Radioactivity What is Radioactivity Radioactivity the release of



- Slides: 10

Radioactivity

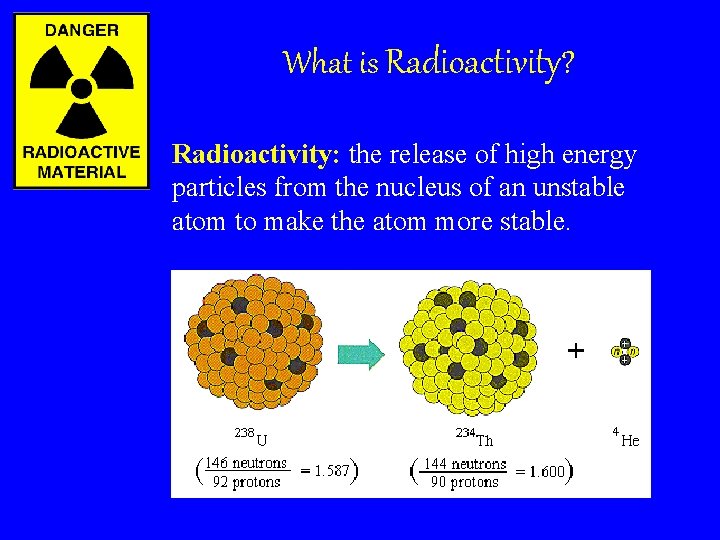
What is Radioactivity? Radioactivity: the release of high energy particles from the nucleus of an unstable atom to make the atom more stable.

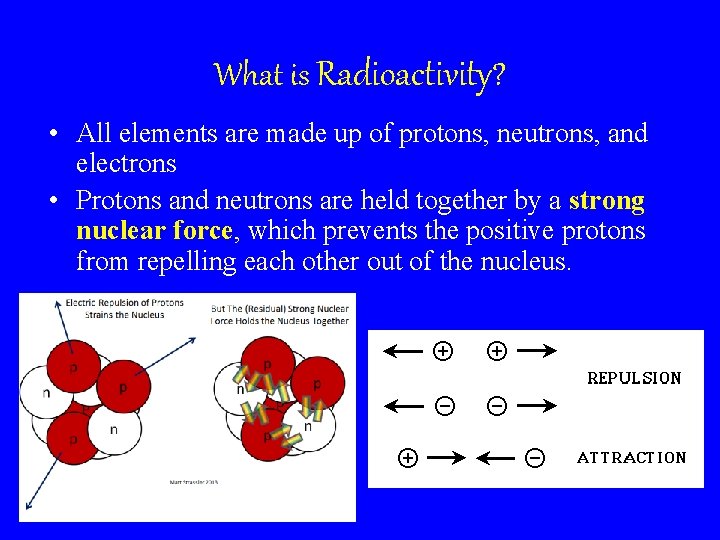
What is Radioactivity? • All elements are made up of protons, neutrons, and electrons • Protons and neutrons are held together by a strong nuclear force, which prevents the positive protons from repelling each other out of the nucleus.

What is Radioactivity? • • BUT!…the Strong Nuclear Force only works for small distances. As atomic number increases, the nucleus increases in size, and the strength of the Strong Force decreases. Atomic Number = Strong Force

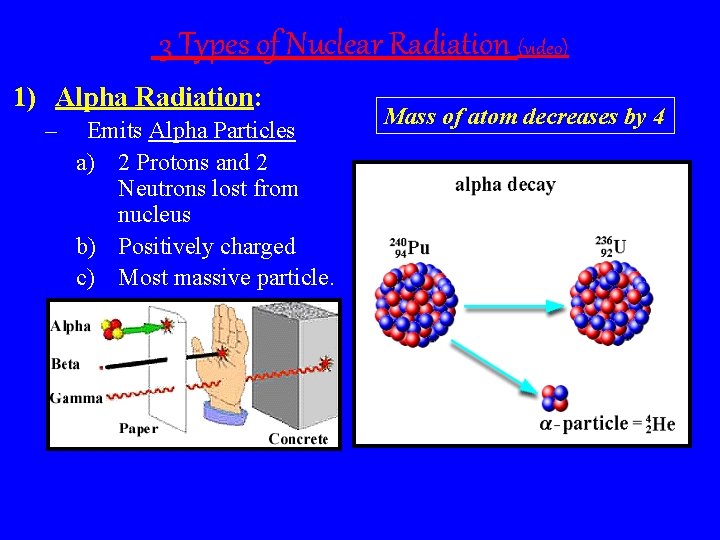
3 Types of Nuclear Radiation (video) 1) Alpha Radiation: – Emits Alpha Particles a) 2 Protons and 2 Neutrons lost from nucleus b) Positively charged c) Most massive particle. Mass of atom decreases by 4

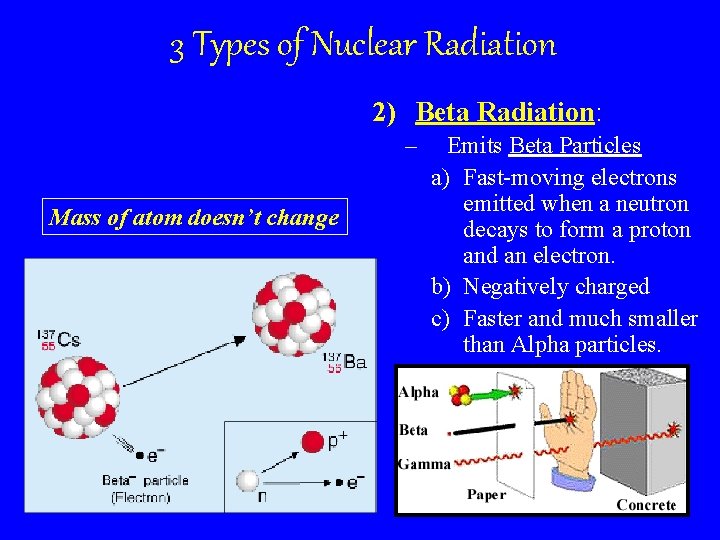
3 Types of Nuclear Radiation 2) Beta Radiation: – Mass of atom doesn’t change Emits Beta Particles a) Fast-moving electrons emitted when a neutron decays to form a proton and an electron. b) Negatively charged c) Faster and much smaller than Alpha particles.

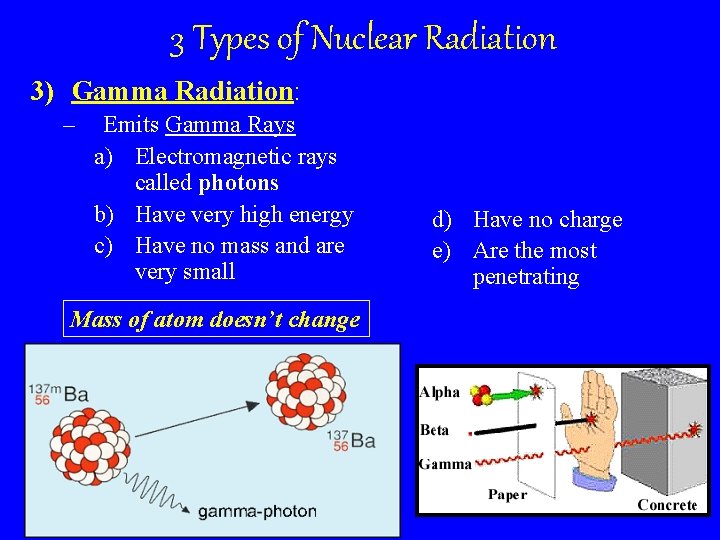
3 Types of Nuclear Radiation 3) Gamma Radiation: – Emits Gamma Rays a) Electromagnetic rays called photons b) Have very high energy c) Have no mass and are very small Mass of atom doesn’t change d) Have no charge e) Are the most penetrating

Nuclear Fission (video) • Nuclear Fission: the splitting of an atomic nucleus into 2 smaller nuclei. – Chain Reaction: On-going series of fission reactions Nagasaki, Japan Aug. 9, 1945 Harris Nuclear Facility Raleigh, NC

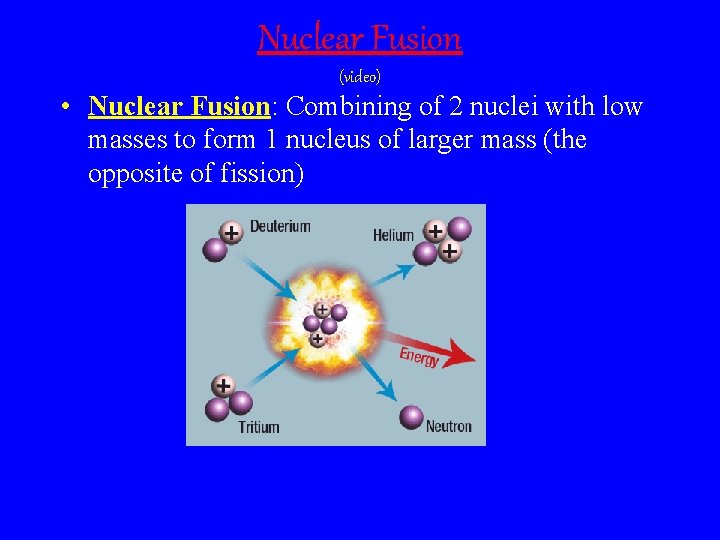
Nuclear Fusion (video) • Nuclear Fusion: Combining of 2 nuclei with low masses to form 1 nucleus of larger mass (the opposite of fission)

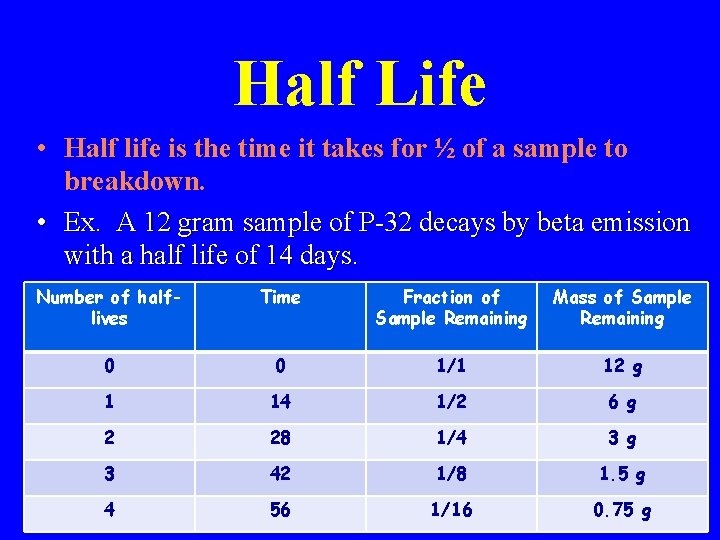
Half Life • Half life is the time it takes for ½ of a sample to breakdown. • Ex. A 12 gram sample of P-32 decays by beta emission with a half life of 14 days. Number of halflives Time Fraction of Sample Remaining Mass of Sample Remaining 0 0 1/1 12 g 1 14 1/2 6 g 2 28 1/4 3 g 3 42 1/8 1. 5 g 4 56 1/16 0. 75 g
 Extended release vs sustained release
Extended release vs sustained release Immediate release dosage form
Immediate release dosage form Osmotic pump
Osmotic pump Natural vs artificial radioactivity
Natural vs artificial radioactivity How to calculate radioactive decay
How to calculate radioactive decay Radioactivity definition geology
Radioactivity definition geology Who discovered radioactivity
Who discovered radioactivity Alpha particle in electric field
Alpha particle in electric field Radioactivity phenomenon
Radioactivity phenomenon Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Defination of radioactivity
Defination of radioactivity


















