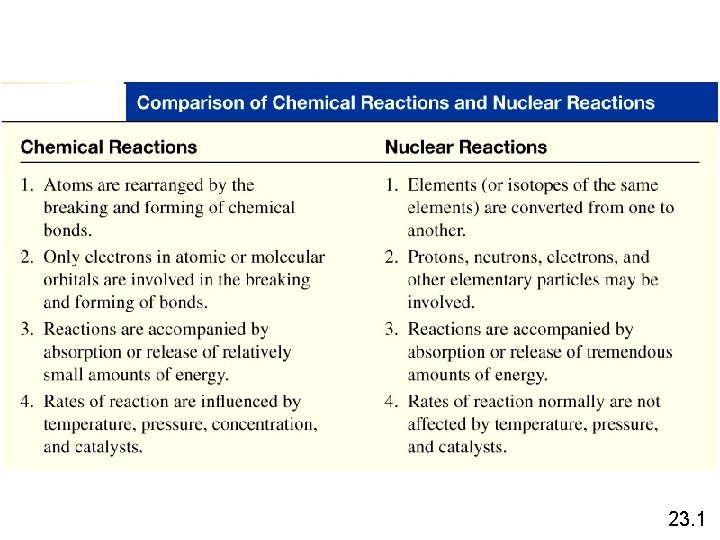
Nuclear Chemistry Nuclear Reactions Nuclear reactions involve the




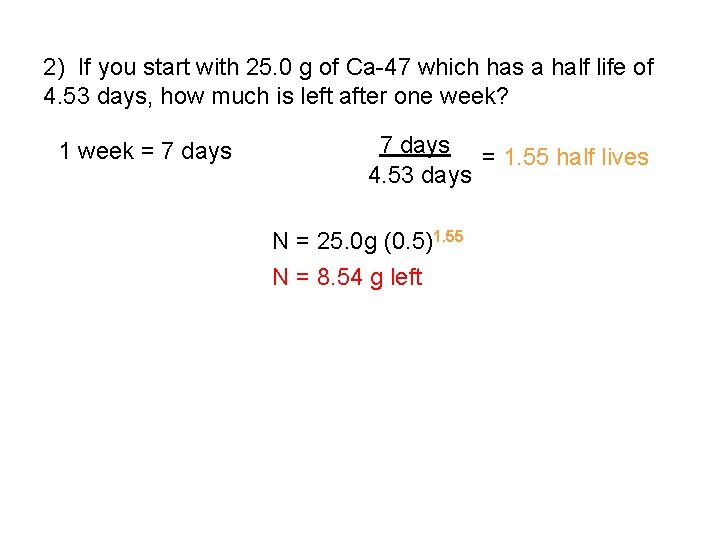
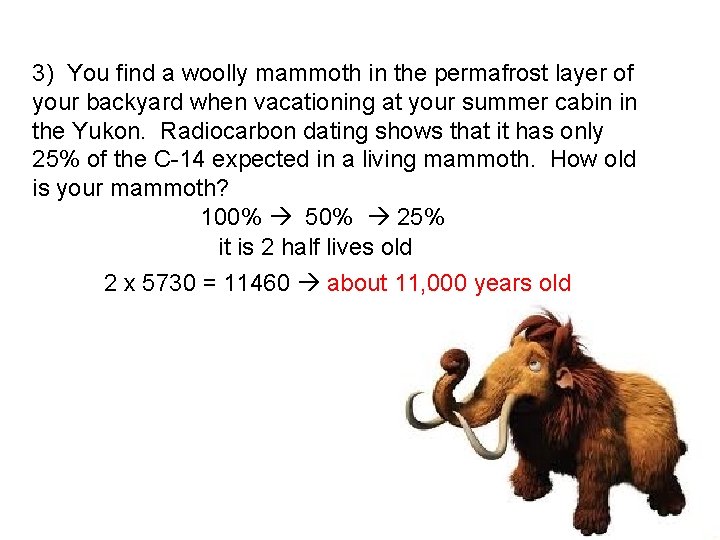













- Slides: 46

Nuclear Chemistry

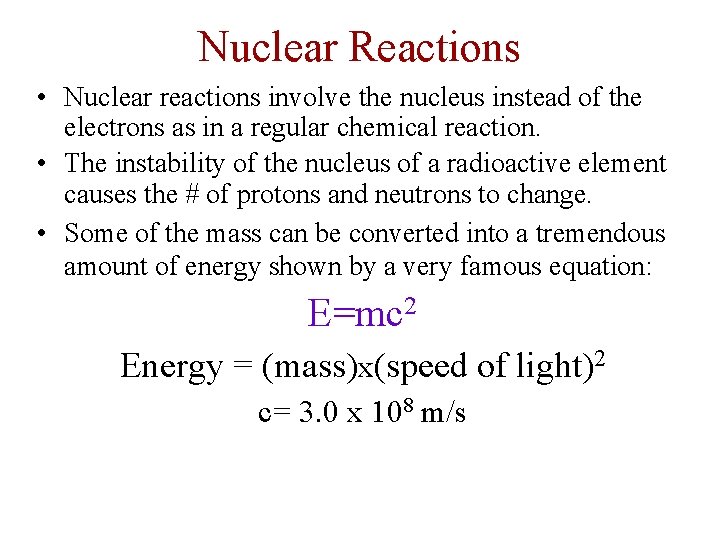
Nuclear Reactions • Nuclear reactions involve the nucleus instead of the electrons as in a regular chemical reaction. • The instability of the nucleus of a radioactive element causes the # of protons and neutrons to change. • Some of the mass can be converted into a tremendous amount of energy shown by a very famous equation: E=mc 2 Energy = (mass)x(speed of light)2 c= 3. 0 x 108 m/s

23. 1

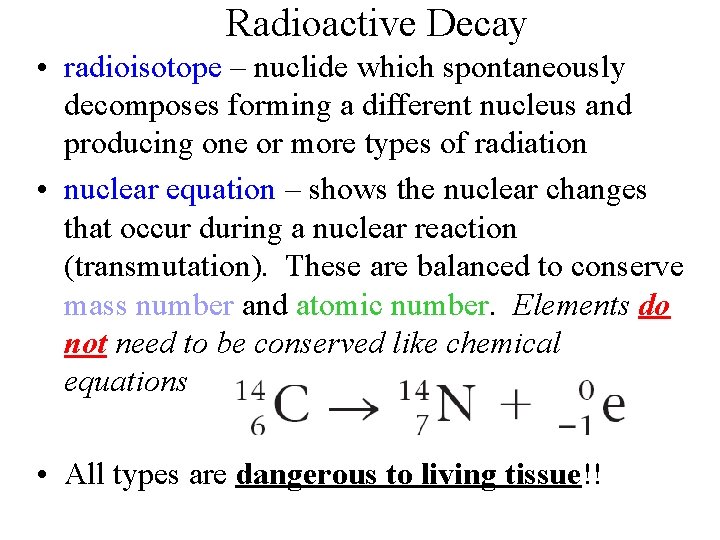
Radioactive Decay • radioisotope – nuclide which spontaneously decomposes forming a different nucleus and producing one or more types of radiation • nuclear equation – shows the nuclear changes that occur during a nuclear reaction (transmutation). These are balanced to conserve mass number and atomic number. Elements do not need to be conserved like chemical equations • All types are dangerous to living tissue!!


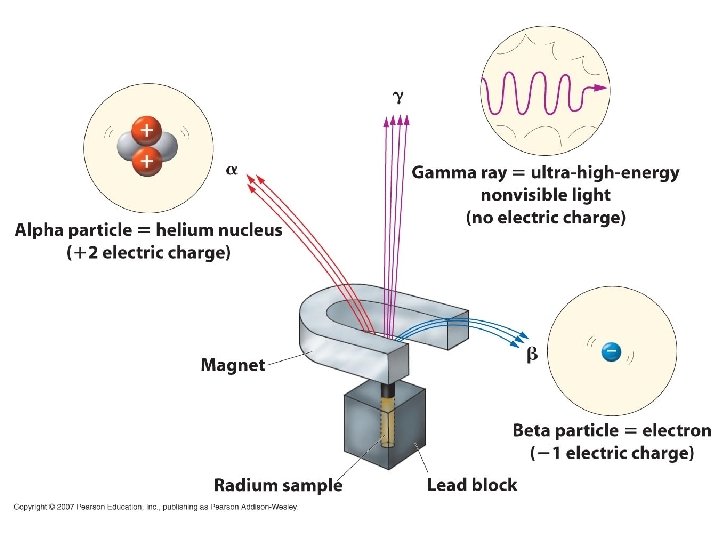
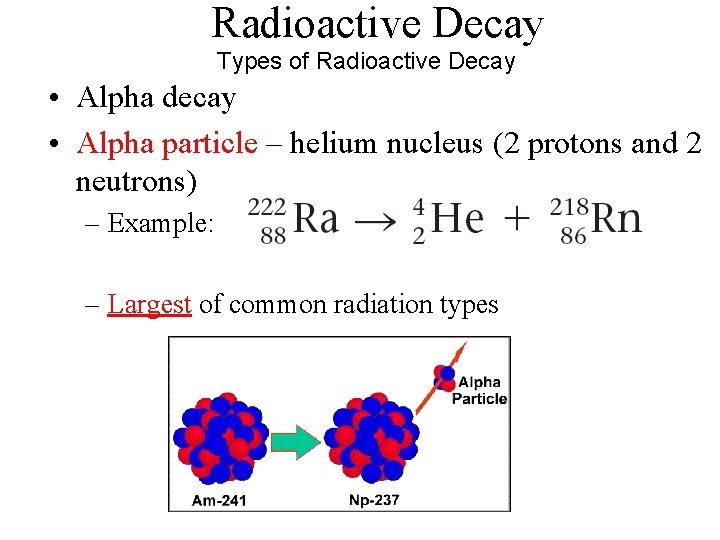
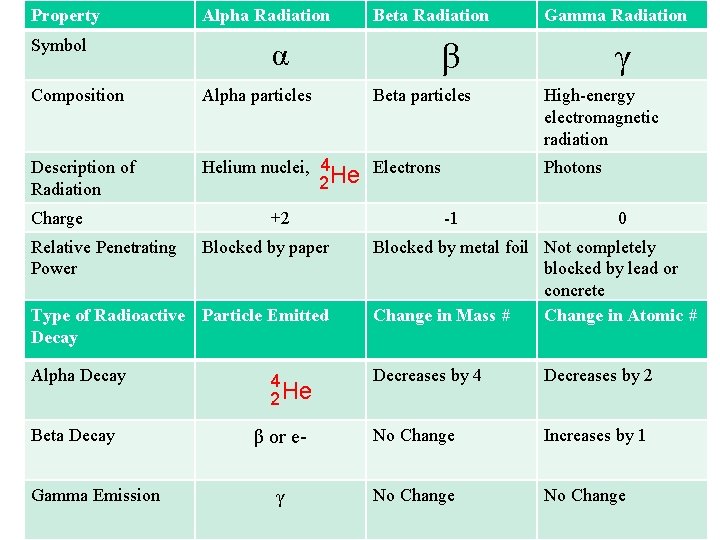
Radioactive Decay Types of Radioactive Decay • Alpha decay • Alpha particle – helium nucleus (2 protons and 2 neutrons) – Example: – Largest of common radiation types

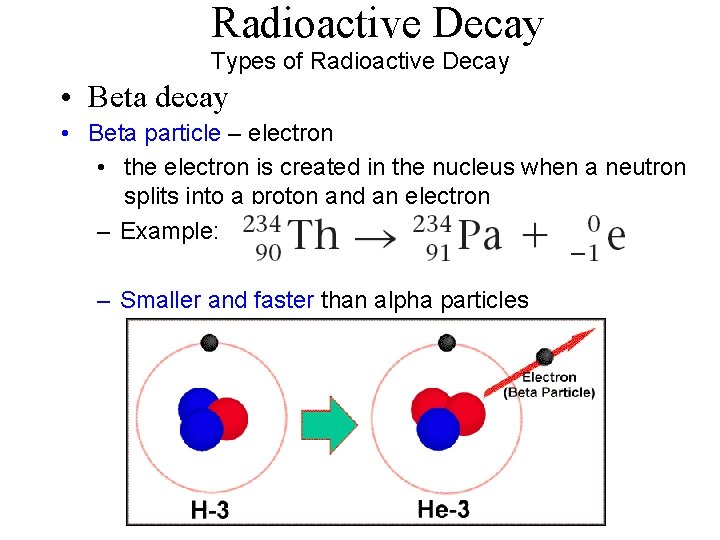
Radioactive Decay Types of Radioactive Decay • Beta decay • Beta particle – electron • the electron is created in the nucleus when a neutron splits into a proton and an electron – Example: – Smaller and faster than alpha particles

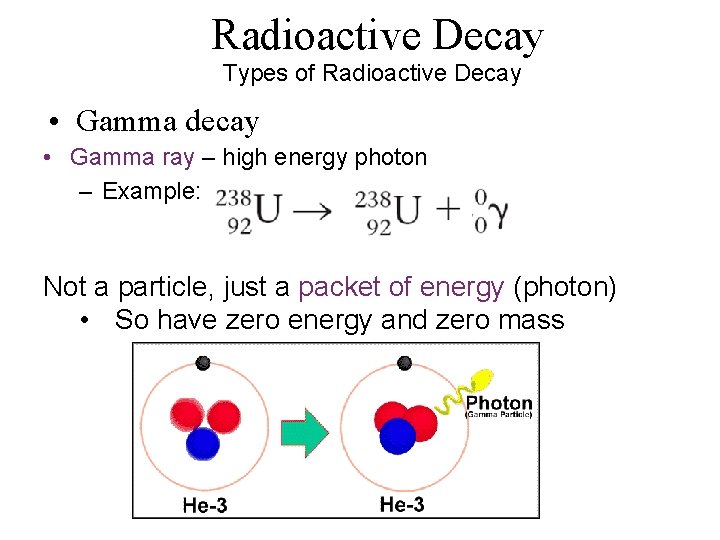
Radioactive Decay Types of Radioactive Decay • Gamma decay • Gamma ray – high energy photon – Example: Not a particle, just a packet of energy (photon) • So have zero energy and zero mass

Property Symbol Alpha Radiation Beta Radiation α β Composition Alpha particles Description of Radiation Helium nuclei, 4 Electrons He 2 Charge Relative Penetrating Power +2 Blocked by paper Type of Radioactive Particle Emitted Decay Alpha Decay Beta Decay Gamma Emission Gamma Radiation Beta particles γ High-energy electromagnetic radiation Photons -1 0 Blocked by metal foil Not completely blocked by lead or concrete Change in Mass # Change in Atomic # Decreases by 4 Decreases by 2 β or e- No Change Increases by 1 γ No Change 4 2 He

Examples • An alpha decay for Iodine-136 • A beta decay for Be-8

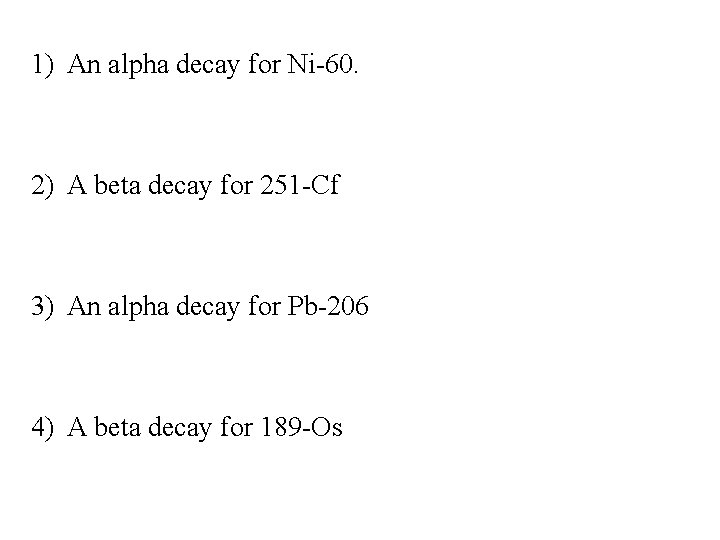
1) An alpha decay for Ni-60. 2) A beta decay for 251 -Cf 3) An alpha decay for Pb-206 4) A beta decay for 189 -Os

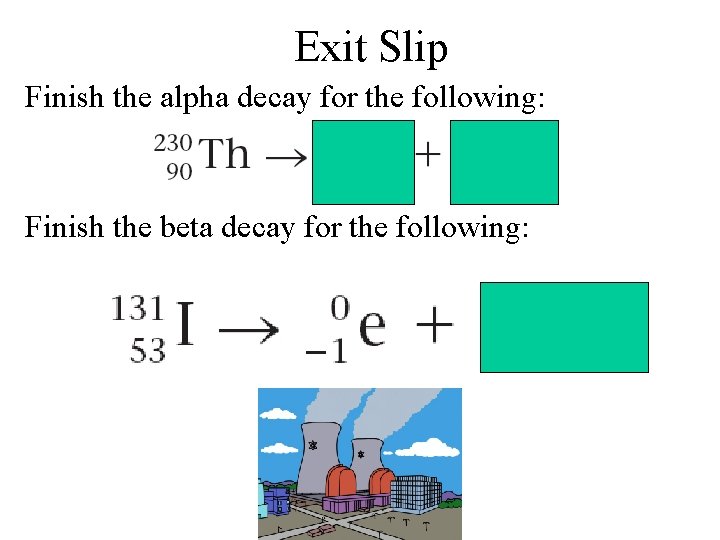
Exit Slip Finish the alpha decay for the following: Finish the beta decay for the following:

Chapter 24. 2 Nuclear Applications

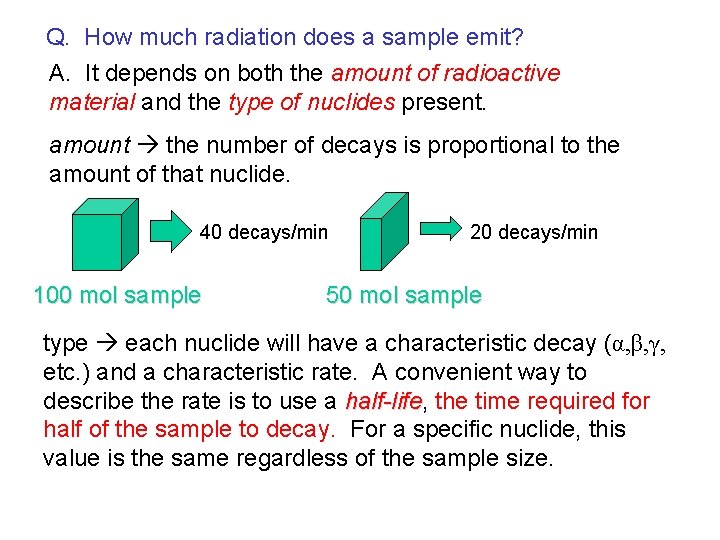
Q. How much radiation does a sample emit? A. It depends on both the amount of radioactive material and the type of nuclides present. amount the number of decays is proportional to the amount of that nuclide. 40 decays/min 100 mol sample 20 decays/min 50 mol sample type each nuclide will have a characteristic decay (α, β, γ, etc. ) and a characteristic rate. A convenient way to describe the rate is to use a half-life, half-life the time required for half of the sample to decay. For a specific nuclide, this value is the same regardless of the sample size.

Half Life Examples Br-84 has a half-life of 32 min. If you start with 12 moles of 84 Br, after 32 minutes you will only have 6. 0 moles left. After another 32 minutes you will have 3. 0 moles left. Every 32 minutes half of the 84 Br nuclides will decay. How much will be left after another 32 minutes? 1. 5 moles

Half-Life Problems When you are setting-up a half life problem, ask yourself which of these pieces of information you know: 1. 2. 3. 4. The starting amount of the nuclide. The amount of nuclide that remains. The amount of time that has passed. The half-life of the nuclide.

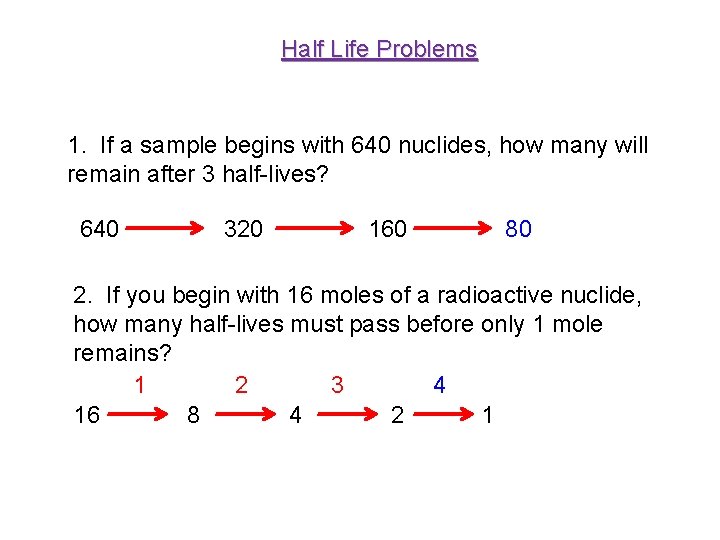
Half Life Problems 1. If a sample begins with 640 nuclides, how many will remain after 3 half-lives? 640 320 160 80 2. If you begin with 16 moles of a radioactive nuclide, how many half-lives must pass before only 1 mole remains? 1 2 3 4 16 8 4 2 1

Half Life Problems 3. A radioisotope has a half-life of 15 minutes. How long will it take for 97% of the sample to decay? 97% decayed means 3% is left 100% 50% 25% 12. 5% 6. 25% 5 half lives x 15 min = 75 min 3. 1%

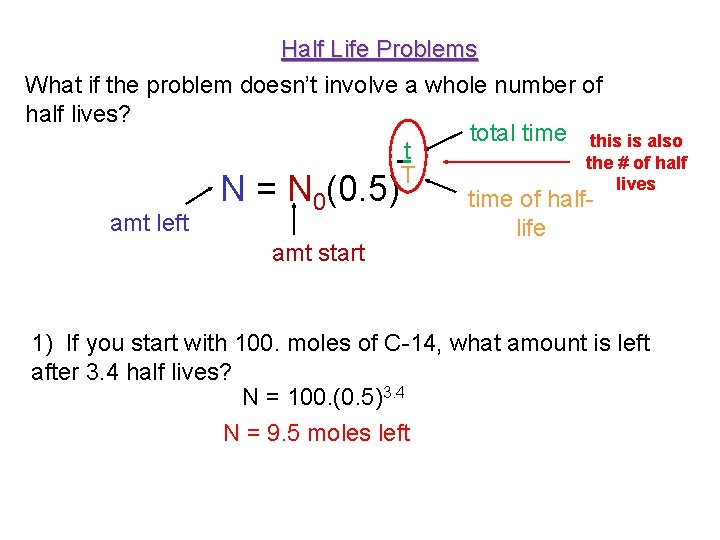
Half Life Problems What if the problem doesn’t involve a whole number of half lives? total time this is also t the # of half T lives N = N 0(0. 5) time of halfamt left life amt start 1) If you start with 100. moles of C-14, what amount is left after 3. 4 half lives? N = 100. (0. 5)3. 4 N = 9. 5 moles left
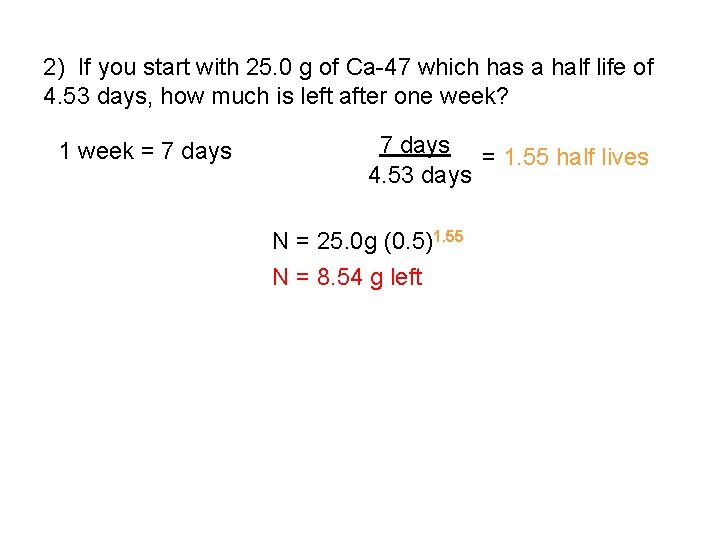
2) If you start with 25. 0 g of Ca-47 which has a half life of 4. 53 days, how much is left after one week? 1 week = 7 days = 1. 55 half lives 4. 53 days N = 25. 0 g (0. 5)1. 55 N = 8. 54 g left

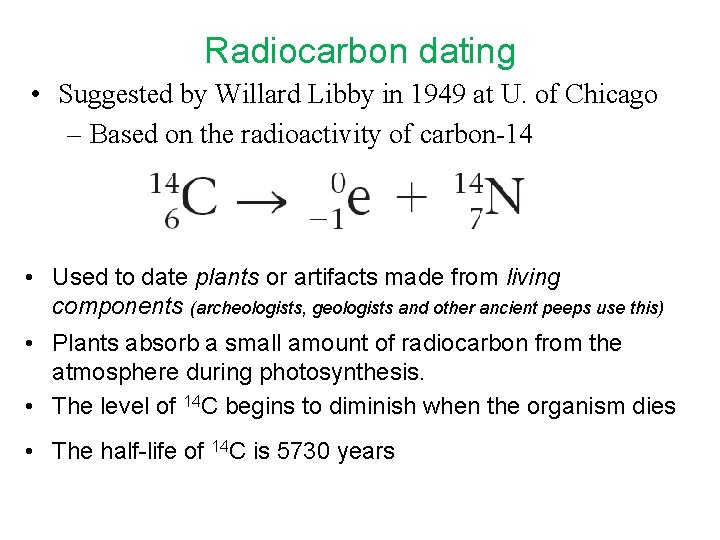
Radiocarbon dating • Suggested by Willard Libby in 1949 at U. of Chicago – Based on the radioactivity of carbon-14 • Used to date plants or artifacts made from living components (archeologists, geologists and other ancient peeps use this) • Plants absorb a small amount of radiocarbon from the atmosphere during photosynthesis. • The level of 14 C begins to diminish when the organism dies • The half-life of 14 C is 5730 years

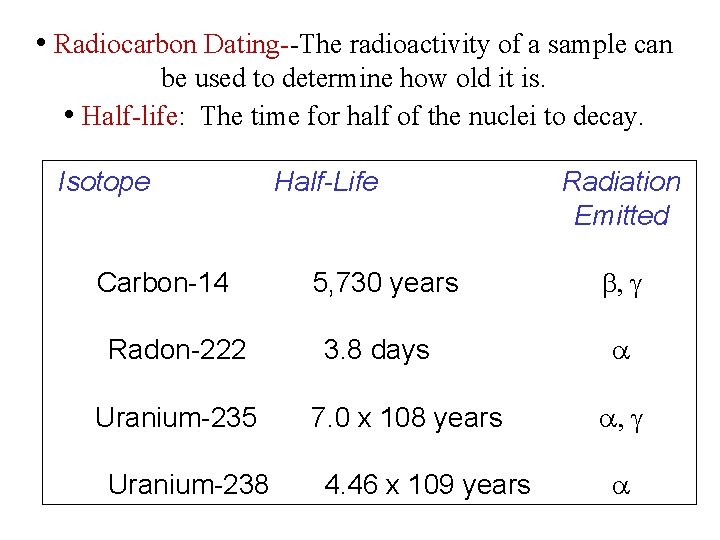
• Radiocarbon Dating--The radioactivity of a sample can be used to determine how old it is. • Half-life: The time for half of the nuclei to decay. Isotope Carbon-14 Radon-222 Uranium-235 Uranium-238 Half-Life 5, 730 years 3. 8 days 7. 0 x 108 years 4. 46 x 109 years Radiation Emitted b, g a a, g a
3) You find a woolly mammoth in the permafrost layer of your backyard when vacationing at your summer cabin in the Yukon. Radiocarbon dating shows that it has only 25% of the C-14 expected in a living mammoth. How old is your mammoth? 100% 50% 25% it is 2 half lives old 2 x 5730 = 11460 about 11, 000 years old

Exit Slip • If a sample begins with 32 nuclides, how many will remain after 4 half-lives? • If you start with 60. moles of an unknown substance, what amount is left if 2. 3 halflives have passed?

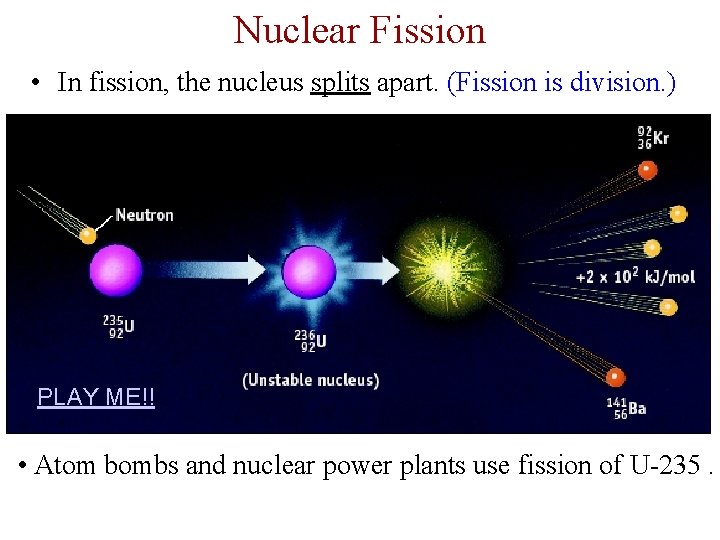
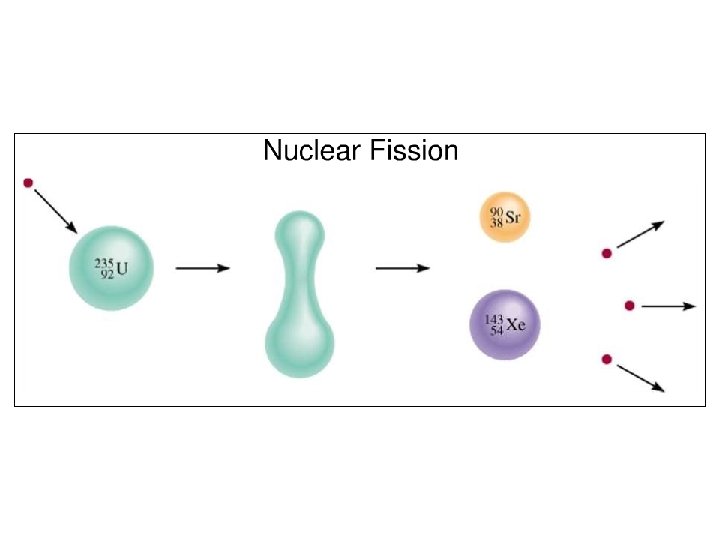
Nuclear Fission • In fission, the nucleus splits apart. (Fission is division. ) PLAY ME!! • Atom bombs and nuclear power plants use fission of U-235.


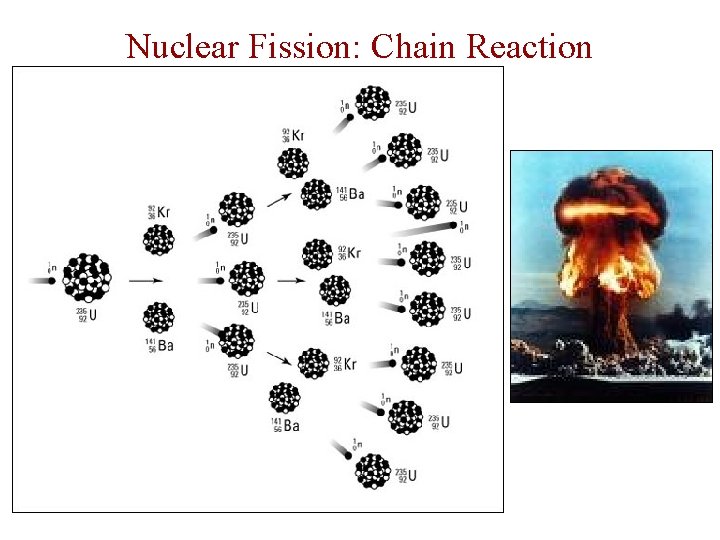
Nuclear Fission: Chain Reaction

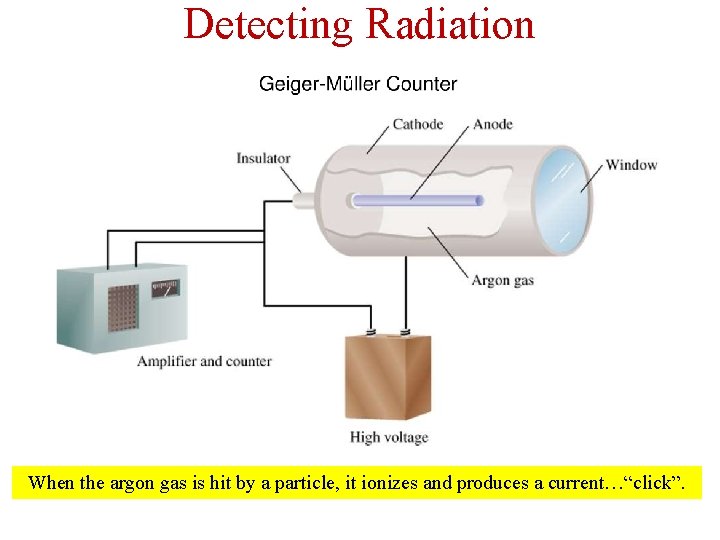
Detecting Radiation When the argon gas is hit by a particle, it ionizes and produces a current…“click”.

Nuclear Power • Currently about 103 nuclear power plants in the U. S. and about 435 worldwide. • 17% of the world’s energy comes from nuclear. • There are 6 nuclear power plants in Illinois. The closest one to us is in Clinton.

Clinton, IL Nuclear Power Plant

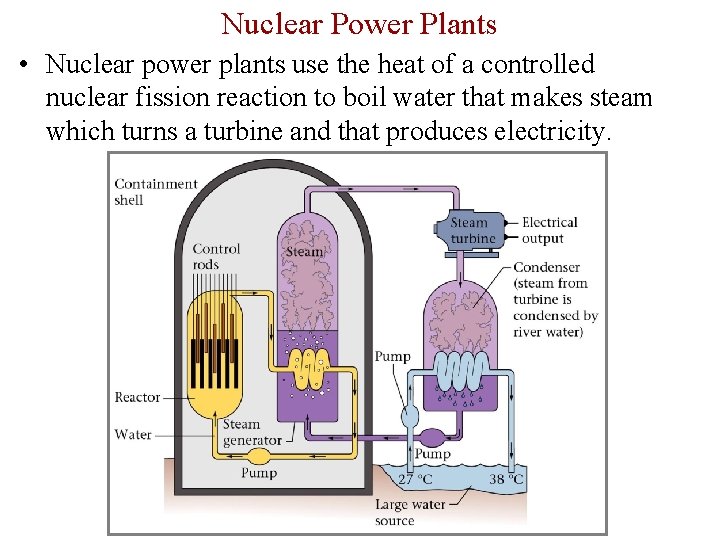
Nuclear Power Plants • Nuclear power plants use the heat of a controlled nuclear fission reaction to boil water that makes steam which turns a turbine and that produces electricity.

Nuclear Power Plant “Disasters” • One possible type of reactor disaster is known as a meltdown. In such an accident, the fission reaction goes out of control, leading to the emission of great amounts of radiation.

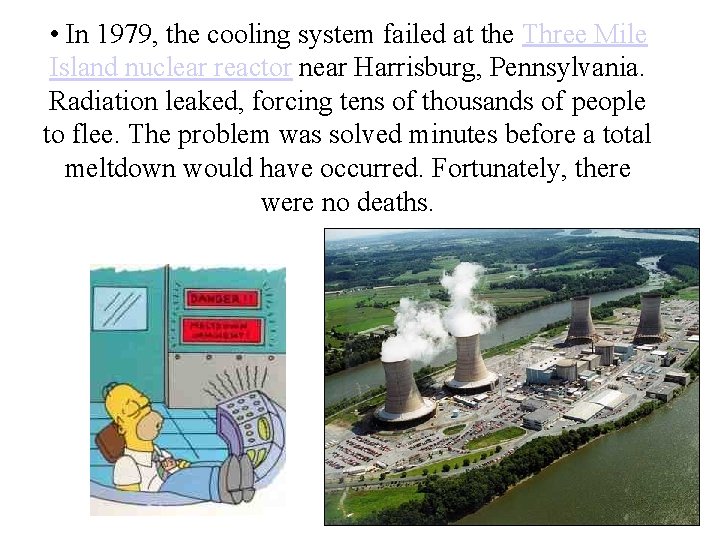
• In 1979, the cooling system failed at the Three Mile Island nuclear reactor near Harrisburg, Pennsylvania. Radiation leaked, forcing tens of thousands of people to flee. The problem was solved minutes before a total meltdown would have occurred. Fortunately, there were no deaths.

• In 1986, a much worse disaster struck Russia's Chernobyl nuclear power plant. In this incident, a large amount of radiation escaped from the reactor. Hundreds of thousands of people were exposed to the radiation. Several dozen died within a few days. In the years to come, thousands more may die of cancers induced by the radiation.

Chernobyl Sarcophagus

Japan 2011 Explanation. .

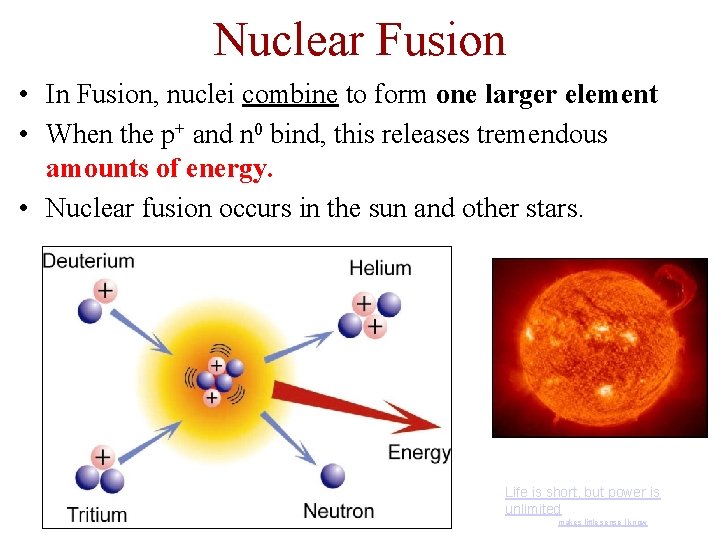
Nuclear Fusion • In Fusion, nuclei combine to form one larger element • When the p+ and n 0 bind, this releases tremendous amounts of energy. • Nuclear fusion occurs in the sun and other stars. Life is short, but power is unlimited makes little sense I know


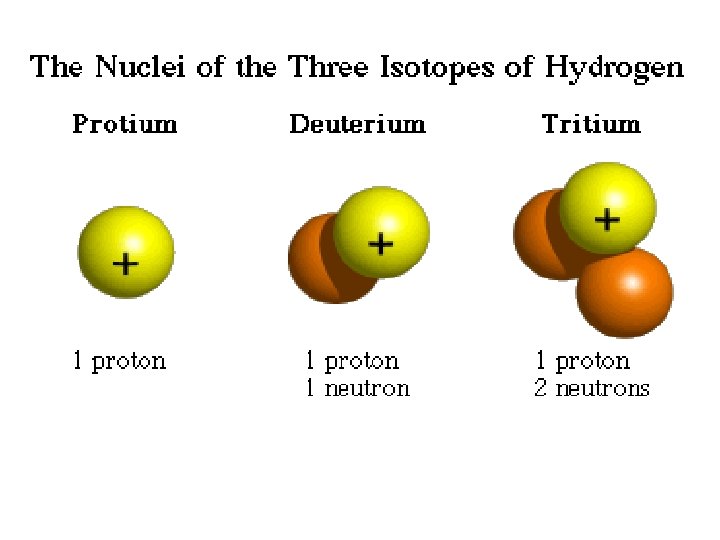
Spiderman 2—Tritium With great power, comes…

Hiroshima

Exit Slip 1) Is fusion or fission involved with splitting an atom? 2) Is fusion or fission involved with the atomic bomb? 3) What happens when you have a nuclear disaster in a power plant? 4) Is fusion or fission the reason we see energy from the sun?

Once I harness the power of the sun, I shall be…. UNSTOPPP ALALBLBLSBKLE LEEL!! bwahaahahhh. HAS HHAHAHH

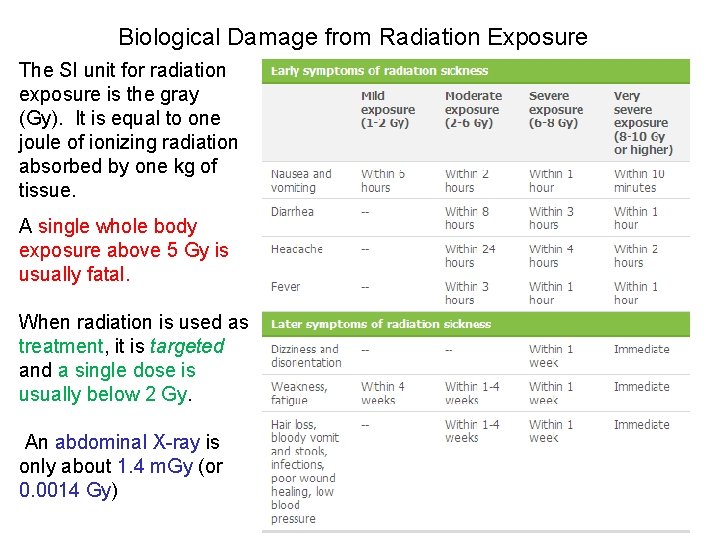
Biological Damage from Radiation Exposure Several factors affect the amount of damage a person experiences when they get exposed to radiation. 1) damage varies based on the type of radiation ex. alpha is 20 x more damaging than gamma 2) damage varies based on the type of tissue ex. rapidly dividing cells (like skin, gut, etc) are more sensitive than slower growing cells (brain) 3) time makes a difference ex. one large dose is much more harmful than an equal amt split into many small exposures (think ‘sunburn’)

Biological Damage from Radiation Exposure The SI unit for radiation exposure is the gray (Gy). It is equal to one joule of ionizing radiation absorbed by one kg of tissue. A single whole body exposure above 5 Gy is usually fatal. When radiation is used as treatment, it is targeted and a single dose is usually below 2 Gy. An abdominal X-ray is only about 1. 4 m. Gy (or 0. 0014 Gy)

Exit Slip • Write the electron capture of Uranium-238 • What nuclear term involves three neutrons being emitted? • Write the gamma decay of Radon-220

Exit Slip • Write the alpha decay of Uranium-238 • What nuclear term involves three neutrons being emitted?