Nuclear Radiation Natural Radioactivity Nuclear Equations Producing Radioactive










- Slides: 28

Nuclear Radiation Natural Radioactivity Nuclear Equations Producing Radioactive Isotopes Half-Life Nuclear Fission and Fusion 1

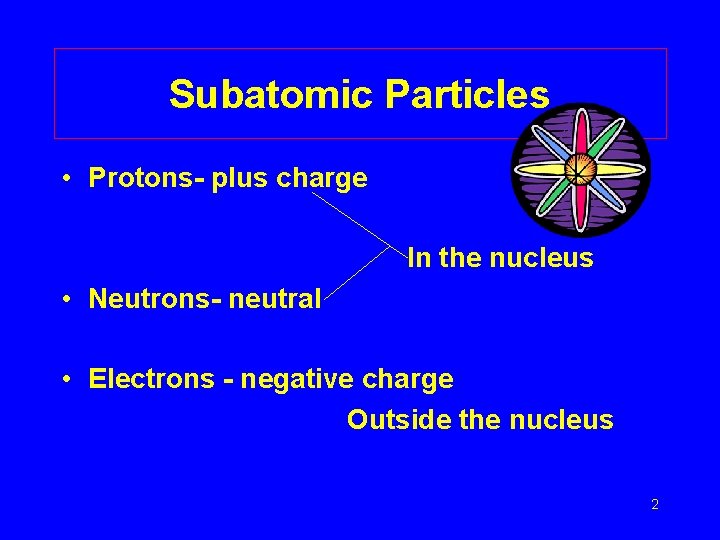
Subatomic Particles • Protons- plus charge In the nucleus • Neutrons- neutral • Electrons - negative charge Outside the nucleus 2

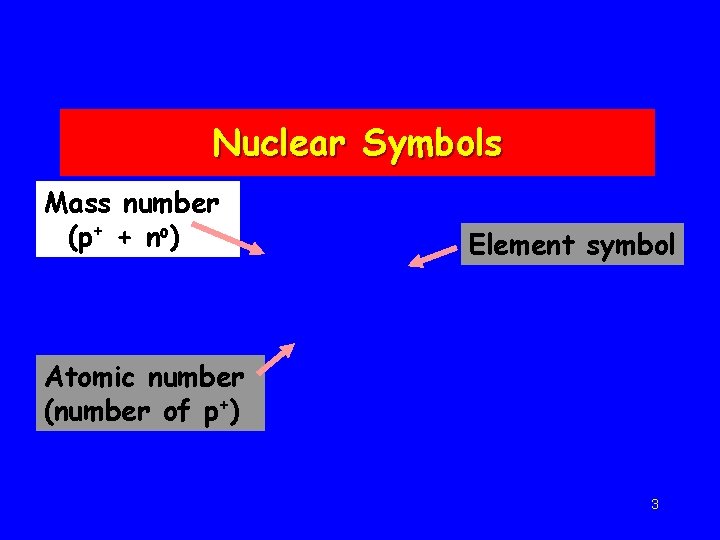
Nuclear Symbols Mass number (p+ + no) Element symbol Atomic number (number of p+) 3

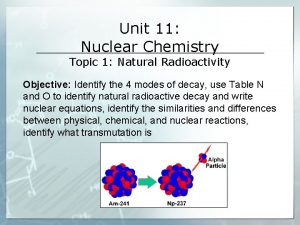
RADIOACTIVITY • Nuclei of unstable isotopes called radioisotopes gain stability by undergoing changes • These changes are always accompanied by large amounts of energy • Discovery of radioactivity dealt a blow to Dalton’s theory that atoms are 4 indivisible

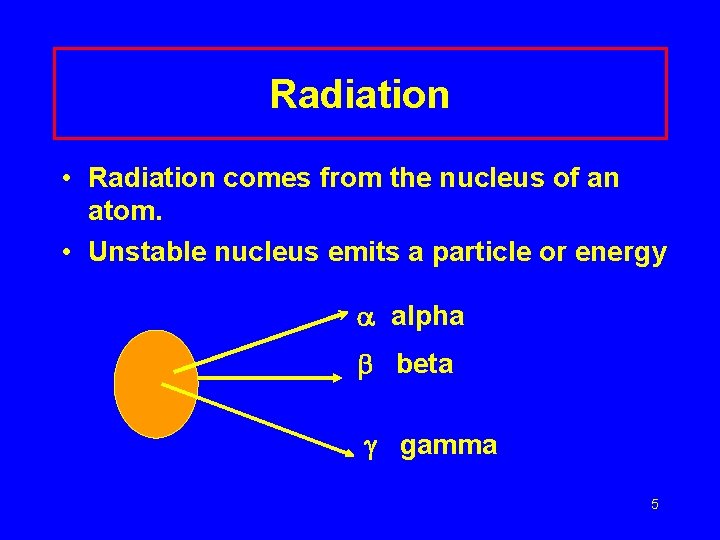
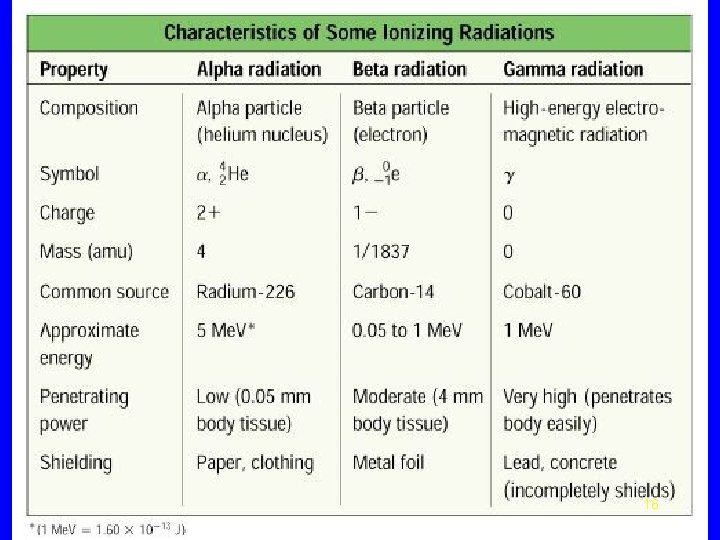
Radiation • Radiation comes from the nucleus of an atom. • Unstable nucleus emits a particle or energy alpha beta gamma 5

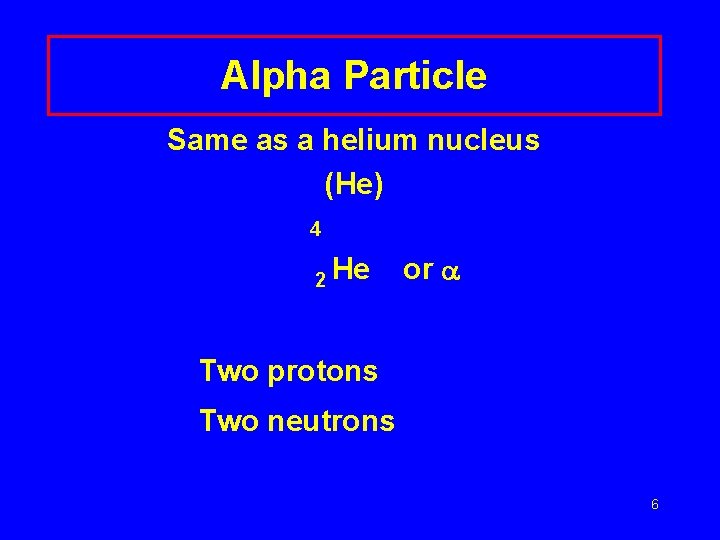
Alpha Particle Same as a helium nucleus (He) 4 2 He or Two protons Two neutrons 6

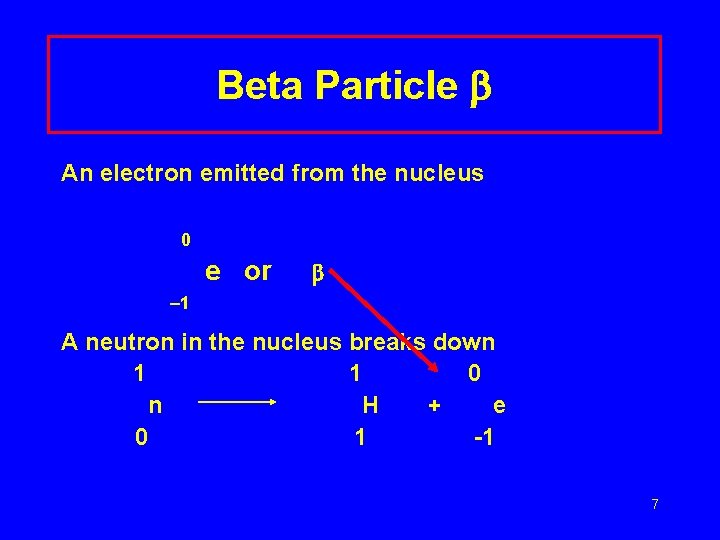
Beta Particle An electron emitted from the nucleus 0 e or 1 A neutron in the nucleus breaks down 1 1 0 n H + e 0 1 -1 7

Gamma Radiation • Pure radiation • Like an X-ray but comes from the nucleus 8

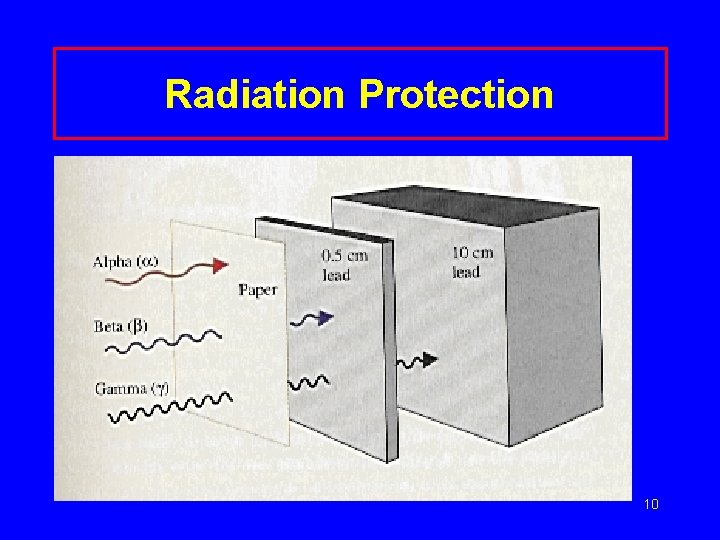
Radiation Protection • Shielding alpha – paper, clothing beta – lab coat, gloves gamma- lead, thick concrete • Limit time exposed • Keep distance from source 9

Radiation Protection 10

Balancing Nuclear Equations In the reactants and products Atomic numbers must balance and Mass numbers must balance 11

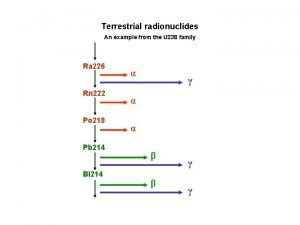
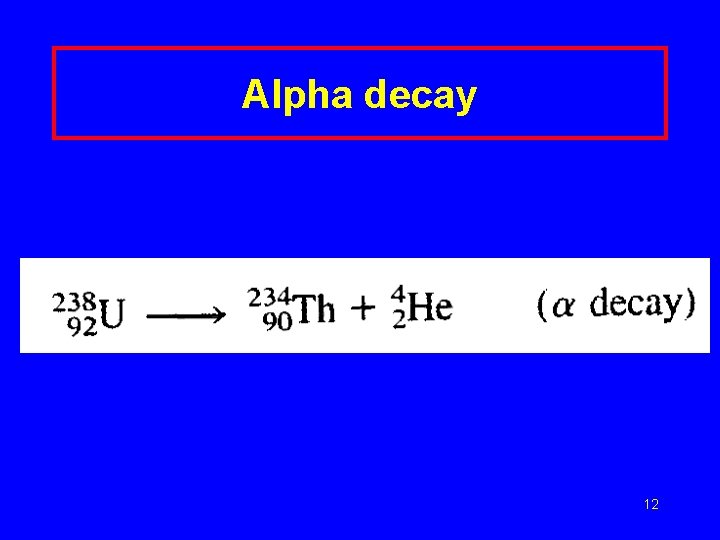
Alpha decay 12

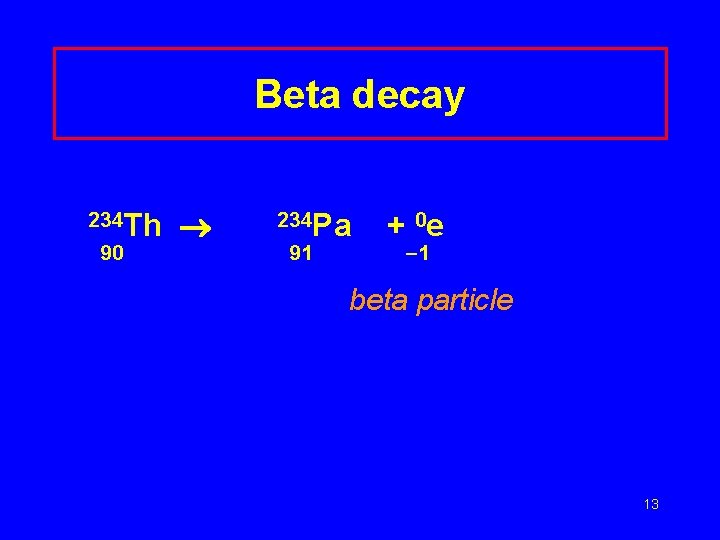
Beta decay 234 Th 90 ® 234 Pa 91 + 0 e 1 beta particle 13

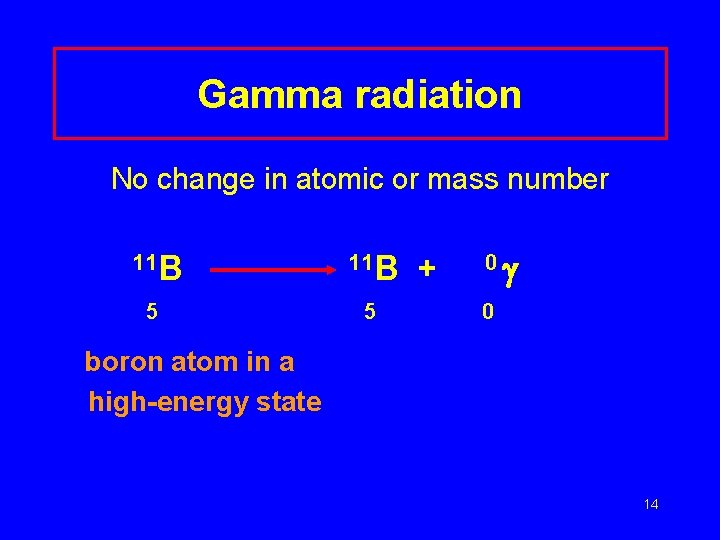
Gamma radiation No change in atomic or mass number 11 B 5 5 + 0 0 boron atom in a high-energy state 14

Types of Radioactive Decay vgamma ray production ( ): • v 0 vpositron production 1 e : • velectron capture: (inner-orbital electron is captured by the nucleus) 15

Types of Radiation 16

Learning Check NR 1 Write the nuclear equation for the beta emitter Co-60. 17

Solution NR 1 Write the nuclear equation for the Beta emitter Co-60. 60 Co 60 Ni 27 28 + 0 e -1 18

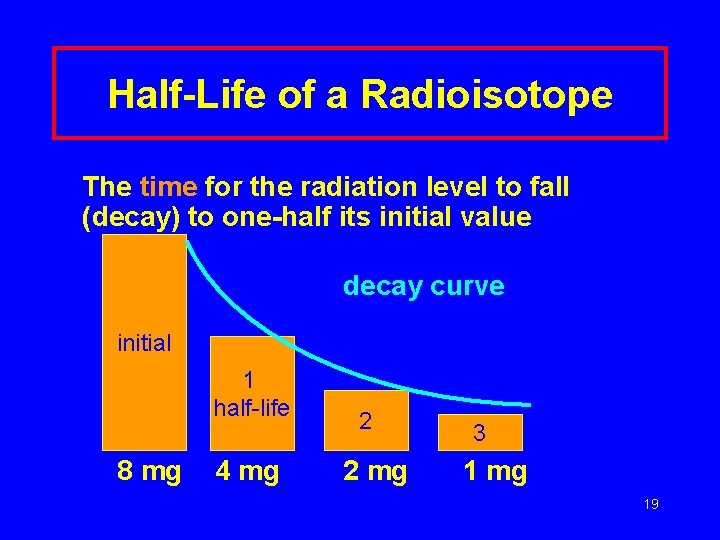
Half-Life of a Radioisotope The time for the radiation level to fall (decay) to one-half its initial value decay curve initial 1 half-life 8 mg 4 mg 2 2 mg 3 1 mg 19

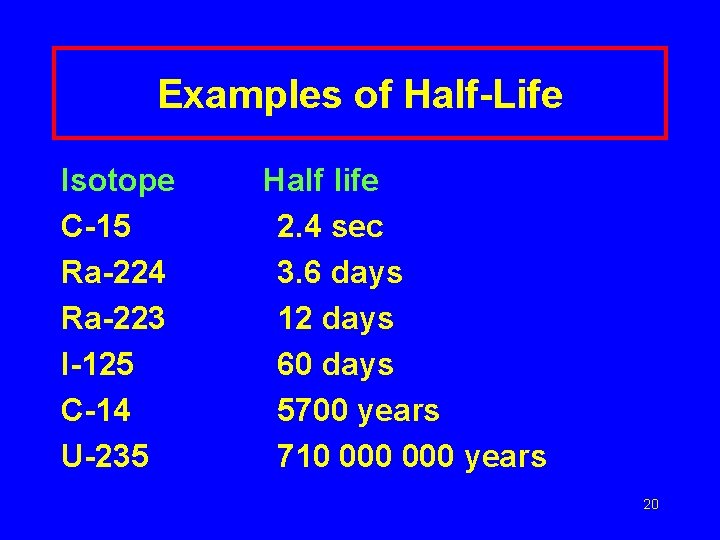
Examples of Half-Life Isotope C-15 Ra-224 Ra-223 I-125 C-14 U-235 Half life 2. 4 sec 3. 6 days 12 days 60 days 5700 years 710 000 years 20

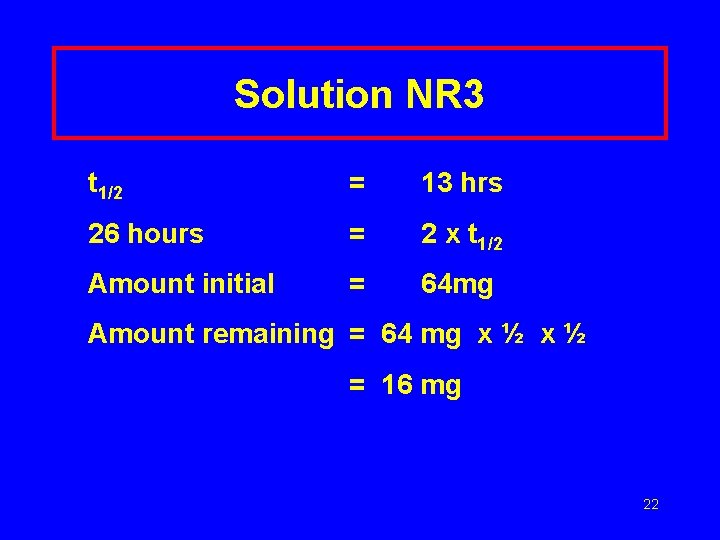
Learning Check NR 3 The half life of I-123 is 13 hr. How much of a 64 mg sample of I-123 is left after 26 hours? 21

Solution NR 3 t 1/2 = 13 hrs 26 hours = 2 x t 1/2 Amount initial = 64 mg Amount remaining = 64 mg x ½ = 16 mg 22

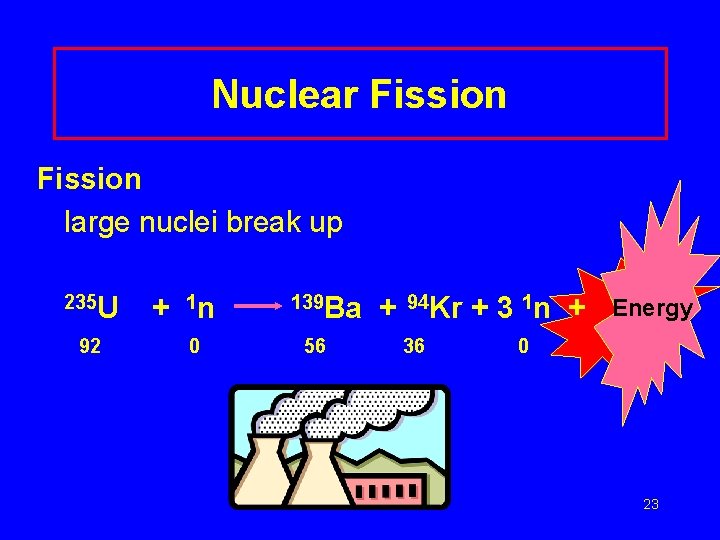
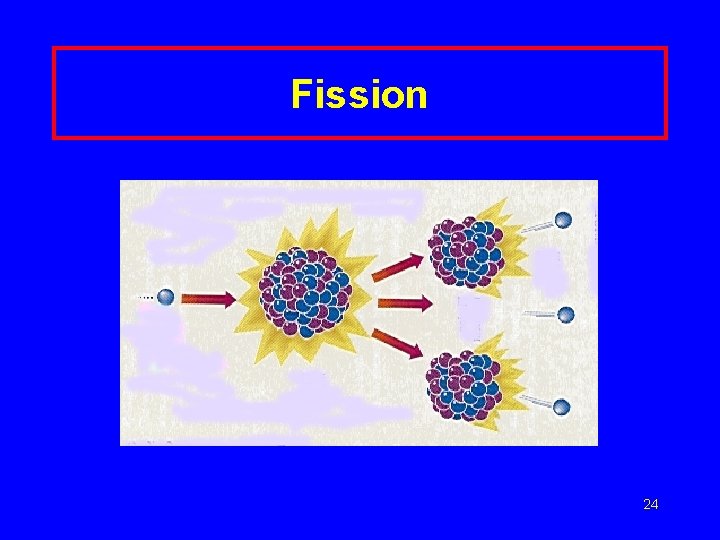
Nuclear Fission large nuclei break up 235 U 92 + 1 n 0 139 Ba 56 + 94 Kr 36 + 3 1 n + Energy 0 23

Fission 24

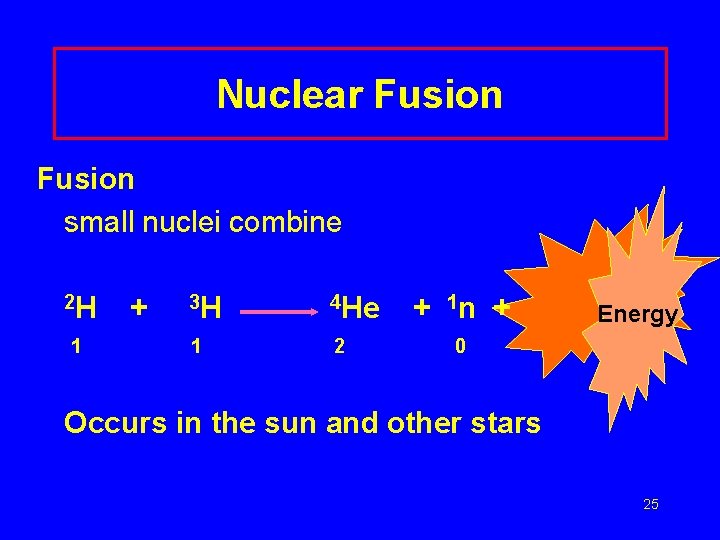
Nuclear Fusion small nuclei combine 2 H 1 + 3 H 4 He 1 2 + 1 n + Energy 0 Occurs in the sun and other stars 25

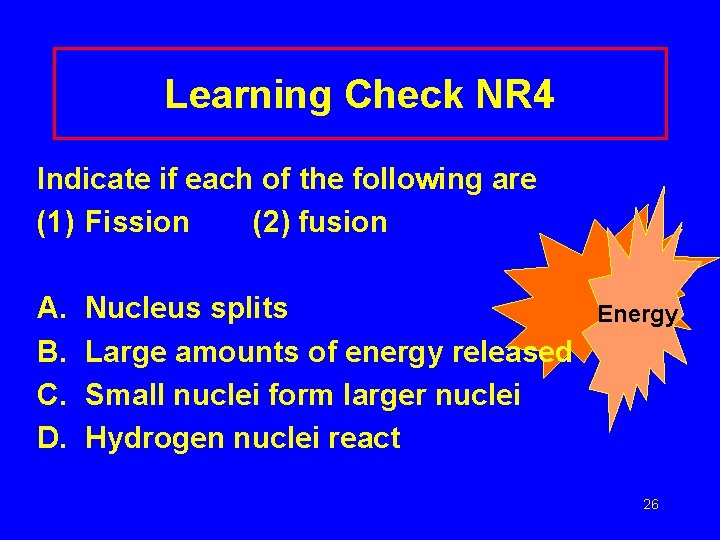
Learning Check NR 4 Indicate if each of the following are (1) Fission (2) fusion A. B. C. D. Nucleus splits Large amounts of energy released Small nuclei form larger nuclei Hydrogen nuclei react Energy 26

Solution NR 4 Indicate if each of the following are (1) Fission (2) fusion A. B. C. D. 1 1+2 2 2 Nucleus splits Large amounts of energy released Small nuclei form larger nuclei Hydrogen nuclei react 27

Energy and Mass Nuclear changes occur with small but measurable losses of mass. The lost mass is called the mass defect, and is converted to energy according to Einstein’s equation: DE = Dmc 2 Dm = mass defect DE = change in energy c = speed of light Because c 2 is so large, even small amounts of mass are converted to enormous amount of energy. 28
 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Natural and artificial radioactivity
Natural and artificial radioactivity Fission and fusion similarities
Fission and fusion similarities Natural and artificial radioactivity
Natural and artificial radioactivity Radioactive decay law
Radioactive decay law Natural radioactivity
Natural radioactivity Natural radioactivity
Natural radioactivity Radioactive nuclear waste
Radioactive nuclear waste Nuclear fusion radiation
Nuclear fusion radiation Types of radiation
Types of radiation What is nuclear radiation
What is nuclear radiation Nuclear radiation
Nuclear radiation Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Who discovered uranium
Who discovered uranium Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Mta ek
Mta ek Radioactivity
Radioactivity Unconformity
Unconformity Datación radiométrica
Datación radiométrica Environmental radioactivity
Environmental radioactivity Radioactivity phenomenon
Radioactivity phenomenon Units of radioactivity
Units of radioactivity Decay equation
Decay equation Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Gamma emission equation
Gamma emission equation Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions