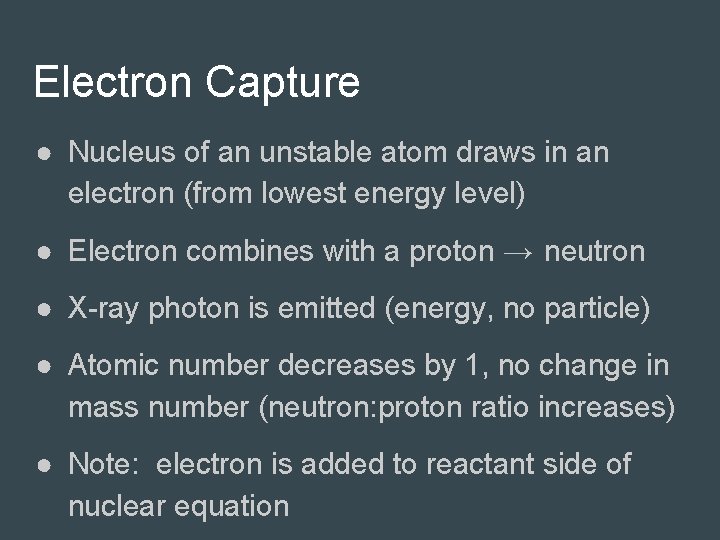
Electron Capture ● Nucleus of an unstable atom draws in an electron (from lowest energy level) ● Electron combines with a proton → neutron ● X-ray photon is emitted (energy, no particle) ● Atomic number decreases by 1, no change in mass number (neutron: proton ratio increases) ● Note: electron is added to reactant side of nuclear equation

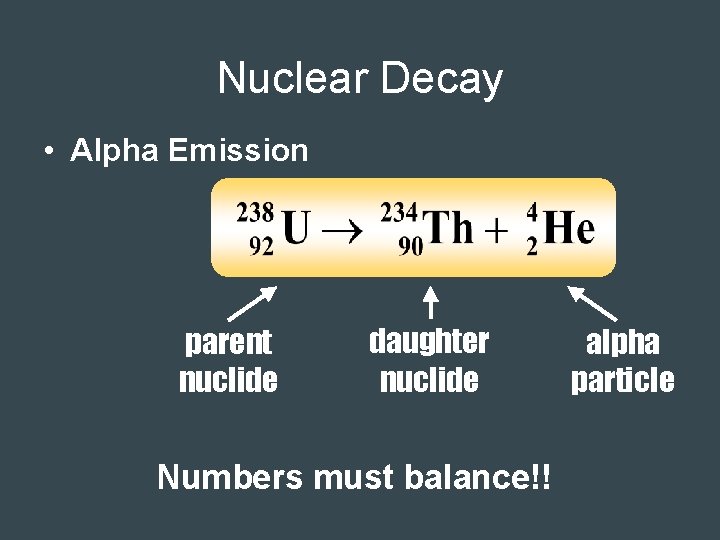
Nuclear Decay • Alpha Emission parent nuclide daughter nuclide Numbers must balance!! alpha particle

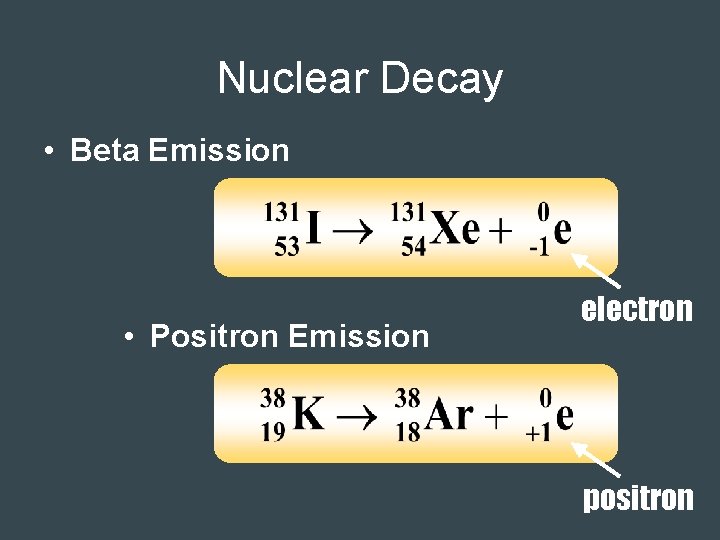
Nuclear Decay • Beta Emission • Positron Emission electron positron

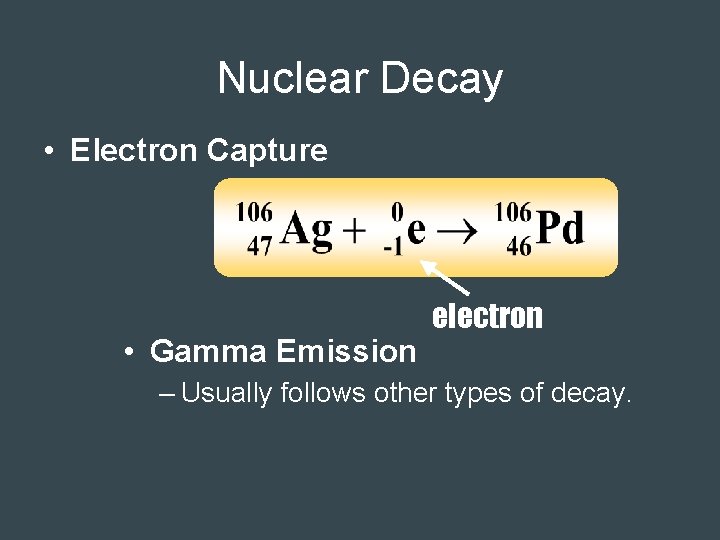
Nuclear Decay • Electron Capture • Gamma Emission electron – Usually follows other types of decay.

• Transmutation: changing one element into another through radioactive decay – Adding or removing a proton changes the atomic number, resulting in a different element

Types of Transmutation • Induced transmutation – Nucleus of an unstable isotope (radionuclide) is struck with a high velocity charged particle • Particle accelerator • Need lots of energy and unstable nucleus – Elements atomic 93 and higher (transuranium elements) • Natural transmutation – Occurs naturally as a radioisotope decays to become more stable

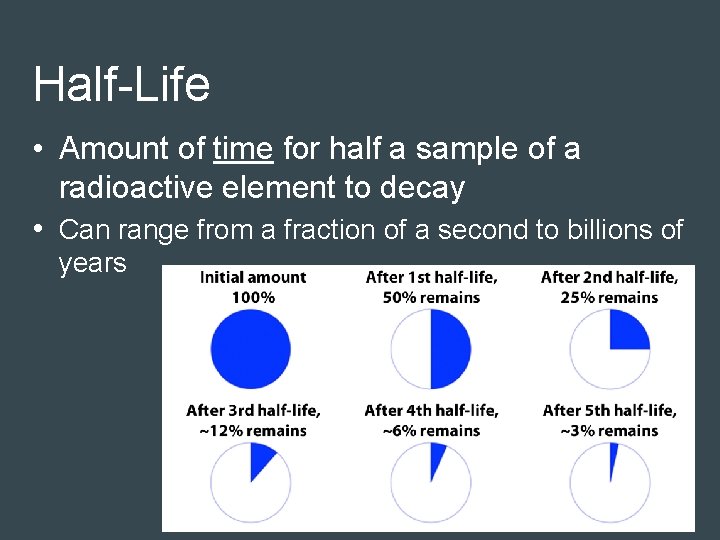
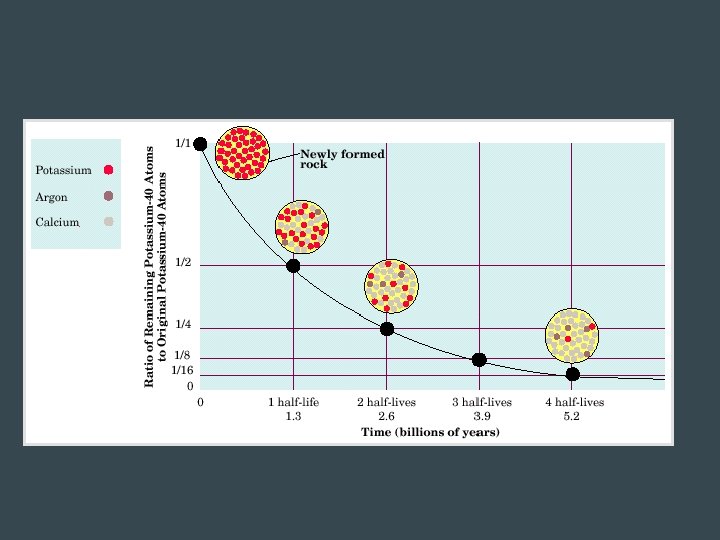
Half-Life • Amount of time for half a sample of a radioactive element to decay • Can range from a fraction of a second to billions of years

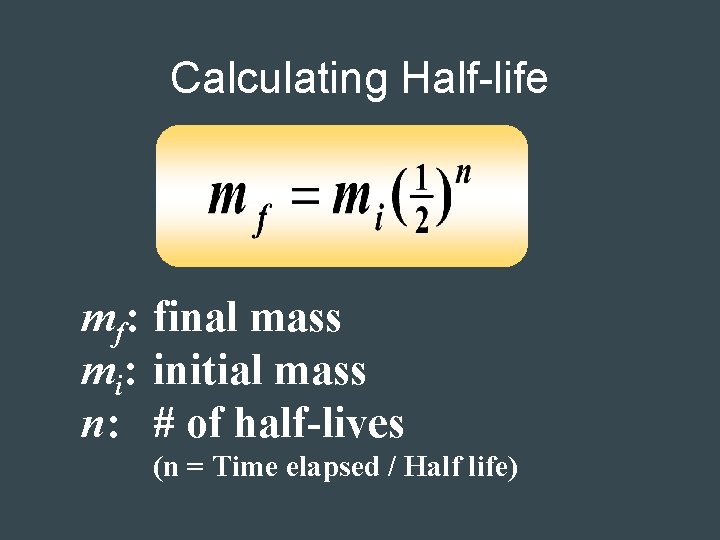
Calculating Half-life mf: final mass mi: initial mass n: # of half-lives (n = Time elapsed / Half life)

Half-life • Fluorine-21 has a half-life of 5. 0 seconds. If you start with 25 g of fluorine-21, how many grams would remain after 60. 0 s?

Topic 26 Basic Assessment Questions Question 1 What element is formed when polonium-214 ( ) radioisotope undergoes alpha decay? Give the atomic number and mass number of the element.

Topic 26 Basic Assessment Questions Question 2 What element is formed when undergoes beta decay? Give the atomic number and mass number of the element.

Topic 26 Basic Assessment Questions Question 4 Write a balanced nuclear equation for the beta decay of the following radioisotope.

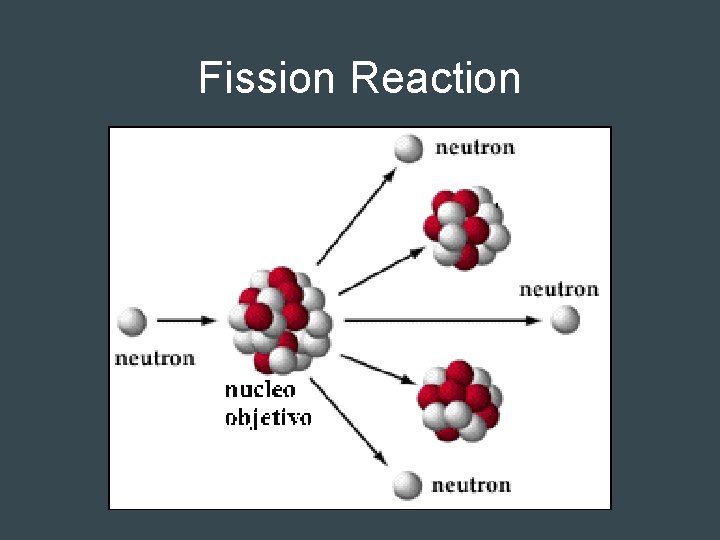
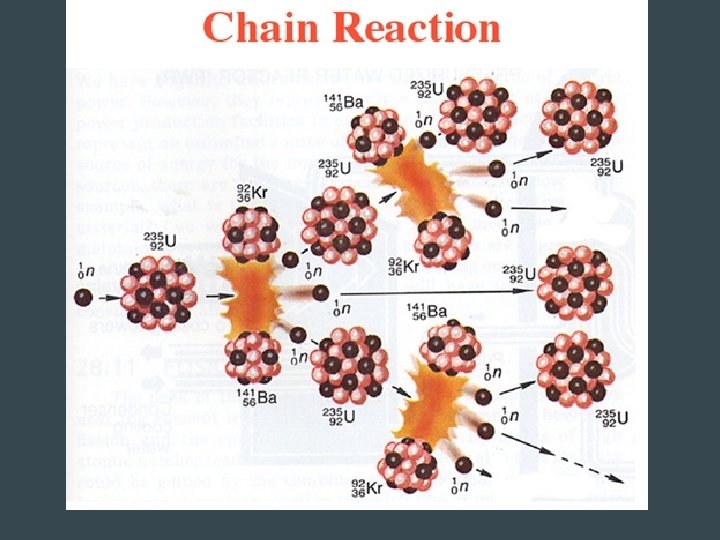
Nuclear Fission • Fission = divide • Neutron hits an unstable atom • Nucleus splits into two fragments of about the same mass – Some single neutrons are released (energy) – These neutrons can smash into other atoms • Causes a chain reaction

Fission Reaction


Nuclear reactors • Nuclear power plants use the process of nuclear fission to produce heat in nuclear reactors. • The heat is used to generate steam, which is then used to drive turbines that produce electricity.

Atomic Bomb- uncontrolled fission reactions

• Little Boy: $2 billion in research; made of Uranium-235; equal to 20, 000 tons of TNT; 140, 000 people died; 2/3 of the city destroyed • Fat Man: Plutonium-239; 70, 000 people died; 40% of the city destroyed

Hydrogen Bomb • 1000 times more powerful than atomic bomb • March 1, 1954; Bikini Atoll in Pacific – Never in war • Fission reaction triggers fusion of Hydrogen isotopes

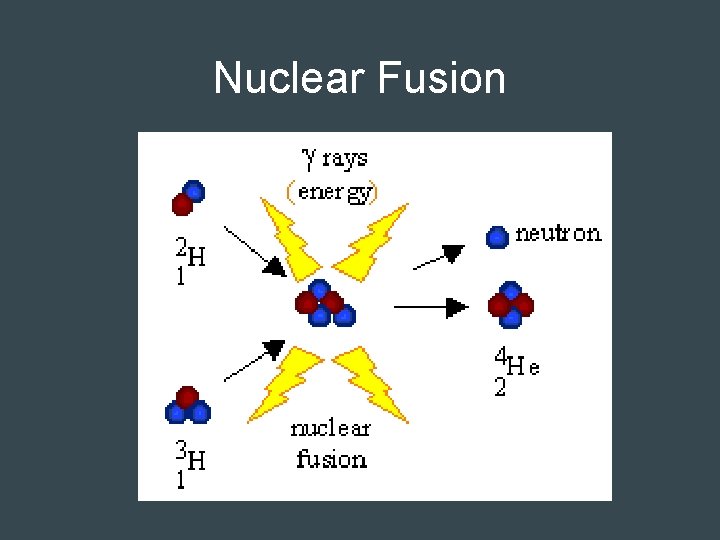
Nuclear Fusion • Opposite of fission • Two nuclei fuse together to form one nucleus with a larger mass – Not simple sum of masses – Some mass lost as energy • Requires high temperature: Thermonuclear reaction • Occurs in the sun and stars – 4 H combine to form one He, 2 e- and energy

Nuclear Fusion

Solar Flare

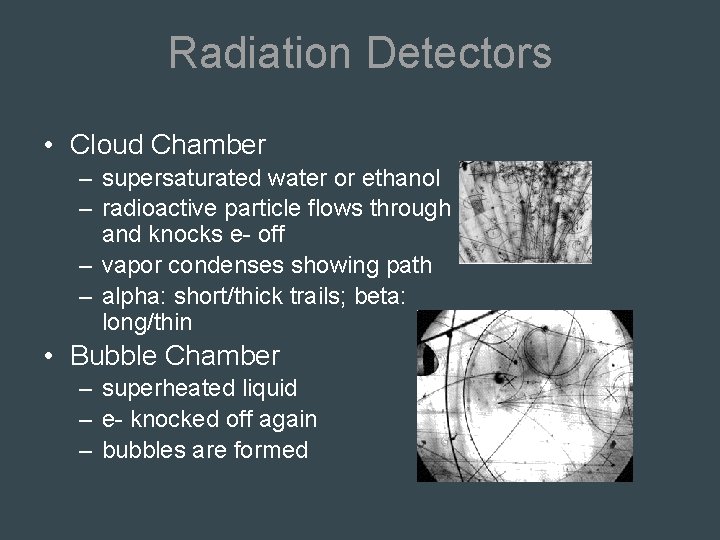
Radiation Detectors • Cloud Chamber – supersaturated water or ethanol – radioactive particle flows through and knocks e- off – vapor condenses showing path – alpha: short/thick trails; beta: long/thin • Bubble Chamber – superheated liquid – e- knocked off again – bubbles are formed

Measuring Radiation • Geiger Counter – produces electric current when near radiation – Results in clicks or a digital reading

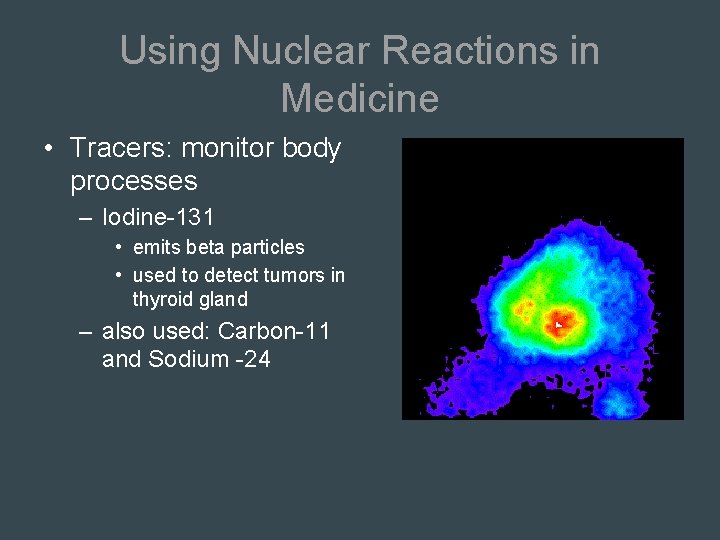
Using Nuclear Reactions in Medicine • Tracers: monitor body processes – Iodine-131 • emits beta particles • used to detect tumors in thyroid gland – also used: Carbon-11 and Sodium -24

• Cancer Treatment • damage cancer cells • Gold -198 or Iridium -192 -- implanted in or near tumor • Cobalt-60 – outside body – emits gamma rays

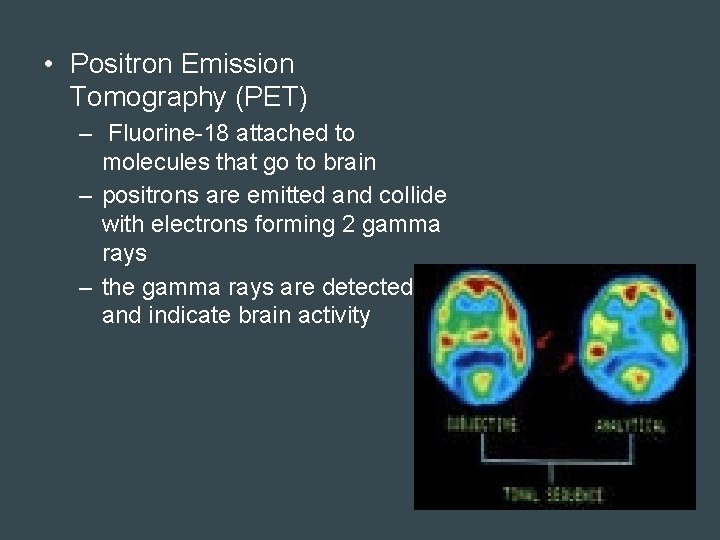
• Positron Emission Tomography (PET) – Fluorine-18 attached to molecules that go to brain – positrons are emitted and collide with electrons forming 2 gamma rays – the gamma rays are detected and indicate brain activity

• http: //www. hpwt. de/Kern 2 e. htm • http: //www. colorado. edu/physics/2000/isoto pes/radioactive_decay 3. html • http: //www. msd. k 12. or. us/schools/mhs/proje cts/Fission/frames. html • http: //www. cnn. com/SPECIALS/cold. war/ex perience/the. bomb/history. science/
