10 1 Radioactivity Nuclear Decay Radioactivity happens when






- Slides: 8

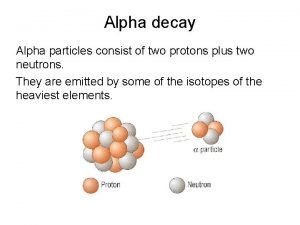
10. 1 Radioactivity • Nuclear Decay – Radioactivity happens when unstable atomic nucleus (called radioactive isotope or radioisotope) emits charged particles/E – Nuclear decay is when atoms of one element change into atoms of another element • Types of Nuclear Radiation – Alpha Decay: U-238 emits + particle (2 p+ and 2 neutrons/ looks like He nucleus) – Beta Decay: Th-234 emits – particle (-1/ like e-) – Gamma Decay: more penetrating and ray of energy emitted (no charge)

• Effects of Nuclear Radiation – Background radiation: radioisotopes occurring naturally in environment – When exceeds background levels nuclear radiation ionizes atoms – ALL damaging to tissues/deeper • How we detect nuclear radiation – Geiger counter: tool to measure amounts – Film: badges: photographic film in paper to monitor radiation exposure

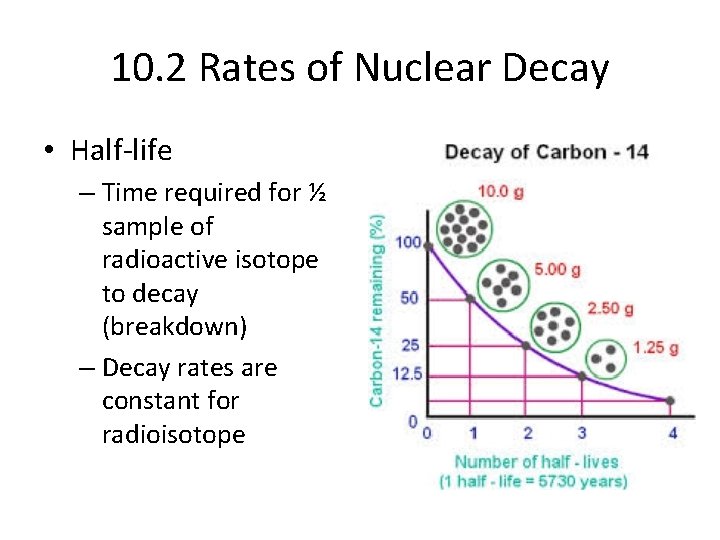
10. 2 Rates of Nuclear Decay • Half-life – Time required for ½ sample of radioactive isotope to decay (breakdown) – Decay rates are constant for radioisotope

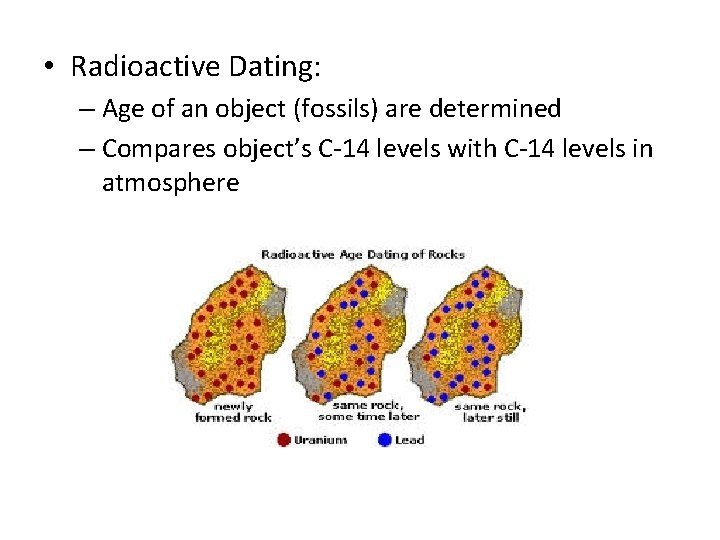
• Radioactive Dating: – Age of an object (fossils) are determined – Compares object’s C-14 levels with C-14 levels in atmosphere

10. 3 Artificial Transmutation • In Lab – Nuclear decay is transmutation that happens naturally – Artificially can hit nucleus with high E particles (p+, neutrons, or alpha particles) • Transuranium Elements – Atomic number above 92 (uranium) – All radioactive and not normally found in nature – Synthesized for lab, industrial, consumer use

• Particle Accelerators – Rutherford (Gold foil guy!) – Used alpha particles – Particle accelerators speed up particles close to speed of light (3. 00 x 108 m/s) – Quark: p+ and neutrons made of even smaller particles, among basic units of matter, 6 types exist

10. 4 Fission and Fusion • Nuclear Forces – Strong nuclear forces bind p+ and neutrons in nucleus – Short distances, depend on # p+ – Nucleus unstable (radioactive) when strong nuclear force can no longer overcome repulsive electric forces of p+ • Fission – German chemists: Otto Hahn and Fritz Strassman – Splitting of atomic nucleus into 2 – Were originally trying to make more massive elements, but made isotopes that were smaller!

• Mass-Energy: E=mc 2 • Chain Reaction – Neutron released during initial split trigger additional splitting • Nuclear E from Fission – Nuclear power plants generate 20% E in US + don’t make pollution, - make radioactive waste – Chernobyl, Ukraine (’ 86) or 2 mile island, PA (’ 79) • Fusion – Nuclei combine to make larger nuclei