Chemistry Notes Nuclear Decay Nuclear Radiation Radioactivity emission




- Slides: 17

Chemistry Notes Nuclear Decay

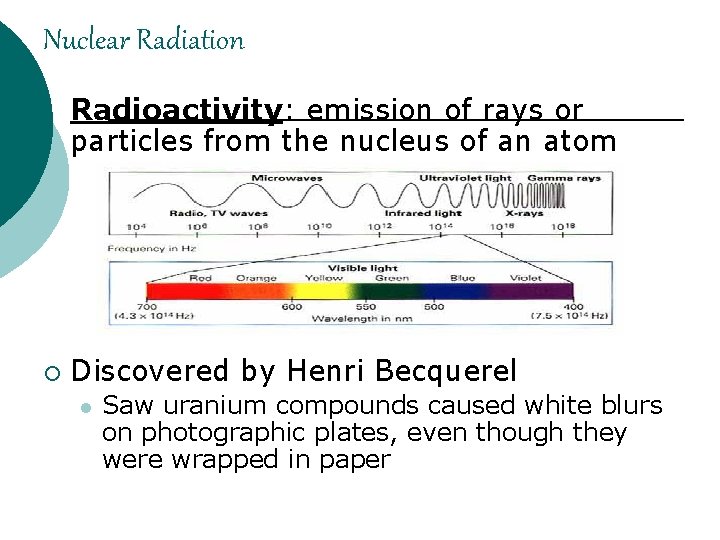
Nuclear Radiation Radioactivity: emission of rays or particles from the nucleus of an atom ¡ Discovered by Henri Becquerel l Saw uranium compounds caused white blurs on photographic plates, even though they were wrapped in paper

Types of Radioactivity ¡ ¡ ¡ Alpaha (α) Beta (β) Gamma (γ)

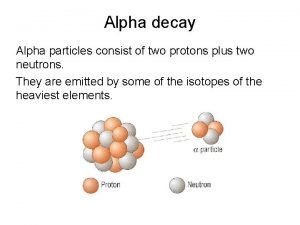
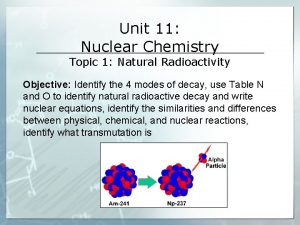
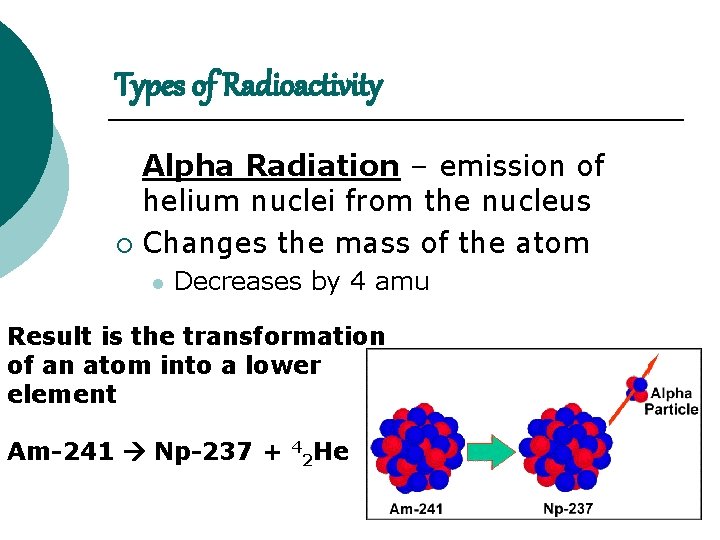
Types of Radioactivity Alpha Radiation – emission of helium nuclei from the nucleus ¡ Changes the mass of the atom l Decreases by 4 amu Result is the transformation of an atom into a lower element Am-241 Np-237 + 4 2 He

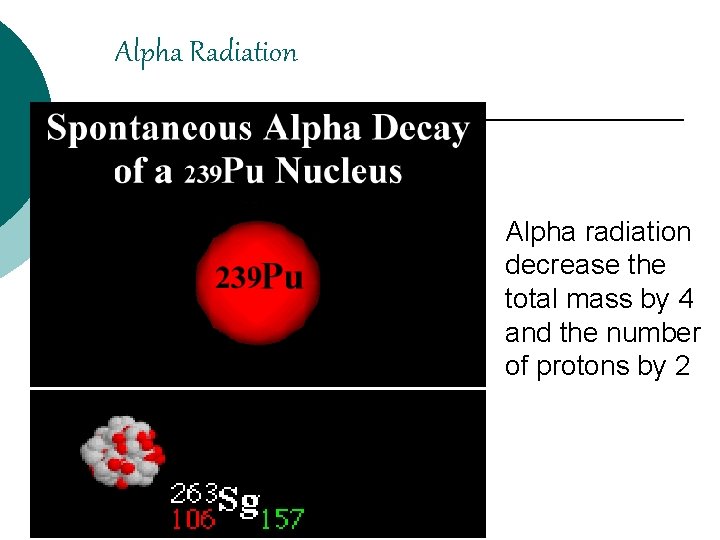
Alpha Radiation Alpha radiation decrease the total mass by 4 and the number of protons by 2

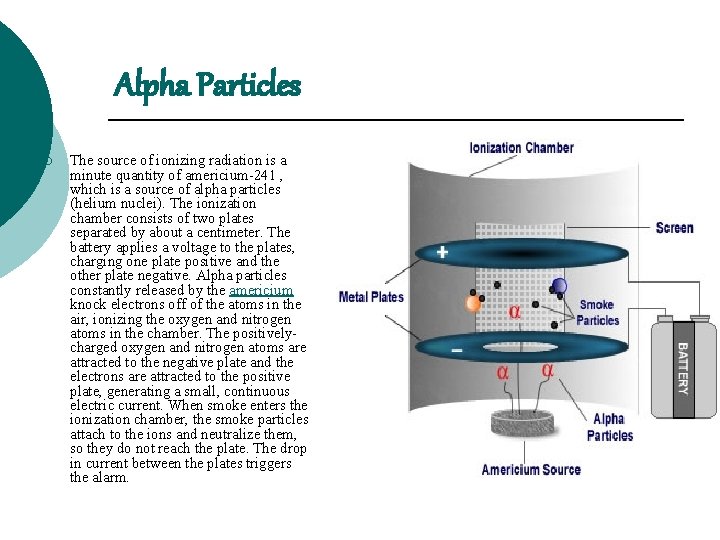
Alpha Particles ¡ The source of ionizing radiation is a minute quantity of americium-241 , which is a source of alpha particles (helium nuclei). The ionization chamber consists of two plates separated by about a centimeter. The battery applies a voltage to the plates, charging one plate positive and the other plate negative. Alpha particles constantly released by the americium knock electrons off of the atoms in the air, ionizing the oxygen and nitrogen atoms in the chamber. The positivelycharged oxygen and nitrogen atoms are attracted to the negative plate and the electrons are attracted to the positive plate, generating a small, continuous electric current. When smoke enters the ionization chamber, the smoke particles attach to the ions and neutralize them, so they do not reach the plate. The drop in current between the plates triggers the alarm.

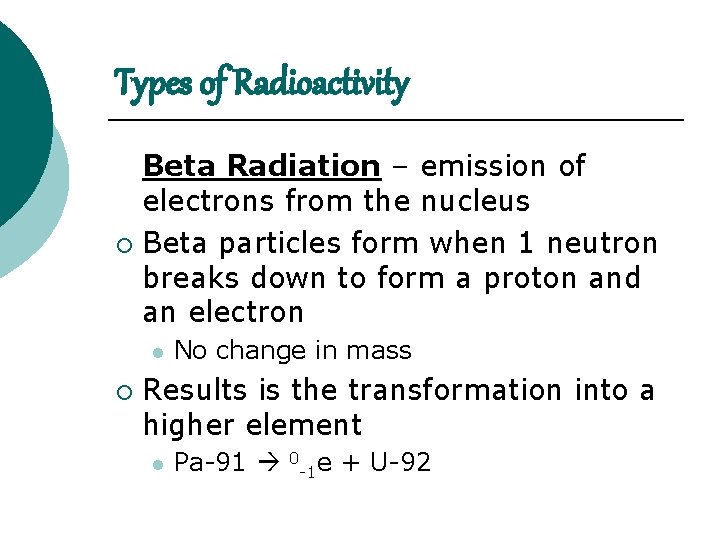
Types of Radioactivity Beta Radiation – emission of electrons from the nucleus ¡ Beta particles form when 1 neutron breaks down to form a proton and an electron l ¡ No change in mass Results is the transformation into a higher element l Pa-91 0 -1 e + U-92

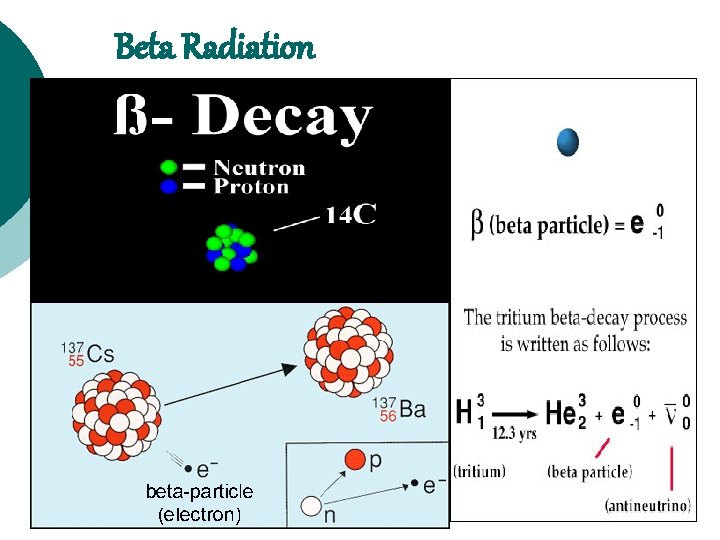
Beta Radiation

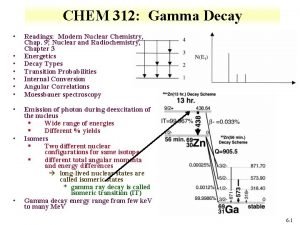
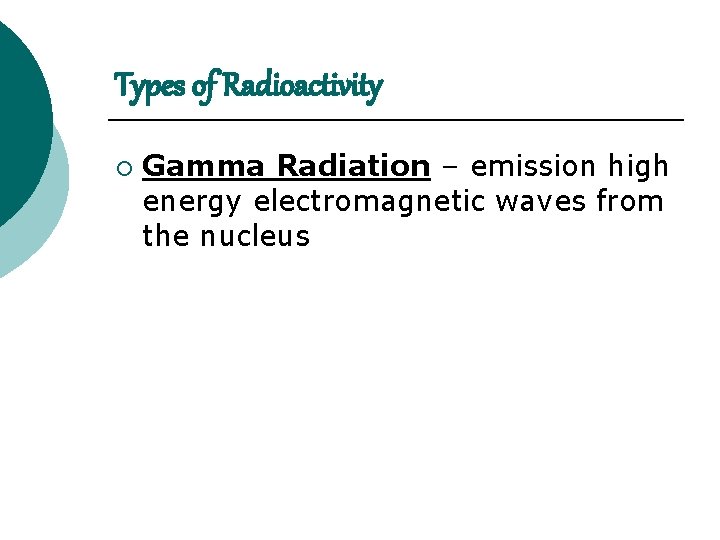
Types of Radioactivity ¡ Gamma Radiation – emission high energy electromagnetic waves from the nucleus

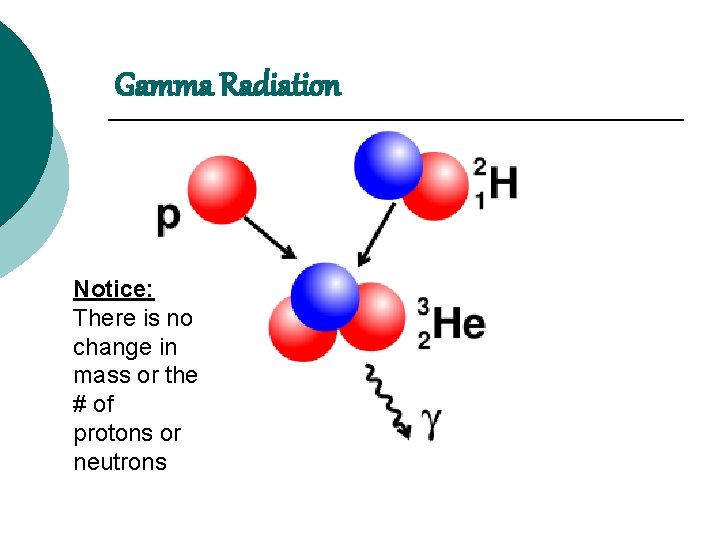
Gamma Radiation Notice: There is no change in mass or the # of protons or neutrons

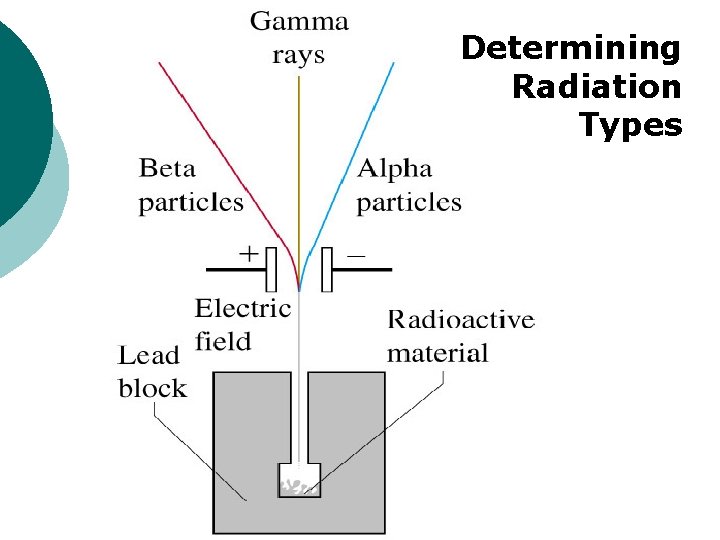
Determining Radiation Types

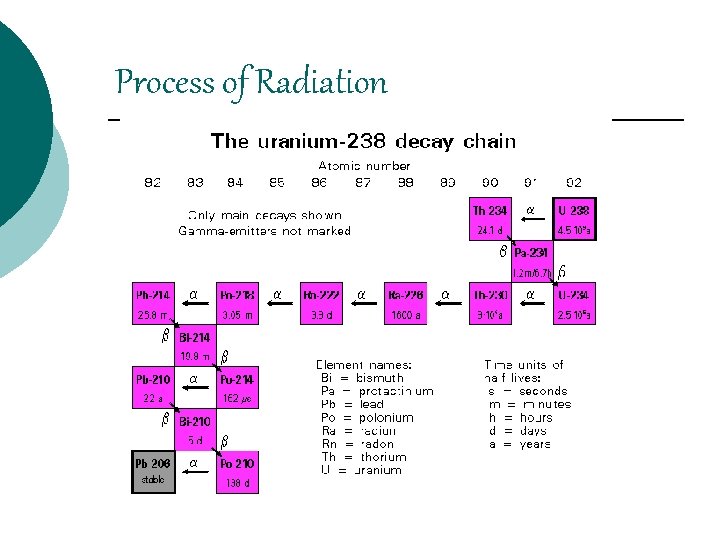
Process of Radiation

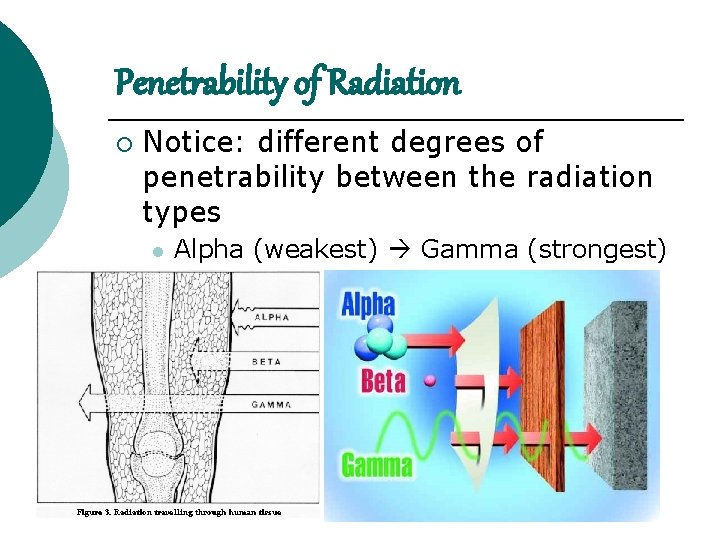
Penetrability of Radiation ¡ Notice: different degrees of penetrability between the radiation types l Alpha (weakest) Gamma (strongest)

Practical Uses of Radioactive Elements ¡ ¡ ¡ Americium -241: Used in many smoke detectors for homes and business. . . to measure levels of toxic lead in dried paint samples. . . to ensure uniform thickness in rolling processes like steel and paper production. . . and to help determine where oil wells should be drilled. Californium - 252: Used to inspect airline luggage for hidden explosives. . . to gauge the moisture content of soil in the road construction and building industries. . . and to measure the moisture of materials stored in silos. Copper - 67: When injected with monoclonal antibodies into a cancer patient, helps the antibodies bind to and destroy the tumor. Krypton - 85: Used in indicator lights in appliances like clothes washer and dryers, stereos and coffee makers. . . to gauge thickness of thin plastics and sheet metal, rubber, textiles and paper. . . and to measure dust and pollutant levels. Thorium - 229: Helps fluorescent lights to last longer.

Medical Uses of Radioactive Elements ¡ ¡ Calcium - 47: Important aid to biomedical researchers studying the cell function and bone formation of mammals. Carbon - 14: Helps in research to ensure that potential new drugs are metabolized without forming harmful by-products. Cesium - 137: Used to treat cancers. . . to measure correct patient dosages of radioactive pharmaceuticals. . . to measure and control the liquid flow in oil pipelines. . . to tell researchers whether oil wells are plugged by sand. . . and to ensure the right fill level for packages of food, drugs and other products. Xenon - 133: Used in nuclear medicine for lung ventilation and blood flow studies.

Industrial Uses of Radioactive Elements ¡ ¡ Curium - 244: Used in mining to analyze material excavated from pits slurries from drilling operations. Iridium - 192: Used to test the integrity of pipeline welds, boilers and aircraft parts. Nickel - 63: Used to detect explosives. . . and as voltage regulators and current surge protectors in electronic devices. Sodium - 24: Used to locate leaks in industrial pipelines. . . and in oil well studies.

Radiation Summary ¡ Definition and Discovery of Radiation ¡ Types of Radiation l Alpha / Beta /Gamma Identifying Radiation Types (diagram) ¡ Process of Radiation ¡ Uses of Radiation ¡
 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Microwave amplification by stimulated emission of radiation
Microwave amplification by stimulated emission of radiation Define metastable state
Define metastable state Beta plus decay
Beta plus decay Exponential decay factor
Exponential decay factor Atomic emission spectroscopy lecture notes
Atomic emission spectroscopy lecture notes Decay nuclear
Decay nuclear Uranium-238 alpha decay equation
Uranium-238 alpha decay equation Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Alpha decay of 150gd64
Alpha decay of 150gd64 Positron emission equation
Positron emission equation Nuclear fission radiation
Nuclear fission radiation Types of radiation
Types of radiation What is nuclear radiation
What is nuclear radiation Nuclear radiation
Nuclear radiation Natural and artificial radioactivity
Natural and artificial radioactivity Fission and fusion similarities
Fission and fusion similarities