25 1 Nuclear Radiation Chapter 25 Nuclear Chemistry

















- Slides: 44

25. 1 Nuclear Radiation > Chapter 25 Nuclear Chemistry 25. 1 Nuclear Radiation 25. 2 Nuclear Transformations 25. 3 Fission and Fusion 25. 4 Radiation in Your Life 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

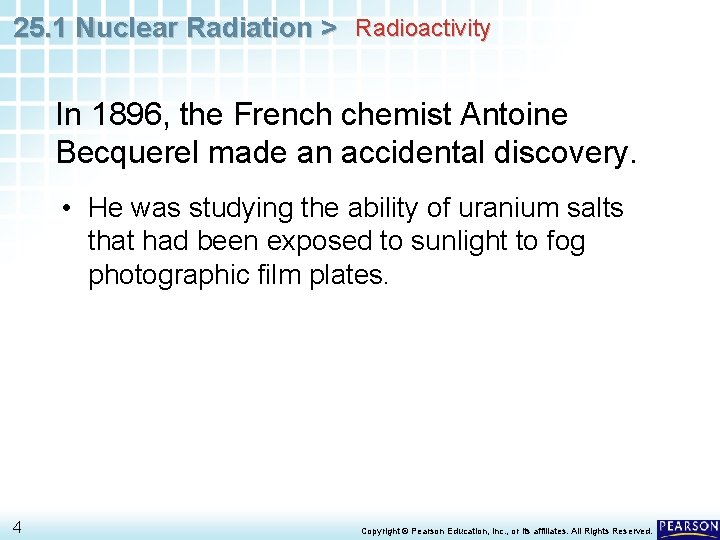
25. 1 Nuclear Radiation > CHEMISTRY & YOU What makes some types of radiation more dangerous than other types? Lengthy or frequent exposure to X-rays can damage cells in your body. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity How do nuclear reactions differ from chemical reactions? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

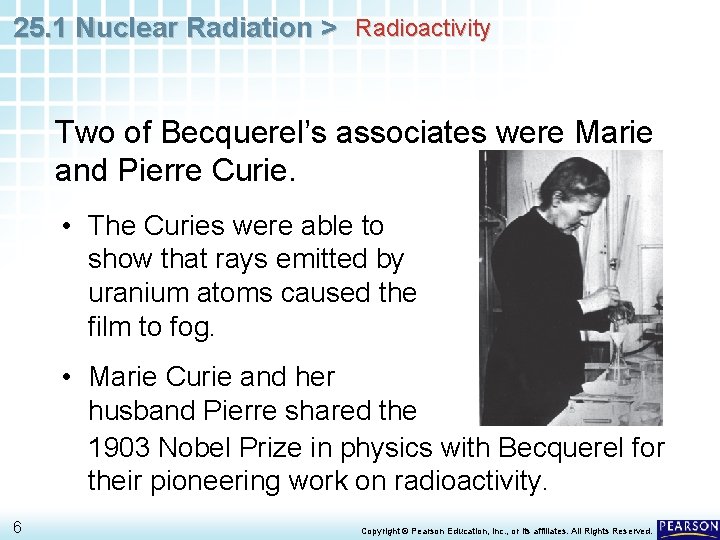
25. 1 Nuclear Radiation > Radioactivity In 1896, the French chemist Antoine Becquerel made an accidental discovery. • He was studying the ability of uranium salts that had been exposed to sunlight to fog photographic film plates. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity In 1896, the French chemist Antoine Becquerel made an accidental discovery. • He was studying the ability of uranium salts that had been exposed to sunlight to fog photographic film plates. • During bad weather, when Becquerel could not expose a sample to sunlight, he left the sample on top of the photographic plate. • When he developed the plate, he discovered that the uranium salt still fogged the film. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity Two of Becquerel’s associates were Marie and Pierre Curie. • The Curies were able to show that rays emitted by uranium atoms caused the film to fog. • Marie Curie and her husband Pierre shared the 1903 Nobel Prize in physics with Becquerel for their pioneering work on radioactivity. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

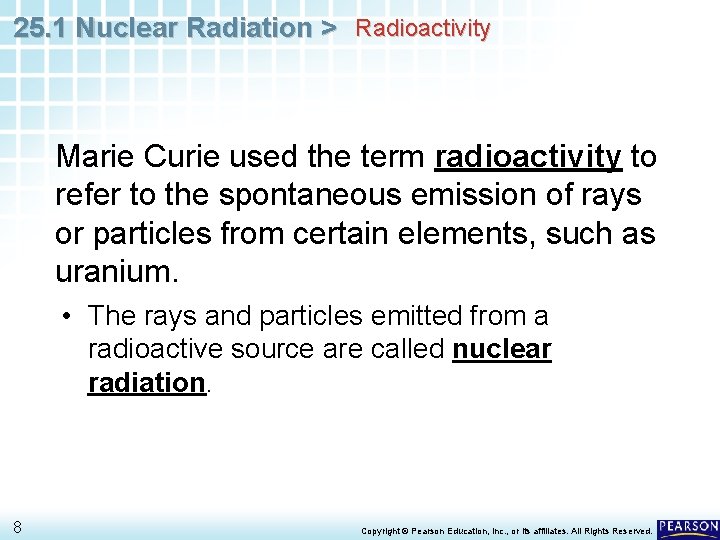
25. 1 Nuclear Radiation > Radioactivity Marie Curie used the term radioactivity to refer to the spontaneous emission of rays or particles from certain elements, such as uranium. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity Marie Curie used the term radioactivity to refer to the spontaneous emission of rays or particles from certain elements, such as uranium. • The rays and particles emitted from a radioactive source are called nuclear radiation. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity, which is also called radioactive decay, is an example of a nuclear reaction. • Nuclear reactions begin with unstable isotopes, or radioisotopes. 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity, which is also called radioactive decay, is an example of a nuclear reaction. • Nuclear reactions begin with unstable isotopes, or radioisotopes. • Atoms of these isotopes become more stable when changes occur in their nuclei. 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity, which is also called radioactive decay, is an example of a nuclear reaction. • Nuclear reactions begin with unstable isotopes, or radioisotopes. • Atoms of these isotopes become more stable when changes occur in their nuclei. • The changes are always accompanied by the emission of large amounts of energy. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

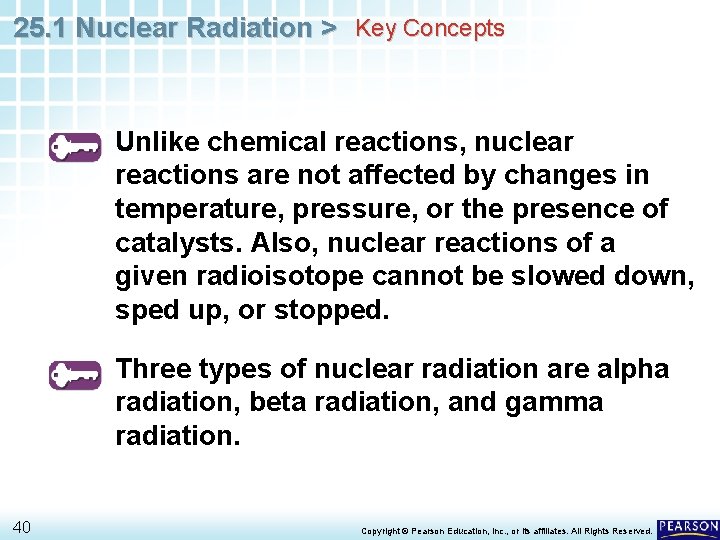
25. 1 Nuclear Radiation > Radioactivity Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. Also, nuclear reactions of a given radioisotope cannot be slowed down, sped up, or stopped. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

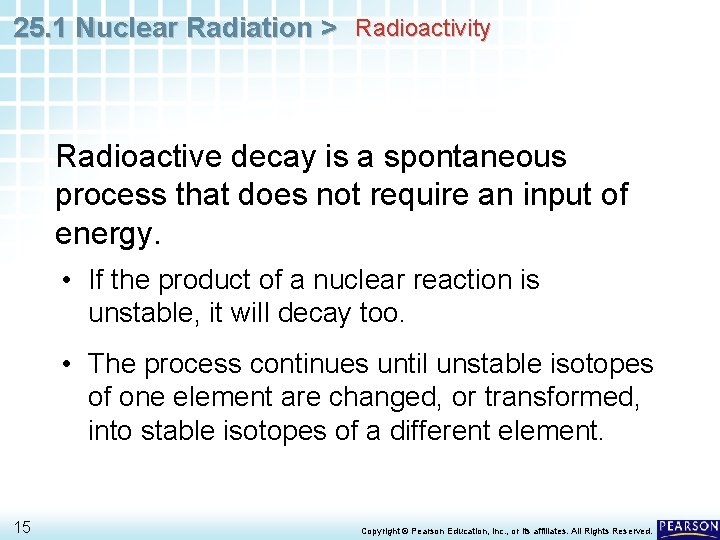
25. 1 Nuclear Radiation > Radioactivity Radioactive decay is a spontaneous process that does not require an input of energy. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity Radioactive decay is a spontaneous process that does not require an input of energy. • If the product of a nuclear reaction is unstable, it will decay too. 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity Radioactive decay is a spontaneous process that does not require an input of energy. • If the product of a nuclear reaction is unstable, it will decay too. • The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Radioactivity Radioactive decay is a spontaneous process that does not require an input of energy. • If the product of a nuclear reaction is unstable, it will decay too. • The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. • These stable isotopes are not radioactive. 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Why do unstable isotopes undergo nuclear reactions? 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Why do unstable isotopes undergo nuclear reactions? Unstable isotopes undergo nuclear reactions so that they may be changed, or transformed, into stable isotopes. 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation What are three types of nuclear radiation? 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation is emitted during radioactive decay. Three types of nuclear radiation are alpha radiation, beta radiation, and gamma radiation. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

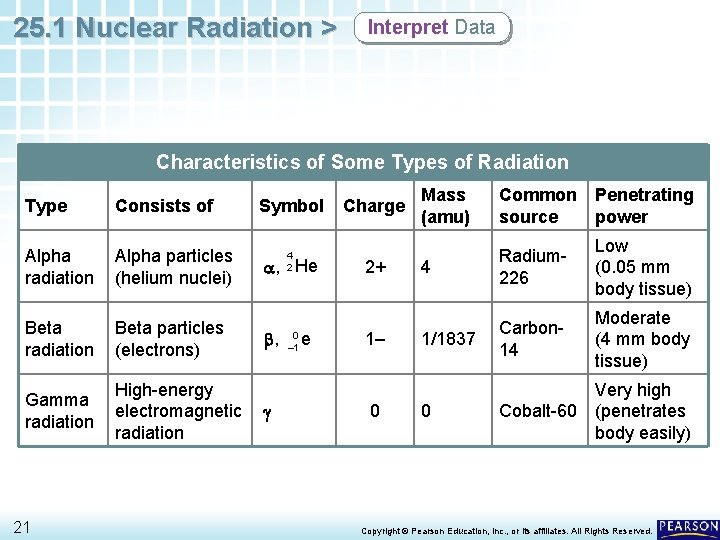
25. 1 Nuclear Radiation > Interpret Data Characteristics of Some Types of Radiation Type Consists of Alpha radiation Alpha particles (helium nuclei) Beta radiation Beta particles (electrons) Gamma radiation High-energy electromagnetic radiation 21 Symbol 4 2 a, He b, g 0 – 1 e Charge 2+ 1– 0 Mass (amu) Common source Penetrating power 4 Radium 226 Low (0. 05 mm body tissue) 1/1837 Carbon 14 Moderate (4 mm body tissue) 0 Very high Cobalt-60 (penetrates body easily) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

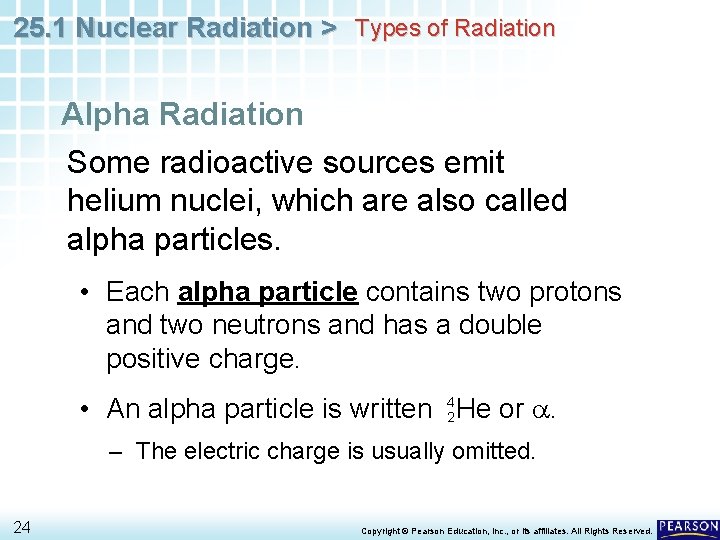
25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation Some radioactive sources emit helium nuclei, which are also called alpha particles. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation Some radioactive sources emit helium nuclei, which are also called alpha particles. • Each alpha particle contains two protons and two neutrons and has a double positive charge. 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation Some radioactive sources emit helium nuclei, which are also called alpha particles. • Each alpha particle contains two protons and two neutrons and has a double positive charge. • An alpha particle is written 42 He or a. – The electric charge is usually omitted. 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

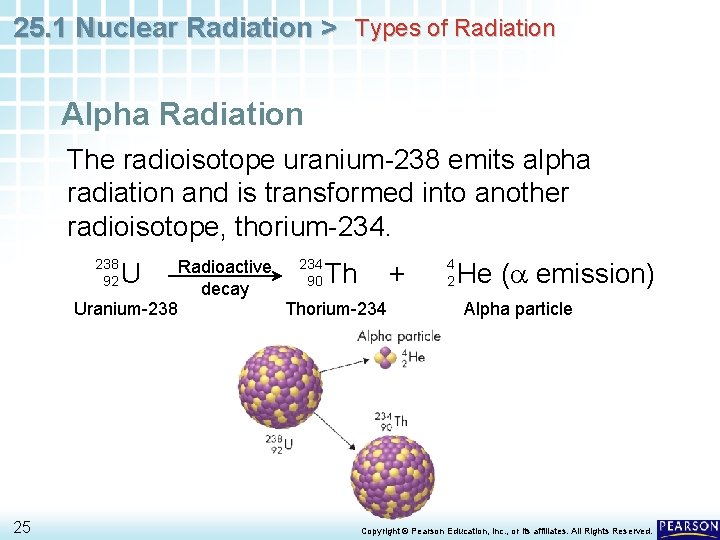
25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation The radioisotope uranium-238 emits alpha radiation and is transformed into another radioisotope, thorium-234. 234 Radioactive + 90 Th decay Thorium-234 Uranium-238 92 25 U 4 2 He (a emission) Alpha particle Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

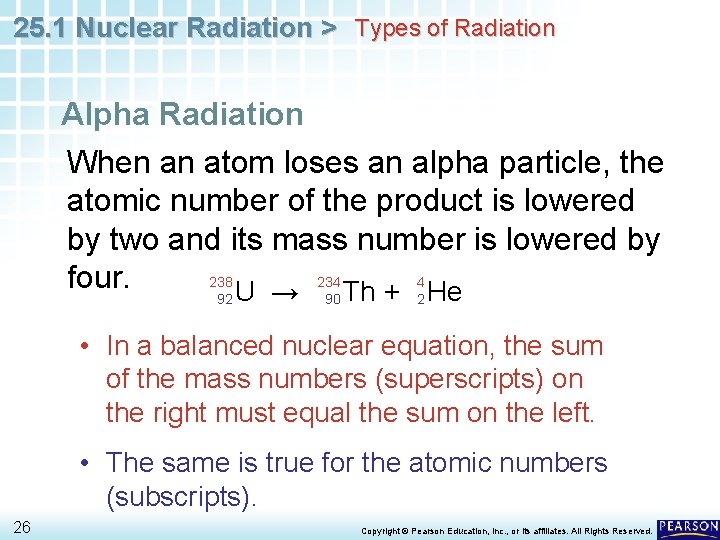
25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation When an atom loses an alpha particle, the atomic number of the product is lowered by two and its mass number is lowered by 234 4 238 four. Th + He U → 92 90 2 • In a balanced nuclear equation, the sum of the mass numbers (superscripts) on the right must equal the sum on the left. • The same is true for the atomic numbers (subscripts). 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation Alpha Radiation Because of their large mass and charge, alpha particles do not travel very far and are not very penetrating. • A sheet of paper or the surface of your skin can stop them. • But radioisotopes that emit alpha particles can cause harm when ingested. – Once inside the body, the particles don’t have to travel far to penetrate soft tissue. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

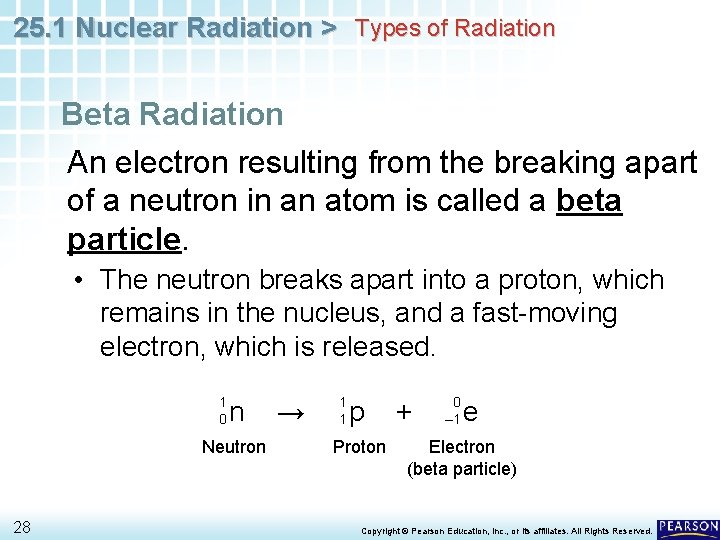
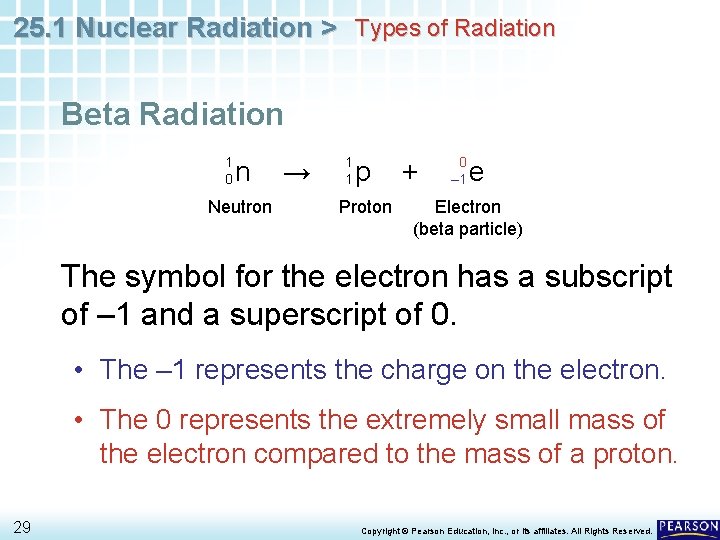
25. 1 Nuclear Radiation > Types of Radiation Beta Radiation An electron resulting from the breaking apart of a neutron in an atom is called a beta particle. • The neutron breaks apart into a proton, which remains in the nucleus, and a fast-moving electron, which is released. 1 0 n Neutron 28 → 1 1 p Proton + 0 – 1 e Electron (beta particle) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Types of Radiation Beta Radiation 1 0 n Neutron → 1 1 p Proton + 0 – 1 e Electron (beta particle) The symbol for the electron has a subscript of – 1 and a superscript of 0. • The – 1 represents the charge on the electron. • The 0 represents the extremely small mass of the electron compared to the mass of a proton. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

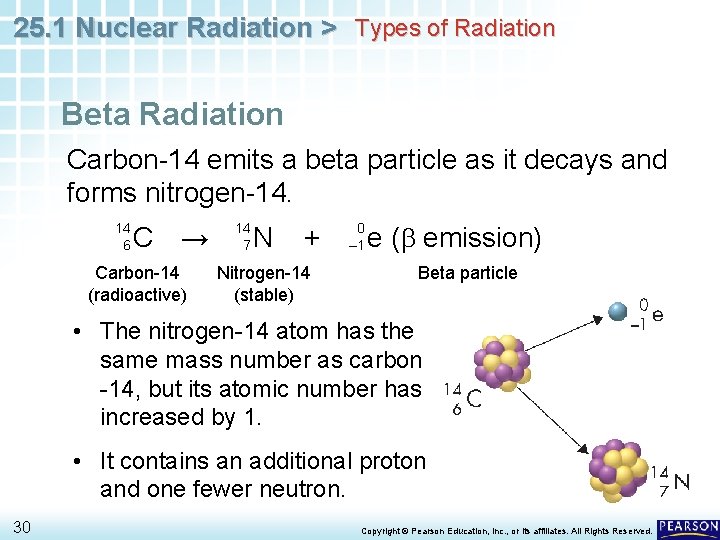
25. 1 Nuclear Radiation > Types of Radiation Beta Radiation Carbon-14 emits a beta particle as it decays and forms nitrogen-14. 14 6 C → Carbon-14 (radioactive) 14 7 N + Nitrogen-14 (stable) 0 – 1 e (b emission) Beta particle • The nitrogen-14 atom has the same mass number as carbon -14, but its atomic number has increased by 1. • It contains an additional proton and one fewer neutron. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

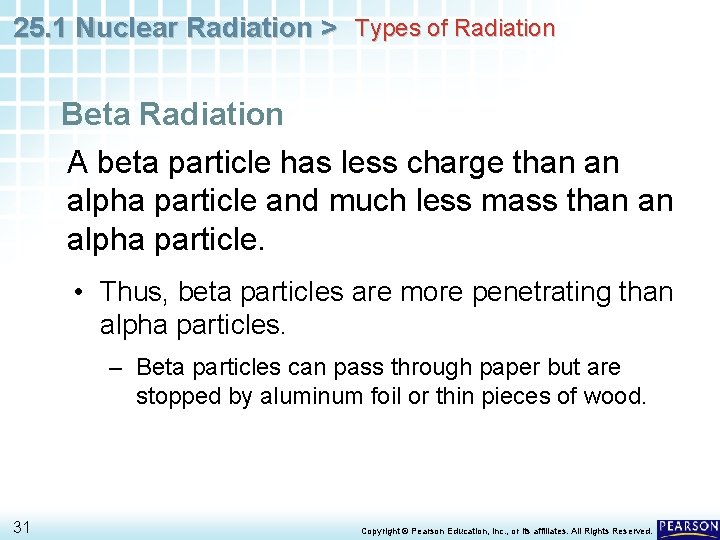
25. 1 Nuclear Radiation > Types of Radiation Beta Radiation A beta particle has less charge than an alpha particle and much less mass than an alpha particle. • Thus, beta particles are more penetrating than alpha particles. – Beta particles can pass through paper but are stopped by aluminum foil or thin pieces of wood. 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

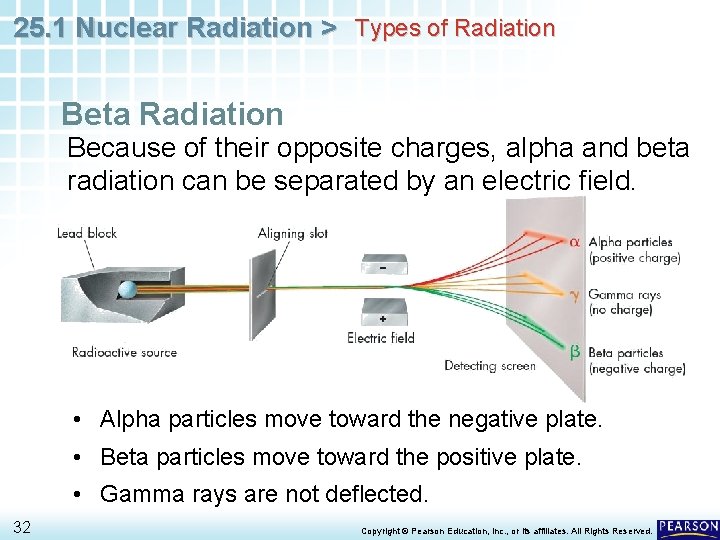
25. 1 Nuclear Radiation > Types of Radiation Beta Radiation Because of their opposite charges, alpha and beta radiation can be separated by an electric field. • Alpha particles move toward the negative plate. • Beta particles move toward the positive plate. • Gamma rays are not deflected. 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

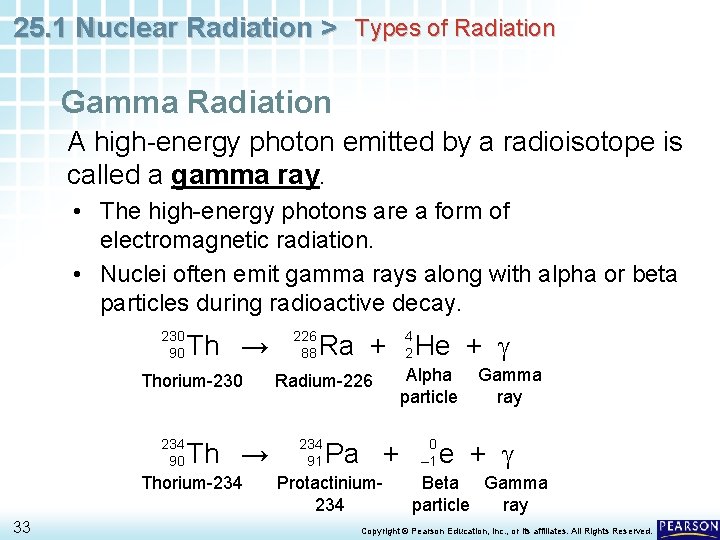
25. 1 Nuclear Radiation > Types of Radiation Gamma Radiation A high-energy photon emitted by a radioisotope is called a gamma ray. • The high-energy photons are a form of electromagnetic radiation. • Nuclei often emit gamma rays along with alpha or beta particles during radioactive decay. 230 90 Th → Thorium-230 234 90 Th → Thorium-234 33 226 88 Ra + Radium-226 234 91 Pa + Protactinium 234 4 2 He + g Alpha particle 0 – 1 Gamma ray e + g Beta Gamma particle ray Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

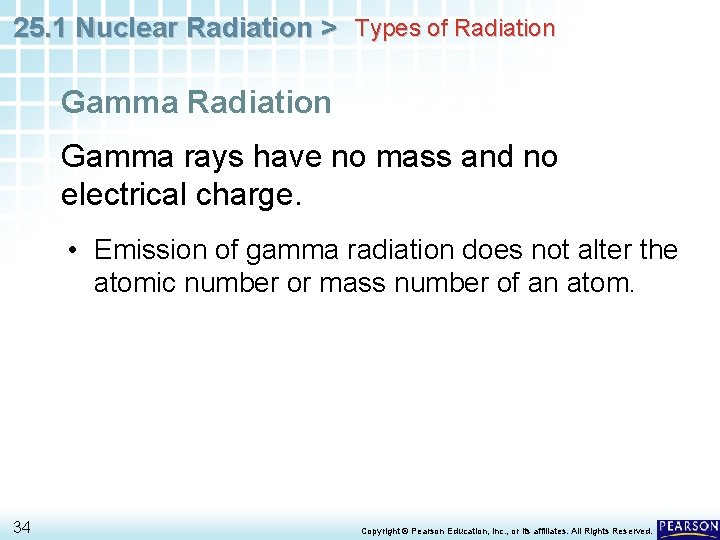
25. 1 Nuclear Radiation > Types of Radiation Gamma rays have no mass and no electrical charge. • Emission of gamma radiation does not alter the atomic number or mass number of an atom. 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

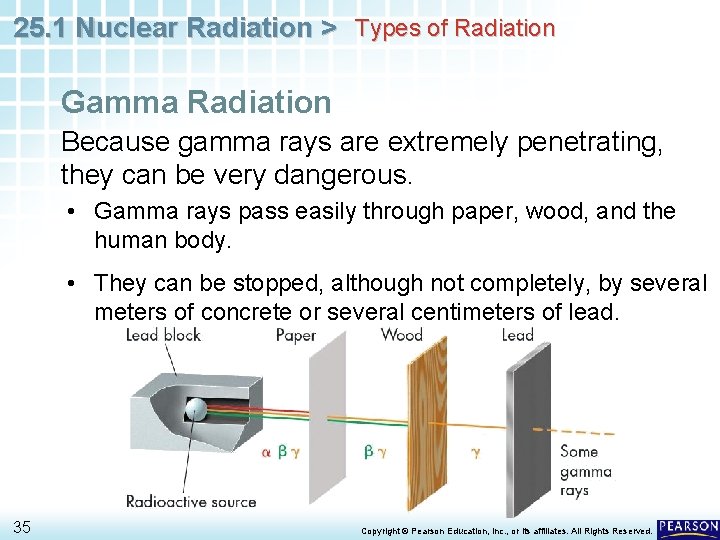
25. 1 Nuclear Radiation > Types of Radiation Gamma Radiation Because gamma rays are extremely penetrating, they can be very dangerous. • Gamma rays pass easily through paper, wood, and the human body. • They can be stopped, although not completely, by several meters of concrete or several centimeters of lead. 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > CHEMISTRY & YOU Gamma rays can be dangerous because of their penetrating power. What property determines the relative penetrating power of electromagnetic radiation? 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > CHEMISTRY & YOU Gamma rays can be dangerous because of their penetrating power. What property determines the relative penetrating power of electromagnetic radiation? The wavelength and energy of electromagnetic radiation determine its relative penetrating power. Gamma rays have a shorter wavelength and higher energy than X-rays or visible light. 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Which process involves a radioactive nucleus releasing a high-speed electron? A. oxidation B. alpha emission C. beta emission D. gamma radiation 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Which process involves a radioactive nucleus releasing a high-speed electron? A. oxidation B. alpha emission C. beta emission D. gamma radiation 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Key Concepts Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. Also, nuclear reactions of a given radioisotope cannot be slowed down, sped up, or stopped. Three types of nuclear radiation are alpha radiation, beta radiation, and gamma radiation. 40 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Glossary Terms • radioactivity: the process by which nuclei emit particles and rays • nuclear radiation: the penetrating rays and particles emitted by a radioactive source • radioisotope: an isotope that has an unstable nucleus and undergoes radioactive decay 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > Glossary Terms • alpha particle: a positively charged particle emitted from certain radioactive nuclei; it consists of two protons and two neutrons and is identical to the nucleus of a helium atom • beta particle: an electron resulting from the breaking apart of neutrons in an atom • gamma ray: a high-energy photon emitted by a radioisotope 42 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > BIG IDEA Electrons and the Structure of Atoms • Unstable atomic nuclei decay by emitting alpha or beta particles. • Often gamma rays are emitted too. 43 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 1 Nuclear Radiation > END OF 25. 1 44 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.