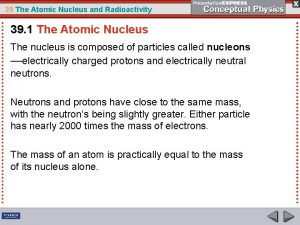
Introduction 1 Radioactivity Next Slide Discovery of radioactivity















- Slides: 29

Introduction 1 Radioactivity Next Slide Discovery of radioactivity • Discovery of radioactivity (1896) : Henri Becquerel Exposure of film by X-ray Photo • Discovery of radioactive material (1898) : Marie Curie and her husband Extract radium from uranium ore Photo

Introduction 2 Radioactivity Next Slide Different kinds of radiation • Three different kinds of radiation : alpha particle ( ), beta particle ( ), gamma ray ( ) • Common sources in school laboratory : i. americium source for alpha radiation ii. strontium source for beta radiation iii. cobalt source for gamma radiation

Nature of radiation Radioactivity Next Slide Nature of different kinds of radiation • Nature of different kinds of radiation Diagram • Emission of radiation from a nucleus Diagram • Penetrating power and range in air Diagram • Ionizing effect Diagram • Effects of electric field and magnetic field on the paths of radiation Diagram

Detection of radiation Radioactivity Next Slide Different methods of detecting radiation • Photographic method : exposure of film • Scintillation counters Diagram • Spark Counter Diagram • Geiger-Muller (GM) tube Diagram • Cloud chamber Diagram

Radiation hazards Radioactivity Next Slide Radiation Hazards • Safety precautions Explanation • Background radiation Explanation

END of Radioactivity

Back to Introduction 1 Radioactivity Click Back to • Henri Becquerel

Back to Introduction 1 Radioactivity Click Back to • Maria Curie

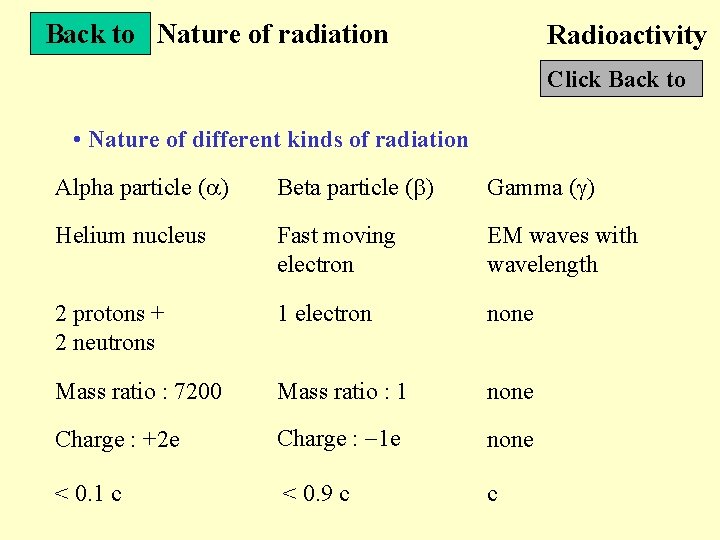
Back to Nature of radiation Radioactivity Click Back to • Nature of different kinds of radiation Alpha particle ( ) Beta particle ( ) Gamma ( ) Helium nucleus Fast moving electron EM waves with wavelength 2 protons + 2 neutrons 1 electron none Mass ratio : 7200 Mass ratio : 1 none Charge : +2 e Charge : 1 e none < 0. 1 c < 0. 9 c c

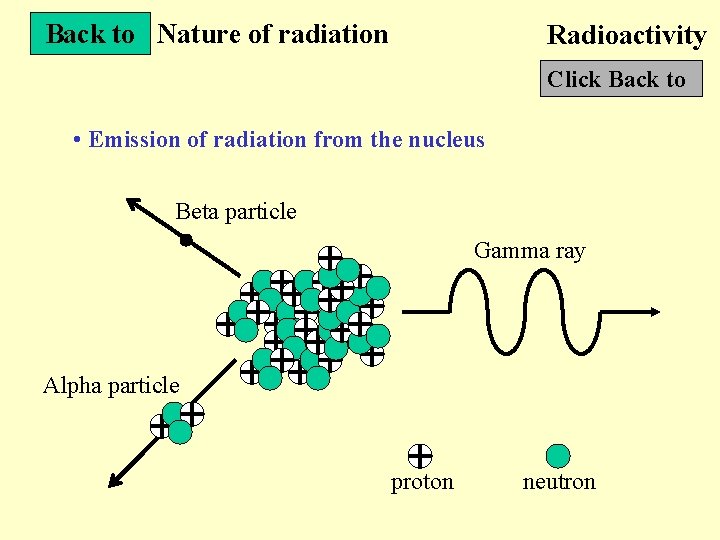
Back to Nature of radiation Radioactivity Click Back to • Emission of radiation from the nucleus Beta particle Gamma ray Alpha particle proton neutron

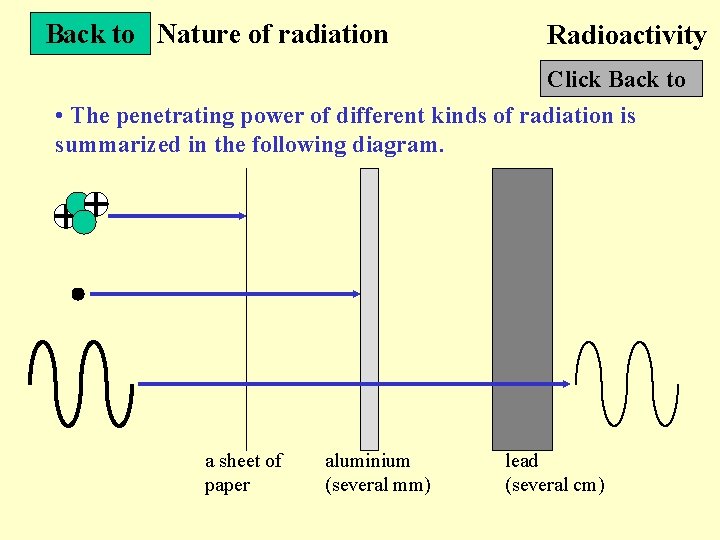
Nature of radiation Radioactivity Next Slide • Alpha particle : Very weak penetrating power (stopped by a piece of paper) Range in air : 5 cm • Beta particle : Weak penetrating power (stopped by aluminum foil of thickness 5 mm) Range in air : 5 m • Gamma ray : Strong penetrating power (cannot be completely absorbed by several cm of lead) Range in air : 100 m

Back to Nature of radiation Radioactivity Click Back to • The penetrating power of different kinds of radiation is summarized in the following diagram. a sheet of paper aluminium (several mm) lead (several cm)

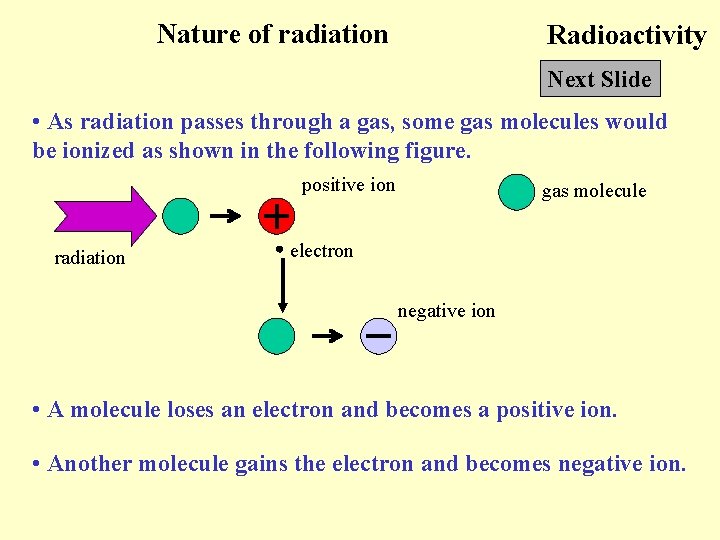
Nature of radiation Radioactivity Next Slide • As radiation passes through a gas, some gas molecules would be ionized as shown in the following figure. positive ion radiation gas molecule electron negative ion • A molecule loses an electron and becomes a positive ion. • Another molecule gains the electron and becomes negative ion.

Back to Nature of radiation Radioactivity Click Back to • Alpha particle : Strong ionizing effect (large amount of ions are produced as it passes through the air) • Beta particle : Weak ionizing effect (small amount of ions are produced as it passes through the air) • Gamma ray : Very weak ionizing effect (very small amount of ions are produced as it passes through the air)

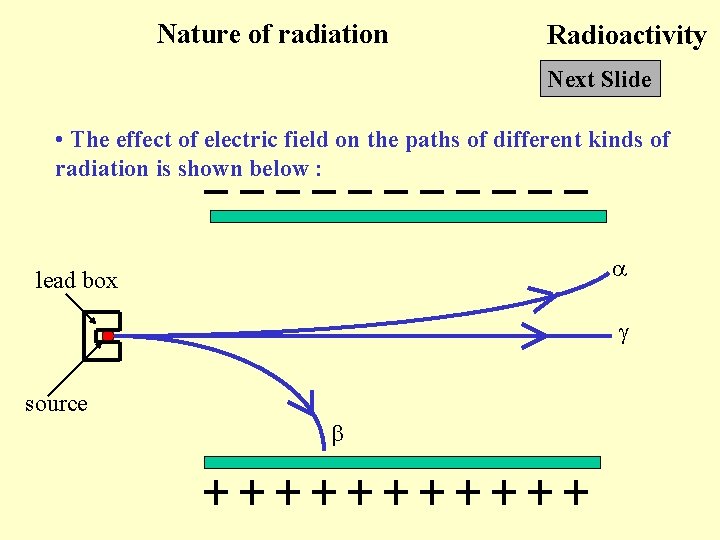
Nature of radiation Radioactivity Next Slide • The effect of electric field on the paths of different kinds of radiation is shown below : lead box source

Nature of radiation Radioactivity Next Slide • Gamma ray : No effect since gamma ray poses no charge • Alpha particle : Deflected to the negative charged plated since an alpha particle poses positive charge • Beta particle : Deflected to the positive charged plated since a beta particle poses negative charge • The experiment must be done in vacuum since the range of alpha particles in air is small. • Since an alpha particle has a larger mass than a beta particle, its deflection is smaller than that of the beta particle.

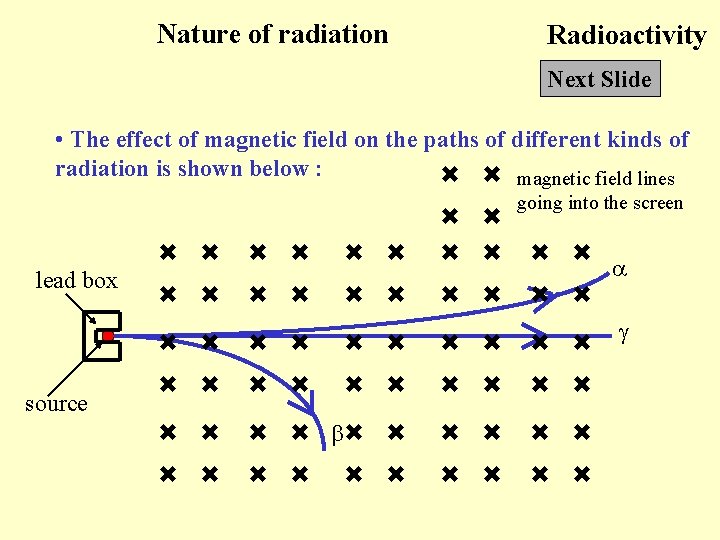
Nature of radiation Radioactivity Next Slide • The effect of magnetic field on the paths of different kinds of radiation is shown below : magnetic field lines going into the screen lead box source

Back to Nature of radiation Radioactivity Click Back to • Gamma ray : No effect since gamma ray poses no charge • Alpha particle and beta particle : Treat the radiation as flows of +ve charge (current) and -ve charge respectively. We can find the directions of the force acting on the particles and hence the directions of deflection. • The experiment must be done in vacuum since the range of alpha particles in air is small. • Since an alpha particle has a larger mass than a beta particle, its deflection is smaller than that of the beta particle.

Back to Detection of radiation Radioactivity Click Back to • A scintillation counter is shown below :

Back to Detection of radiation • A spark counter is shown below : Radioactivity Click Back to

Detection of radiation Radioactivity Next Slide • A Geiger-Muller (GM) tube is shown below :

Detection of radiation Radioactivity Next Slide • A Geiger-Muller (GM) tube with scalar is shown below :

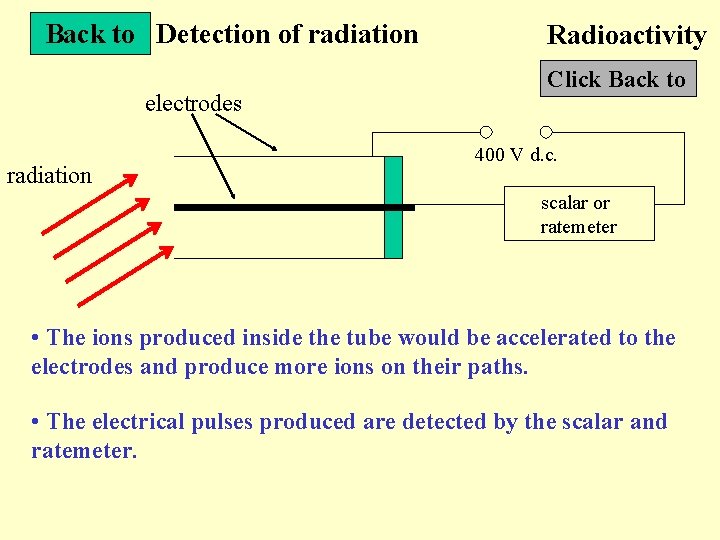
Back to Detection of radiation electrodes radiation Radioactivity Click Back to 400 V d. c. scalar or ratemeter • The ions produced inside the tube would be accelerated to the electrodes and produce more ions on their paths. • The electrical pulses produced are detected by the scalar and ratemeter.

Detection of radiation Radioactivity Next Slide • Cloud chamber: • Cloud chamber with tracks By courtesy of Prof. S. Y. Mak

Detection of radiation Radioactivity Next Slide • Structure of a cloud chamber is shown below :

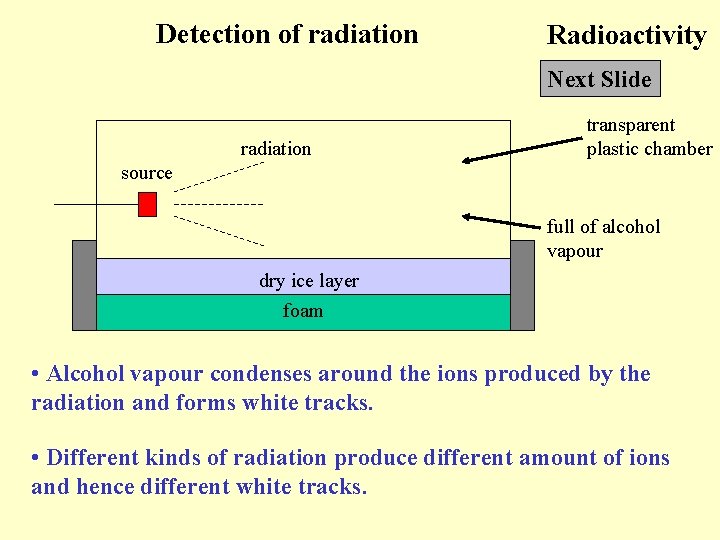
Detection of radiation Radioactivity Next Slide radiation transparent plastic chamber source full of alcohol vapour dry ice layer foam • Alcohol vapour condenses around the ions produced by the radiation and forms white tracks. • Different kinds of radiation produce different amount of ions and hence different white tracks.

Back to Detection of radiation Radioactivity Click Back to • Alpha particles : thick, short and clear tracks • Beta particles : thin and twisted tracks • Gamma ray : very unclear and diffuse tracks

Back to Radiation hazards Radioactivity Click Back to • Lead container should be used to store and transport all radioactive sources. • Do not use bare hand to touch or handle the sources. Forceps should be used instead. • The sources should be at least one arm’s length away from your body and other people’s bodies. Never point the sources towards the eyes.

Back to Radiation hazards Radioactivity Click Back to • Sources of background radiation (natural radiation) i. cosmic rays ii. natural radioactive materials on earth iii. medical diagnosis (X-ray) iv. industrial and scientific nuclear sources and wastes v. radon and thoron (radioactive gases) from rocks and building materials (concrete)
 X.next = x.next.next
X.next = x.next.next Becquerel discovery of radioactivity
Becquerel discovery of radioactivity Heel and toe polka
Heel and toe polka Continued on next slide
Continued on next slide Natural and artificial radioactivity
Natural and artificial radioactivity Artificial transmutation worksheet
Artificial transmutation worksheet Who discovered radioactivity
Who discovered radioactivity Who discovered uranium
Who discovered uranium Natural and artificial radioactivity
Natural and artificial radioactivity Law of radioactive decay
Law of radioactive decay Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Mta ek
Mta ek Radioactivity
Radioactivity Unconformity
Unconformity Atoms and radioactivity
Atoms and radioactivity Natural radioactivity
Natural radioactivity Environmental radioactivity
Environmental radioactivity Natural radioactivity
Natural radioactivity Radioactivity phenomenon
Radioactivity phenomenon Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Units of radioactivity
Units of radioactivity Radioactive decay equation
Radioactive decay equation Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Alpha particle in electric field
Alpha particle in electric field Introduction to data mining and knowledge discovery
Introduction to data mining and knowledge discovery Factoring polynomials
Factoring polynomials What comes after the introduction
What comes after the introduction Growyourtraction
Growyourtraction