Nuclear Radiation Radioactivity Radioactivity The spontaneous release of



- Slides: 7

Nuclear Radiation

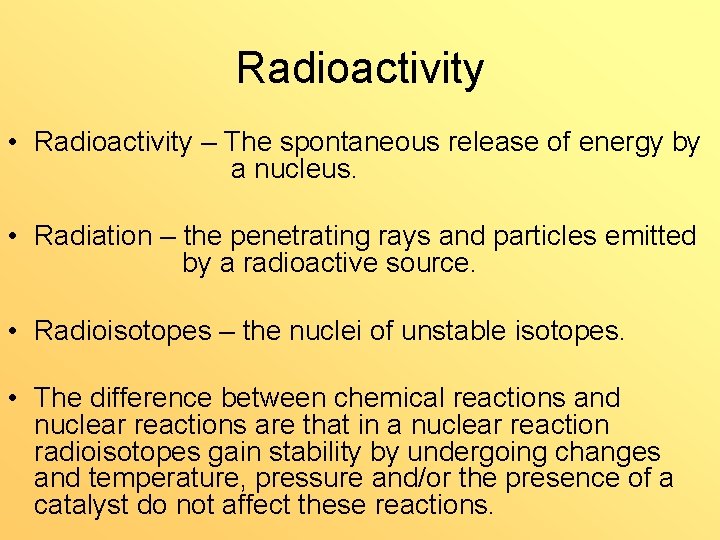
Radioactivity • Radioactivity – The spontaneous release of energy by a nucleus. • Radiation – the penetrating rays and particles emitted by a radioactive source. • Radioisotopes – the nuclei of unstable isotopes. • The difference between chemical reactions and nuclear reactions are that in a nuclear reaction radioisotopes gain stability by undergoing changes and temperature, pressure and/or the presence of a catalyst do not affect these reactions.

Radioactivity (Continued) • Too many or too few neutrons relative to the number of protons makes a nucleus unstable. • An unstable nucleus releases energy by emitting radiation during the process of radioactive decay. Are atoms really indivisible as Dalton said? No, radioactivity disproved Dalton’s assumption.

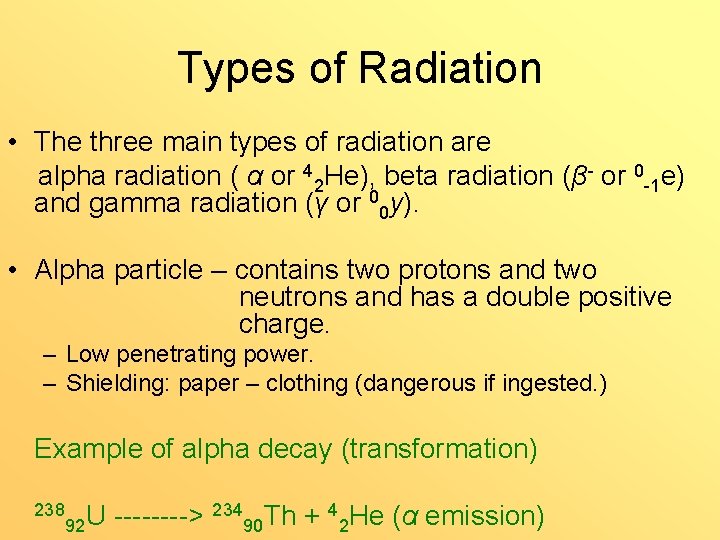
Types of Radiation • The three main types of radiation are alpha radiation ( α or 42 He), beta radiation (β- or 0 -1 e) and gamma radiation (γ or 00 y). • Alpha particle – contains two protons and two neutrons and has a double positive charge. – Low penetrating power. – Shielding: paper – clothing (dangerous if ingested. ) Example of alpha decay (transformation) 238 92 U ----> 23490 Th + 42 He (α emission)

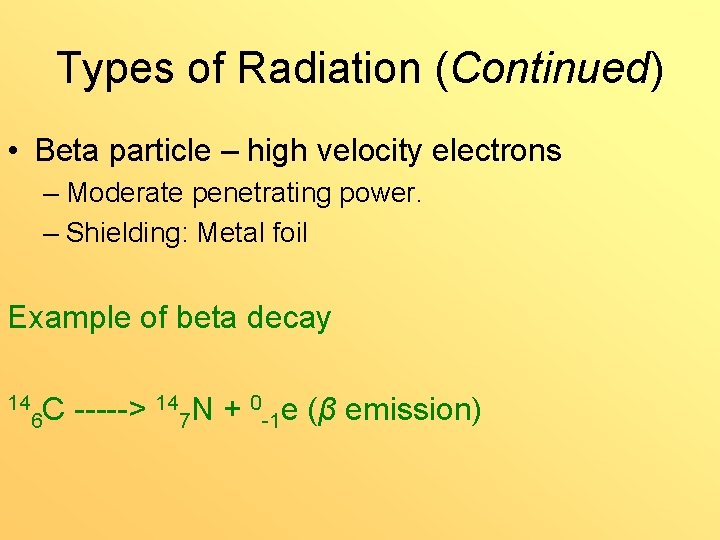
Types of Radiation (Continued) • Beta particle – high velocity electrons – Moderate penetrating power. – Shielding: Metal foil Example of beta decay 14 14 N + 0 e (β emission) C -----> 6 7 -1

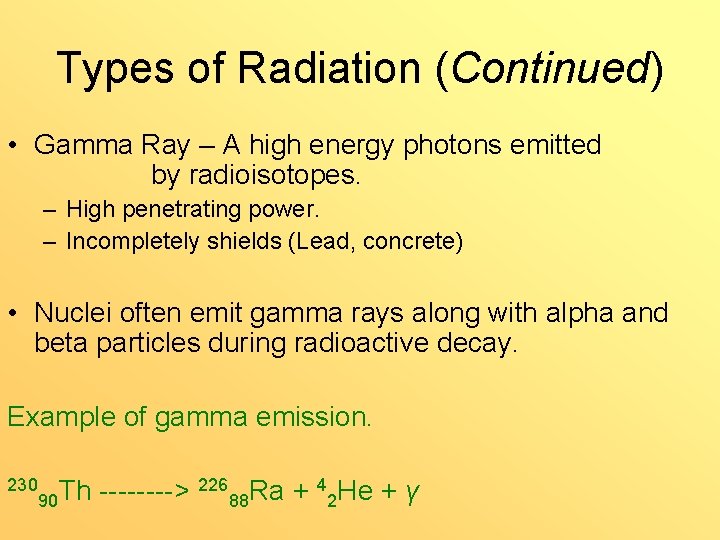
Types of Radiation (Continued) • Gamma Ray – A high energy photons emitted by radioisotopes. – High penetrating power. – Incompletely shields (Lead, concrete) • Nuclei often emit gamma rays along with alpha and beta particles during radioactive decay. Example of gamma emission. 230 226 Ra + 4 He + γ Th ----> 90 88 2

25. 1 Section Assessment pg. 802 #’s 3 -6 Read Section 25. 2 pgs. 803 -808