Nuclear Reactions FISSION FUSION Introduction Nuclear reactions deal










- Slides: 14

Nuclear Reactions: FISSION & FUSION

Introduction ã Nuclear reactions deal with interactions between the nuclei of atoms ã Both fission and fusion processes deal with matter and energy

Matter and Energy ã Previous studies have taught us that “matter and energy cannot be created nor destroyed” ã We now need to understand that Matter and Energy are two forms of the same thing

E = mc 2 ã Remember that matter can be changed into Energy ã This tells us that a small amount of mass can be converted into a very large amount of energy because the speed of light (c) is an extremely large number Energy Mass Light Speed

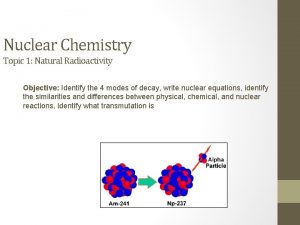
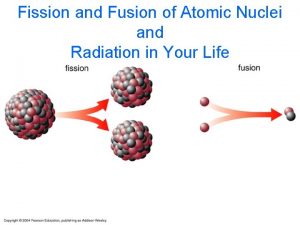
Fission ã Fission may be defined as the process of splitting an atomic nucleus into fission fragments ã The fission fragments are generally in the form of smaller atomic nuclei and neutrons ã Large amounts of energy are produced by the fission process

Fission ã Fissile nuclei are generally atoms with more neutrons than protons ã The nuclei of such heavy atoms are struck by neutrons initiating the fission process ã Fission occurs when the strong nuclear force is disrupted by an incoming projectile (in this case a neutron) ã When the strong nuclear force is disrupted electrostatic repulsion splits the nuclei

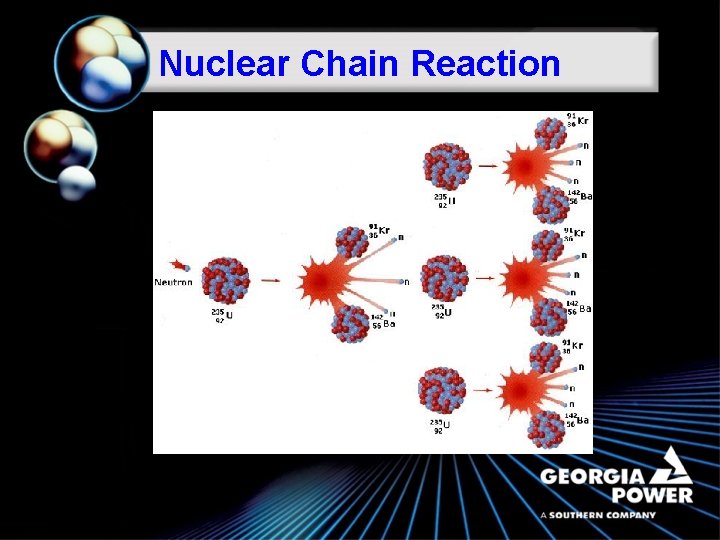
Fission ã A classic example of a fission reaction is that of U-235: U-235 + 1 Neutron 3 Neutrons + Kr-91 + Ba-142 + Energy ã In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission.

Nuclear Chain Reaction

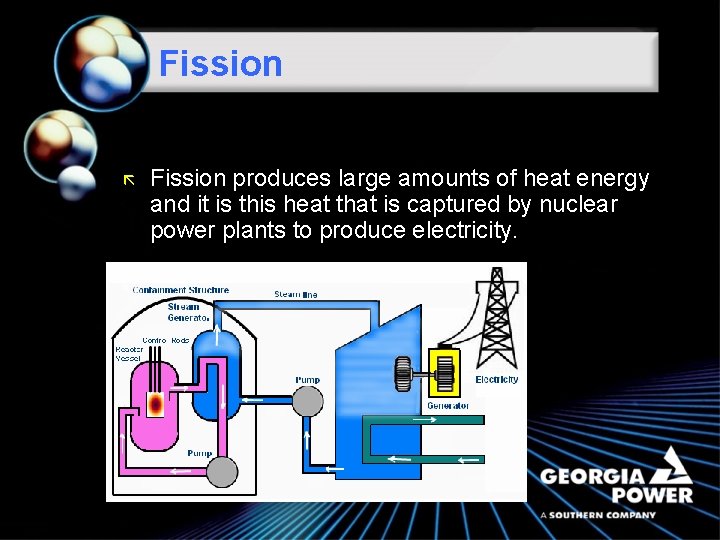
Fission ã Fission produces large amounts of heat energy and it is this heat that is captured by nuclear power plants to produce electricity.

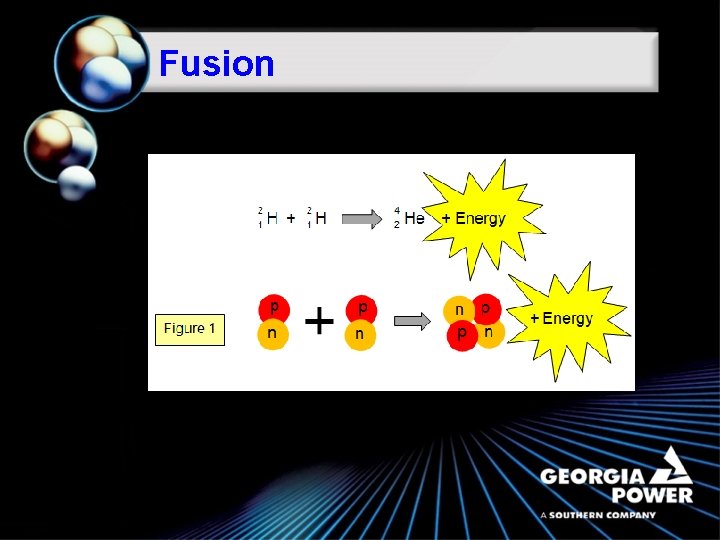
Fusion ã Fusion is a nuclear reaction whereby two light atomic nuclei fuse or combine to form a single larger nuclei which is lighter than the sum of the two that fuse. ã The lost mass is converted to energy. (E ( = mc 2 ) ã For fusion to occur, a large amount of energy is needed to overcome the electrical charges of the nuclei and fuse them together

Fusion

Fusion ã Fusion reactions do not occur naturally on our planet but are the principal type of reaction found in stars ã The large masses, densities, and high temperatures of stars provide the initial energies needed to fuel fusion reactions ã The sun fuses hydrogen atoms to produce helium, subatomic particles, and vast amounts of energy

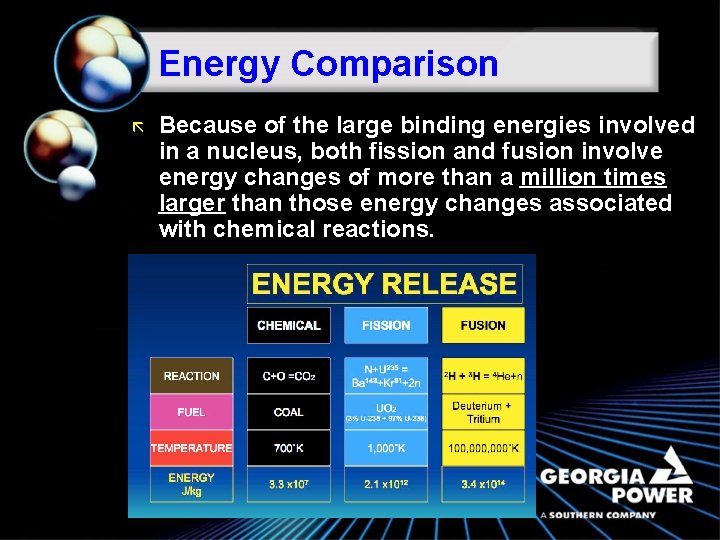
Energy Comparison ã Because of the large binding energies involved in a nucleus, both fission and fusion involve energy changes of more than a million times larger than those energy changes associated with chemical reactions.

Review ã Mass and Energy are two forms of the same thing; neither can be created nor destroyed but mass can be converted into energy (E = mc 2) ã Fission is a nuclear reaction in which a heavy atomic nucleus is split into lighter atomic nuclei ã Fusion is a nuclear reaction in which 2 light atomic nuclei are combined into a single, heavier atomic nucleus
 Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Nuclear fission vs fusion
Nuclear fission vs fusion Who discovered uranium
Who discovered uranium Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Is the sun fusion or fission
Is the sun fusion or fission Fission vs fusion nuclear
Fission vs fusion nuclear Nuclear fission and fusion webquest answer key
Nuclear fission and fusion webquest answer key Fission and fusion venn diagram
Fission and fusion venn diagram Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fission vs fusion
Fission vs fusion Fission vs fusion
Fission vs fusion Fission vs fusion
Fission vs fusion Fission and fusion similarities
Fission and fusion similarities Fission or fusion
Fission or fusion Fusion or fission
Fusion or fission