Nuclear Reactions and Radioactivity Part I 1 Discovery






- Slides: 36

Nuclear Reactions and Radioactivity Part I 1

Discovery of Radioactivity Antoine-Henri Becquerel (1896) While experimenting with uranium compounds, he discovered that: • The compounds emit penetrating radiation that produces images on photographic film • This phenomenon occurs even when wrapped in paper and stored in the dark • Radiation creates an electric discharge in air, providing a way to measure its intensity 2

Discovery of Radioactivity Marie & Pierre Curie (Early 1900 s) • Found that thorium minerals also emit radiation • Showed that the intensity of radiation is directly proportional to the concentration of the element in the mineral, not the nature of the compound in which element occurs • Named this behavior radioactivity • Discovered the elements polonium and radium 3

Discovery of Radioactivity Rutherford & Colleagues (1902) • Discovered that elements other than radium formed when radium emitted radioactive emissions • Proposed that radioactive emissions cause one element to change into another • This proposal was met with skepticism (sounded similar to alchemy) • Led to an understanding of the three types of radioactive emissions: alpha, beta, and gamma 4

Radioactivity The spontaneous breakdown of the nuclei of atoms accompanied by a release of some type of radiation. (The atom’s nuclei are trying to become more stable. 5

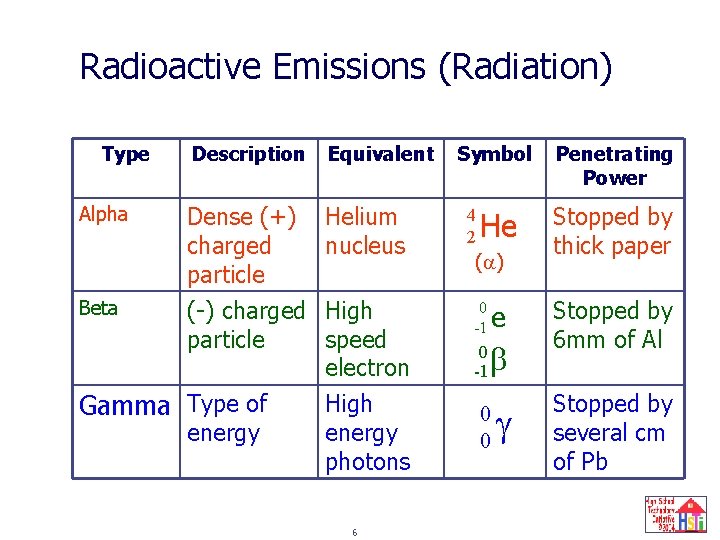
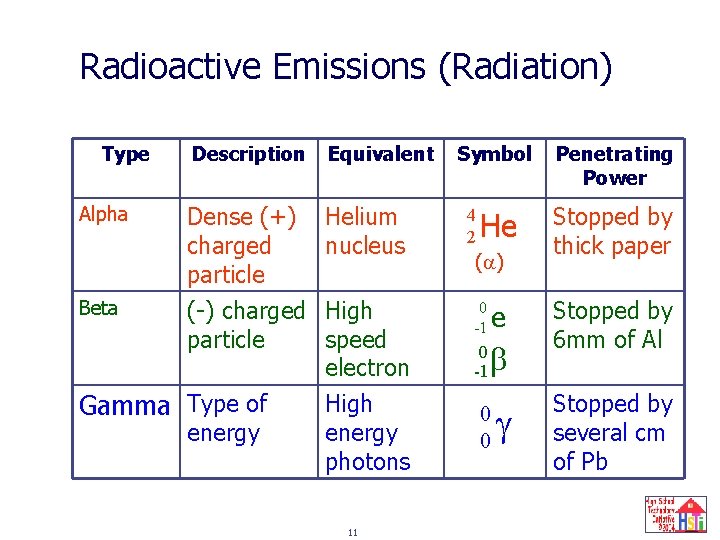
Radioactive Emissions (Radiation) Type Alpha Beta Description Equivalent Dense (+) charged particle (-) charged particle Helium nucleus Gamma Type of energy High speed electron High energy photons 6 Symbol 4 2 He ( ) 0 -1 e 0 b -1 0 0 Penetrating Power Stopped by thick paper Stopped by 6 mm of Al Stopped by several cm of Pb

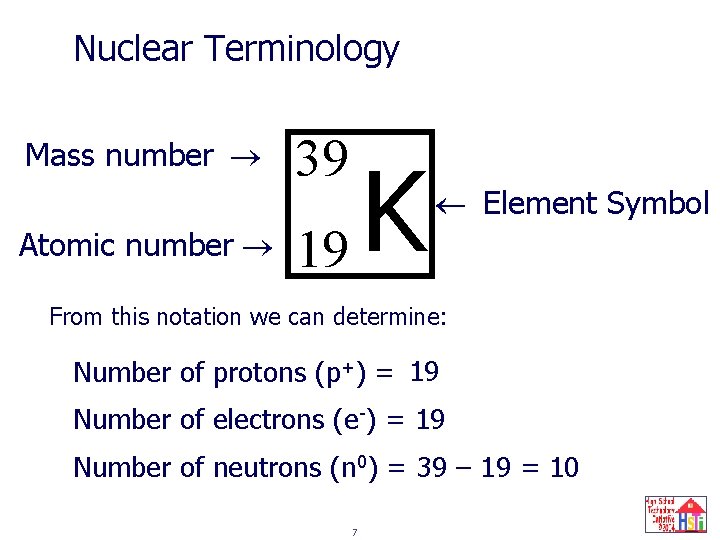
Nuclear Terminology Mass number Atomic number 39 K 19 Element Symbol From this notation we can determine: Number of protons (p+) = 19 Number of electrons (e-) = 19 Number of neutrons (n 0) = 39 – 19 = 10 7

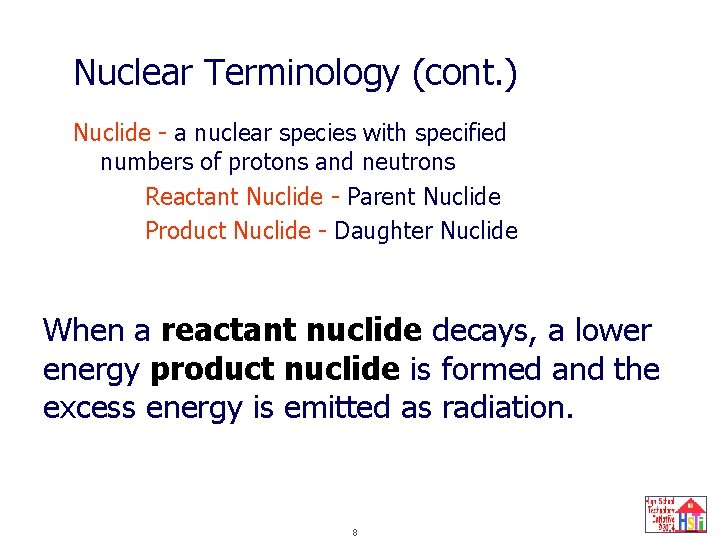
Nuclear Terminology (cont. ) Nuclide - a nuclear species with specified numbers of protons and neutrons Reactant Nuclide - Parent Nuclide Product Nuclide - Daughter Nuclide When a reactant nuclide decays, a lower energy product nuclide is formed and the excess energy is emitted as radiation. 8

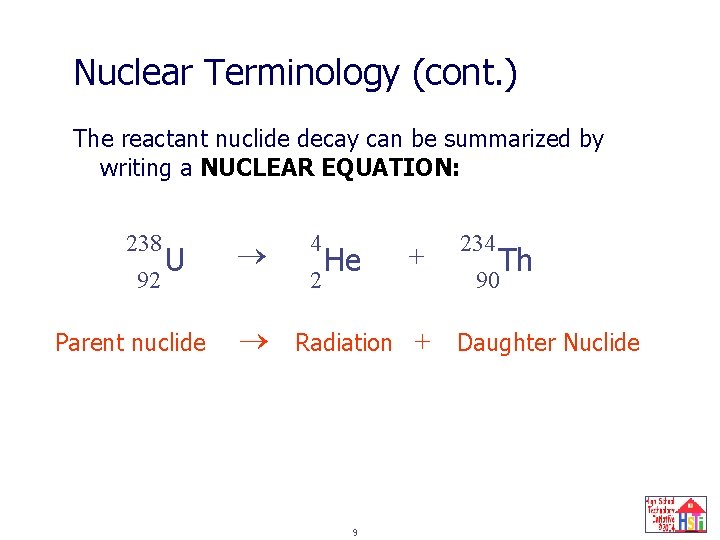
Nuclear Terminology (cont. ) The reactant nuclide decay can be summarized by writing a NUCLEAR EQUATION: 238 92 U Parent nuclide 4 He + 234 Radiation + Daughter Nuclide 2 9 Th 90

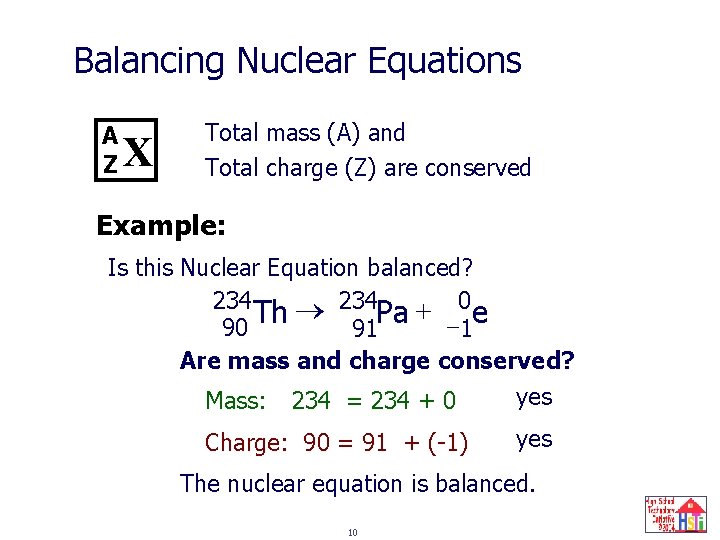
Balancing Nuclear Equations A Z X Total mass (A) and Total charge (Z) are conserved Example: Is this Nuclear Equation balanced? 234 Pa + -0 e Th 90 91 1 Are mass and charge conserved? yes Mass: 234 = 234 + 0 Charge: 90 = 91 + (-1) yes The nuclear equation is balanced. 10

Radioactive Emissions (Radiation) Type Alpha Beta Description Equivalent Dense (+) charged particle (-) charged particle Helium nucleus Gamma Type of energy High speed electron High energy photons 11 Symbol 4 2 He ( ) 0 -1 e 0 b -1 0 0 Penetrating Power Stopped by thick paper Stopped by 6 mm of Al Stopped by several cm of Pb

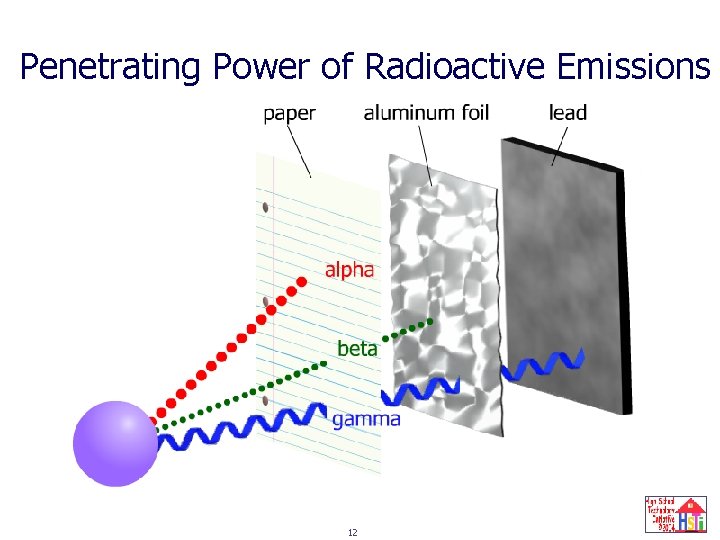
Penetrating Power of Radioactive Emissions 12

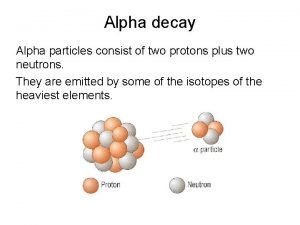
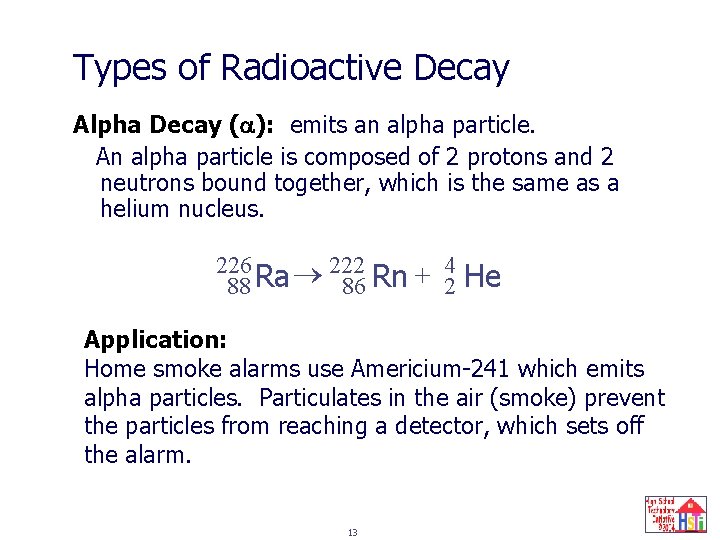
Types of Radioactive Decay Alpha Decay ( ): emits an alpha particle. An alpha particle is composed of 2 protons and 2 neutrons bound together, which is the same as a helium nucleus. 226 222 4 + Ra Rn 88 86 2 He Application: Home smoke alarms use Americium-241 which emits alpha particles. Particulates in the air (smoke) prevent the particles from reaching a detector, which sets off the alarm. 13

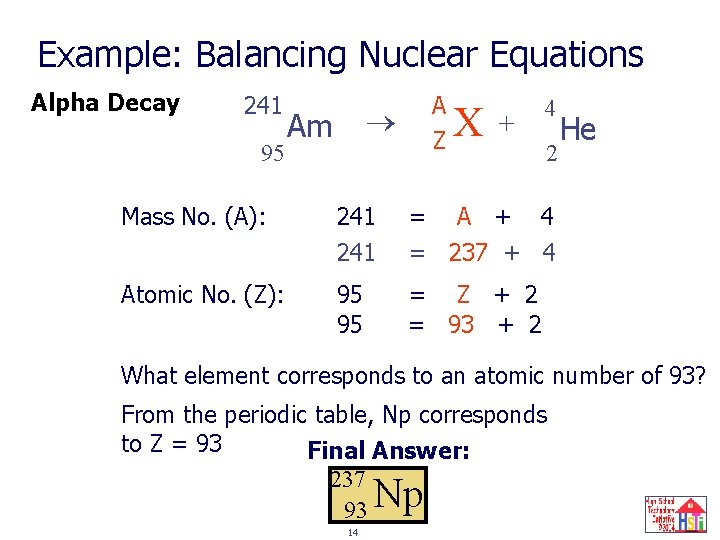
Example: Balancing Nuclear Equations Alpha Decay 241 95 A ? Z X Am + 4 2 Mass No. (A): 241 = A + 4 = 237 + 4 Atomic No. (Z): 95 95 = Z + 2 = 93 + 2 He What element corresponds to an atomic number of 93? From the periodic table, Np corresponds to Z = 93 Final Answer: 237 93 Np 14

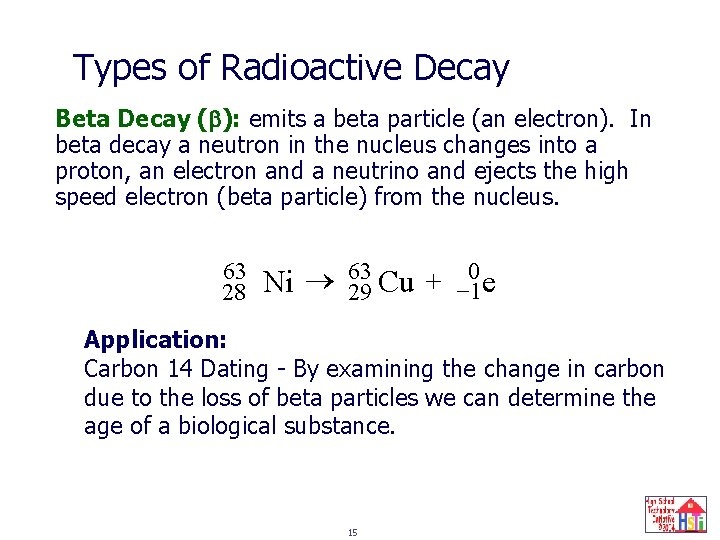
Types of Radioactive Decay Beta Decay ( ): emits a beta particle (an electron). In beta decay a neutron in the nucleus changes into a proton, an electron and a neutrino and ejects the high speed electron (beta particle) from the nucleus. 63 28 Ni 63 29 Cu + 0 -1 e Application: Carbon 14 Dating - By examining the change in carbon due to the loss of beta particles we can determine the age of a biological substance. 15

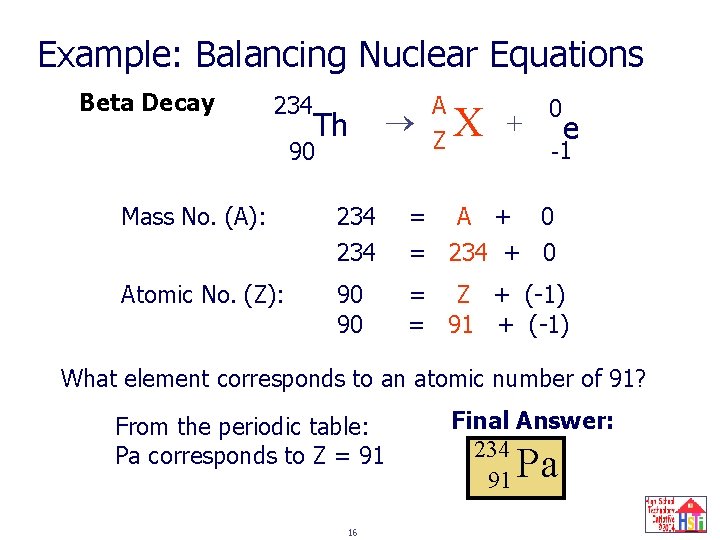
Example: Balancing Nuclear Equations Beta Decay 234 Th 90 A ? Z X + 0 e -1 Mass No. (A): 234 = A + 0 = 234 + 0 Atomic No. (Z): 90 90 = Z + (-1) = 91 + (-1) What element corresponds to an atomic number of 91? From the periodic table: Pa corresponds to Z = 91 16 Final Answer: 234 91 Pa

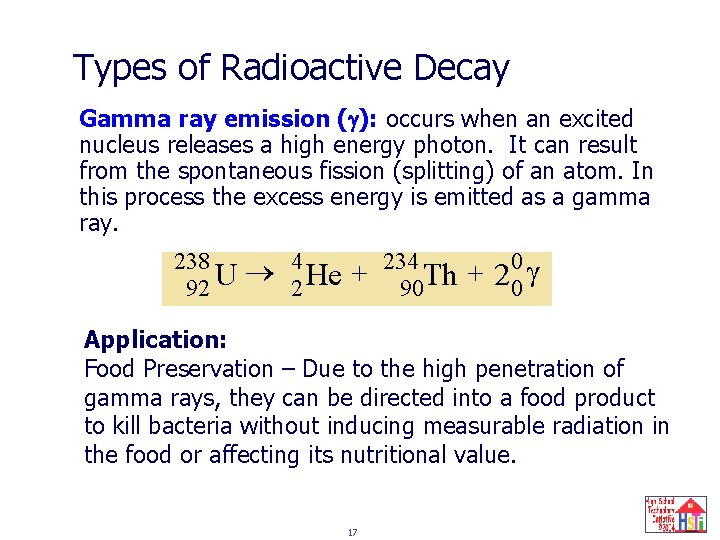
Types of Radioactive Decay Gamma ray emission ( ): occurs when an excited nucleus releases a high energy photon. It can result from the spontaneous fission (splitting) of an atom. In this process the excess energy is emitted as a gamma ray. 238 92 U 42 He + 234 90 Th + 2 00 Application: Food Preservation – Due to the high penetration of gamma rays, they can be directed into a food product to kill bacteria without inducing measurable radiation in the food or affecting its nutritional value. 17

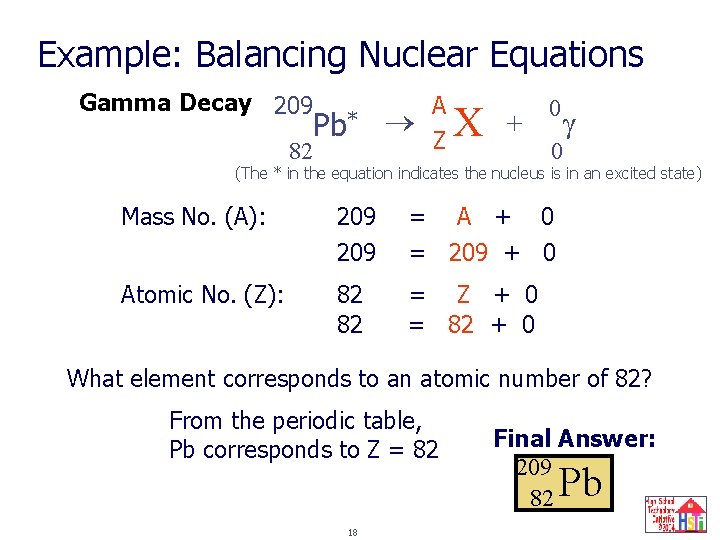
Example: Balancing Nuclear Equations Gamma Decay 209 Pb* 82 A ? Z X + 0 0 (The * in the equation indicates the nucleus is in an excited state) Mass No. (A): 209 = A + 0 = 209 + 0 Atomic No. (Z): 82 82 = Z + 0 = 82 + 0 What element corresponds to an atomic number of 82? From the periodic table, Pb corresponds to Z = 82 18 Final Answer: 209 82 Pb

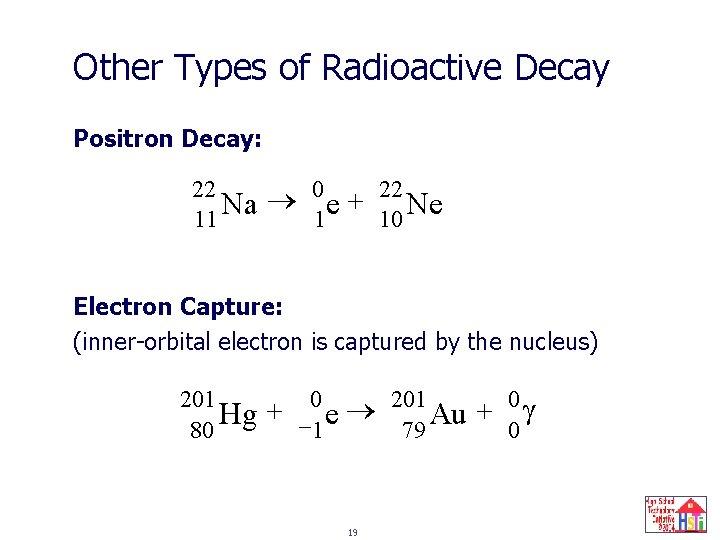
Other Types of Radioactive Decay Positron Decay: 22 Na 11 0 e + 1 22 Ne 10 Electron Capture: (inner-orbital electron is captured by the nucleus) 201 Hg 80 + 0 - 1 e 19 201 Au 79 + 0 0

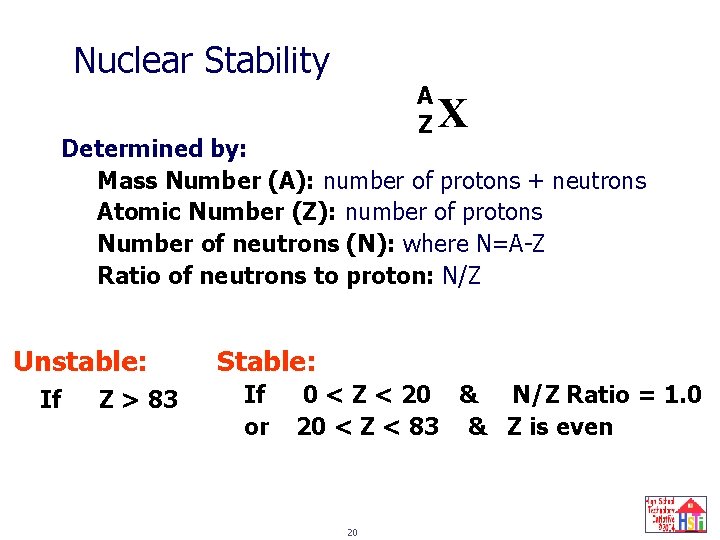
Nuclear Stability A Z X Determined by: Mass Number (A): number of protons + neutrons Atomic Number (Z): number of protons Number of neutrons (N): where N=A-Z Ratio of neutrons to proton: N/Z Unstable: If Z > 83 Stable: If or 0 < Z < 20 & N/Z Ratio = 1. 0 20 < Z < 83 & Z is even 20

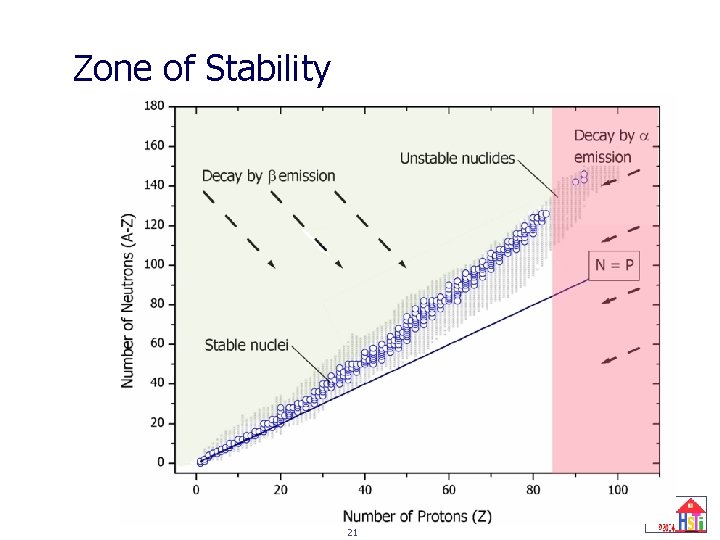
Zone of Stability 21

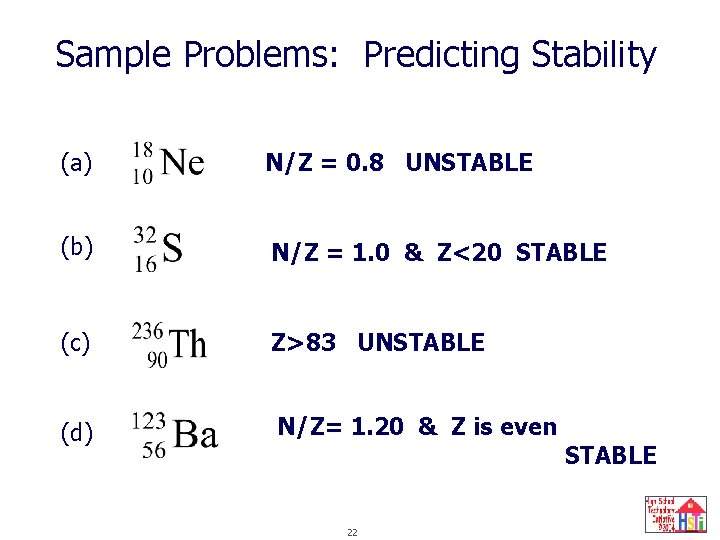
Sample Problems: Predicting Stability (a) N/Z = 0. 8 UNSTABLE (b) N/Z = 1. 0 & Z<20 STABLE (c) Z>83 UNSTABLE (d) N/Z= 1. 20 & Z is even 22 STABLE

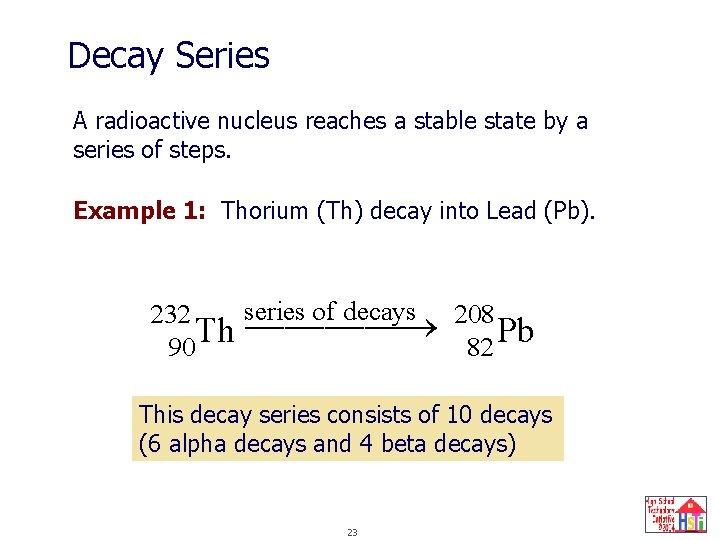
Decay Series A radioactive nucleus reaches a stable state by a series of steps. Example 1: Thorium (Th) decay into Lead (Pb). series of decays 208 232 ¾¾¾¾ ¾ Th Pb 90 82 This decay series consists of 10 decays (6 alpha decays and 4 beta decays) 23

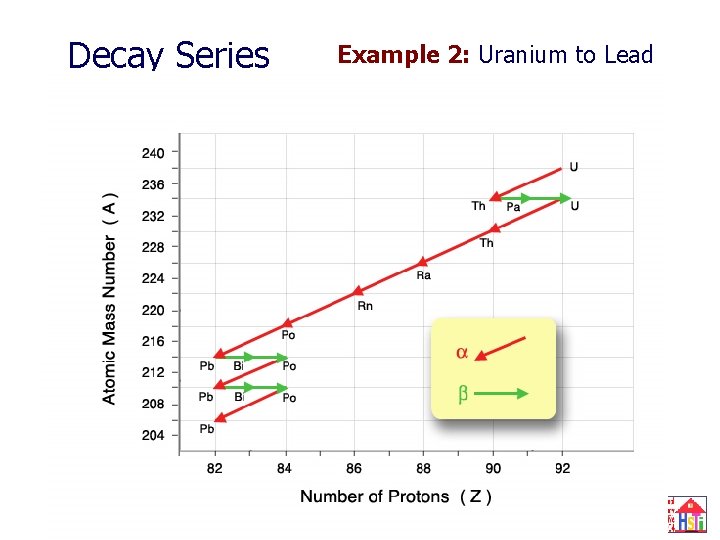
Decay Series Example 2: Uranium to Lead 24

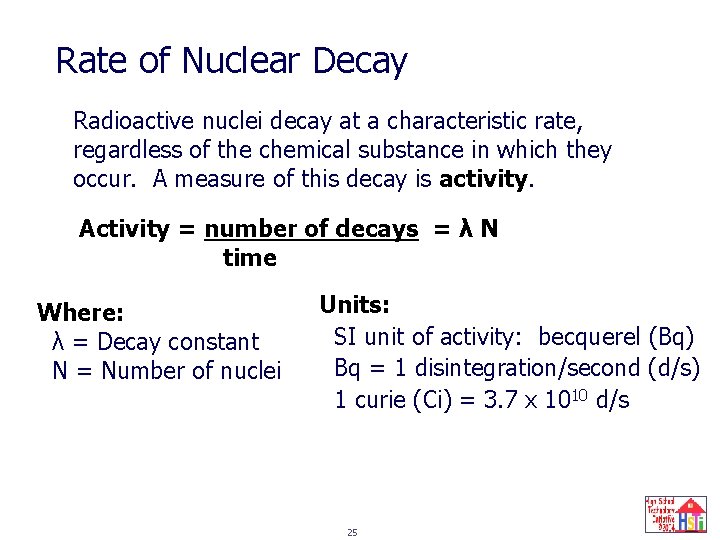
Rate of Nuclear Decay Radioactive nuclei decay at a characteristic rate, regardless of the chemical substance in which they occur. A measure of this decay is activity. Activity = number of decays = λ N time Where: λ = Decay constant N = Number of nuclei Units: SI unit of activity: becquerel (Bq) Bq = 1 disintegration/second (d/s) 1 curie (Ci) = 3. 7 x 1010 d/s 25

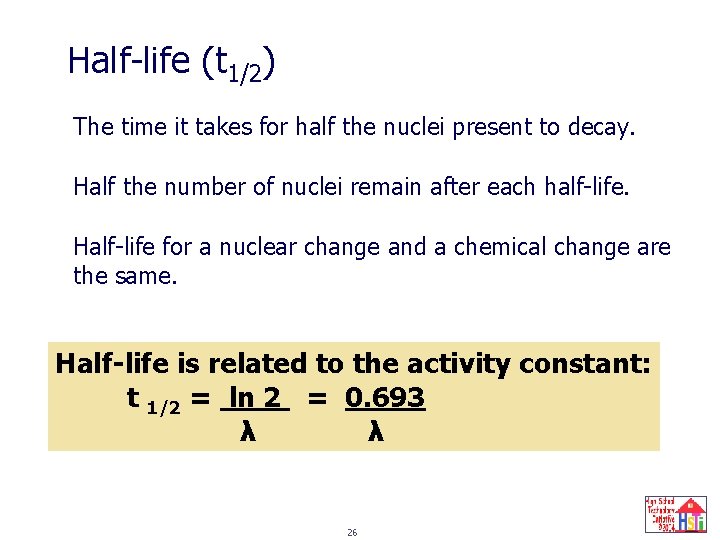
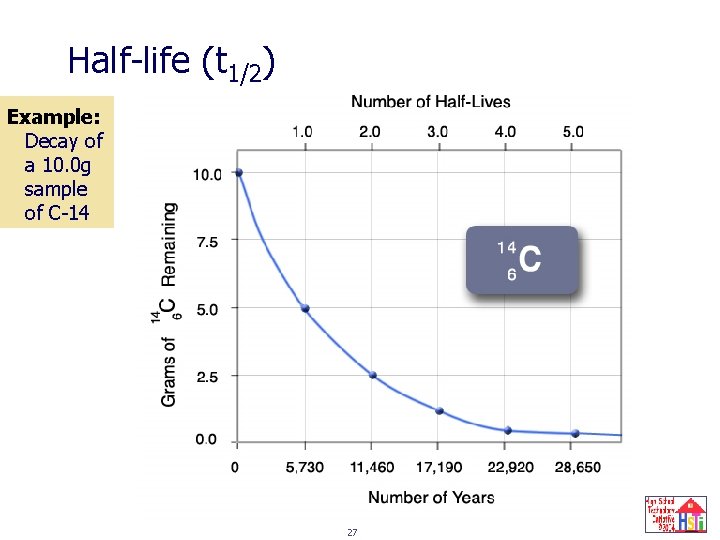
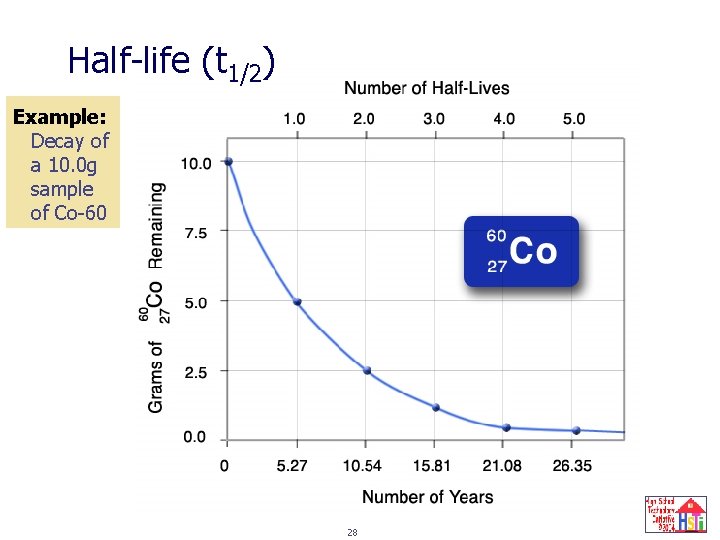
Half-life (t 1/2) The time it takes for half the nuclei present to decay. Half the number of nuclei remain after each half-life. Half-life for a nuclear change and a chemical change are the same. Half-life is related to the activity constant: t 1/2 = ln 2 = 0. 693 λ λ 26

Half-life (t 1/2) Example: Decay of a 10. 0 g sample of C-14 27

Half-life (t 1/2) Example: Decay of a 10. 0 g sample of Co-60 28

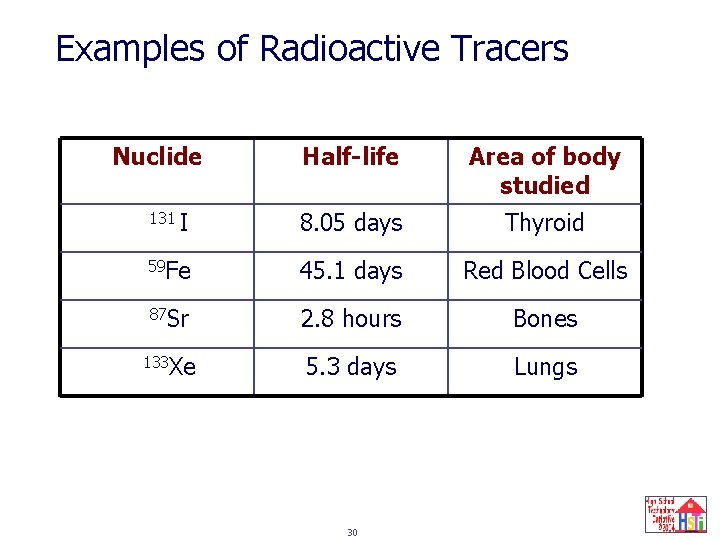
Medical Applications of Radioactive Nuclides as Radioactive Tracers Radiotracers: radioactive nuclides that are introduced into organisms via food or drugs; the pathway of the radiotracer can be “traced” by monitoring their radioactivity. Examples: • By incorporating 14 C and 32 P into foods, metabolic pathways can be studied. • The thyroid gland can be monitored by a scanner after patients drink a solution containing Na 131 I. • 201 Th can be used to assess damage to heart caused by a heart attack by determining the amount of Th present in heart muscle tissue because Th is concentrated in healthy muscle tissue. 29

Examples of Radioactive Tracers Nuclide Half-life Area of body studied I 8. 05 days Thyroid 59 Fe 45. 1 days Red Blood Cells 87 Sr 2. 8 hours Bones 133 Xe 5. 3 days Lungs 131 30

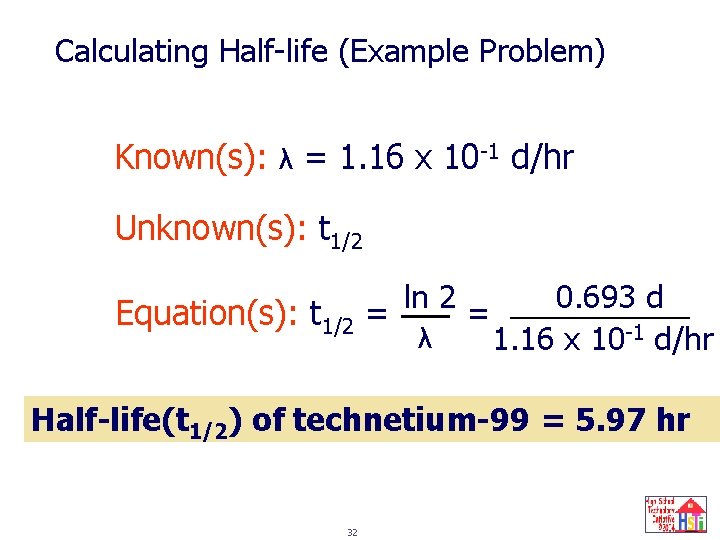
Calculating Half-life (Example Problem) Technetium-99 is used to form images of internal organs in the body and is often used to determine heart damage. This nuclide, 99 Tc decays to ground state by gamma emission. The rate constant for decay is 1. 16 x 10 -1 d/hr. What is the half-life of this nuclide? 31

Calculating Half-life (Example Problem) Known(s): λ = 1. 16 x 10 -1 d/hr Unknown(s): t 1/2 Equation(s): t 1/2 = ln 2 λ 0. 693 d = 1. 16 x 10 -1 d/hr Half-life(t 1/2) of technetium-99 = 5. 97 hr 32

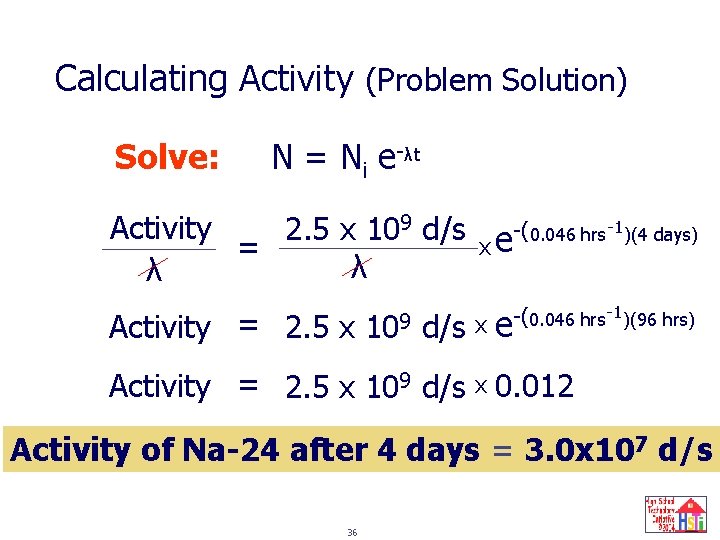
Calculating Activity (Example Problem) Sodium-24 has a half-life of 15 hours and is used to study blood circulation. If a patient is injected with a 24 Na. Cl solution whose activity is 2. 5 x 10 9 d/s, how much of the activity is present in the patient’s body and excreted fluids after 4. 0 days? 33

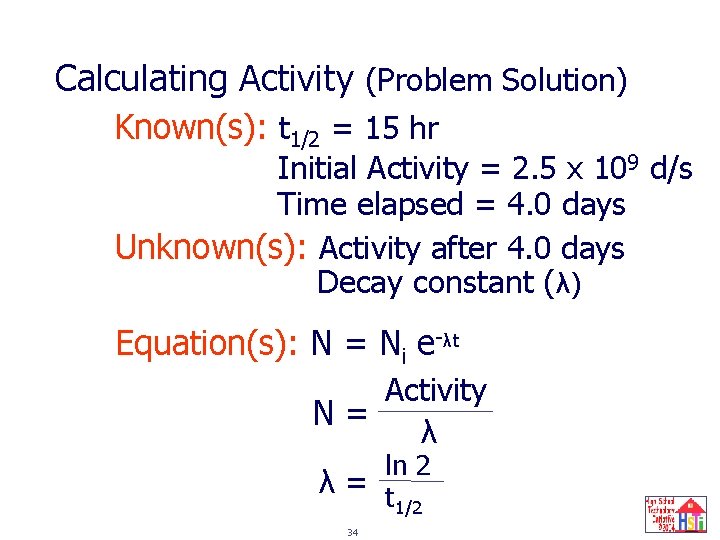
Calculating Activity (Problem Solution) Known(s): t 1/2 = 15 hr Initial Activity = 2. 5 x 109 d/s Time elapsed = 4. 0 days Unknown(s): Activity after 4. 0 days Decay constant (λ) Equation(s): N = Ni e-λt Activity N= λ ln 2 λ= t 1/2 34

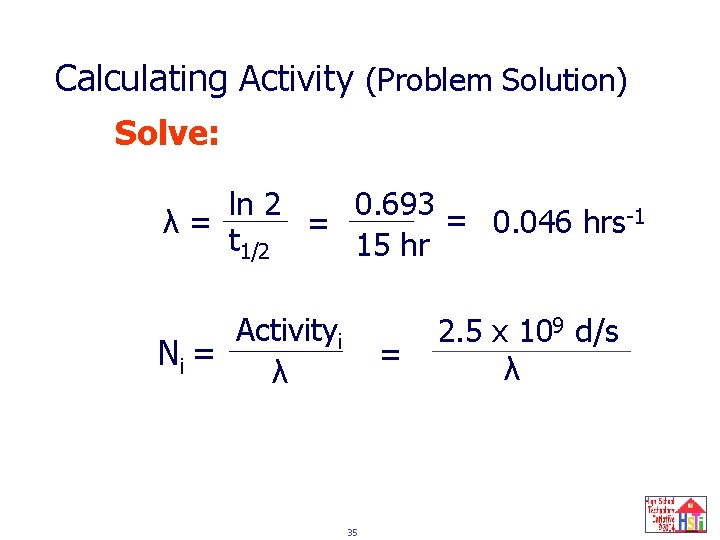
Calculating Activity (Problem Solution) Solve: 0. 693 = ln 2 0. 046 hrs-1 λ= = t 1/2 15 hr Activityi Ni = λ = 35 2. 5 x 109 d/s λ

Calculating Activity (Problem Solution) Solve: N = Ni e-λt Activity 2. 5 x 109 d/s x e-(0. 046 hrs-1)(4 days) = λ λ Activity = 2. 5 x 109 d/s x e -(0. 046 hrs-1)(96 hrs) Activity = 2. 5 x 109 d/s x 0. 012 Activity of Na-24 after 4 days = 3. 0 x 107 d/s 36
 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Who discovered radioactivity
Who discovered radioactivity Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Geiger counter
Geiger counter Nuclear transmutation equation
Nuclear transmutation equation Balancing nuclear reactions
Balancing nuclear reactions Balance nuclear equations
Balance nuclear equations Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Alpha emission equation
Alpha emission equation Two types of nuclear reactions
Two types of nuclear reactions Nuclear bombardment equation
Nuclear bombardment equation Nuclear reactions are at
Nuclear reactions are at Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Redox reaction example
Redox reaction example Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Natural and artificial radioactivity
Natural and artificial radioactivity Natural transmutation example
Natural transmutation example Natural and artificial radioactivity
Natural and artificial radioactivity Atoms and radioactivity
Atoms and radioactivity Who discovered radioactivity
Who discovered radioactivity Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Natural vs artificial radioactivity
Natural vs artificial radioactivity Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Radioactivity
Radioactivity Radioactivity
Radioactivity The great unconformity
The great unconformity Natural radioactivity
Natural radioactivity Environmental radioactivity
Environmental radioactivity Natural radioactivity
Natural radioactivity Radioactivity phenomenon
Radioactivity phenomenon Units of radioactivity
Units of radioactivity Isotope decay formula
Isotope decay formula Defination of radioactivity
Defination of radioactivity