Nuclear Radiation Nuclear Radiation Transformations Nuclear Radiation Marie

















- Slides: 30

Nuclear Radiation > Nuclear Radiation & Transformations

Nuclear Radiation > Marie Curie was a Polish scientist whose research led to many discoveries about radiation and radioactive elements. In 1934 she died from leukemia caused by her long-term exposure to radiation.

Nuclear Radiation > Radioactivity Marie Curie (1867 -1934) and Pierre Curie (1859 -1906) were able to show that rays emitted by uranium atoms caused fogging in photographic plates. Marie Curie named the process by which materials give off such rays radioactivity. The penetrating rays and particles emitted by a radioactive source are called radiation.

Nuclear Radiation > Radioactivity In nuclear reactions, the nuclei of unstable isotopes, called radioisotopes, gain stability by undergoing changes. An unstable nucleus releases energy by emitting radiation during the process of radioactive decay.

Nuclear Radiation > Types of Radiation Alpha Beta Gamma

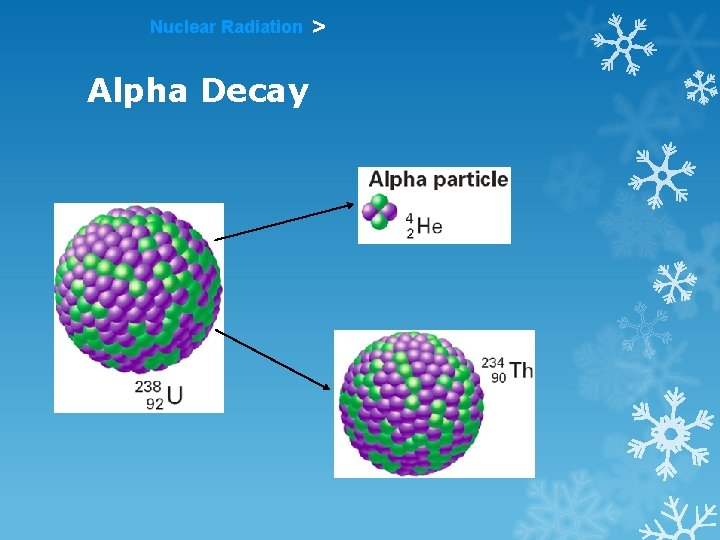
Nuclear Radiation > Alpha Radiation Nuclear Symbol: 4 2 He Made of: 2 p+ and 2 n 0 Mass: 4 Charge: +2 or 4 2α

Nuclear Radiation Alpha Decay >

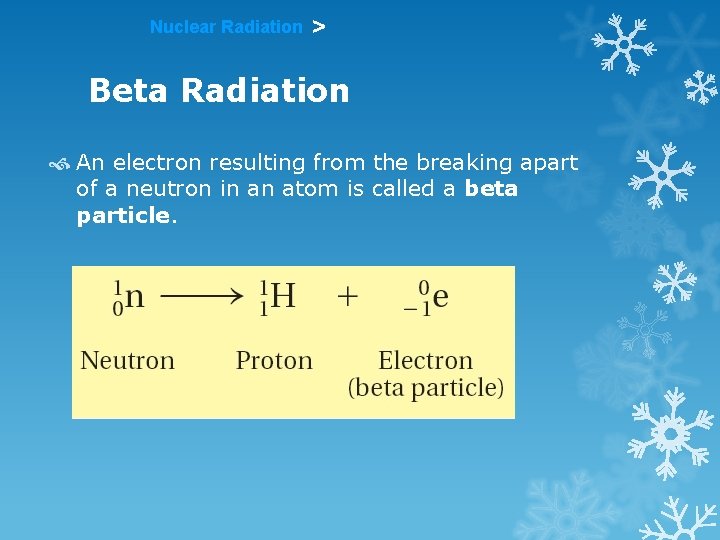
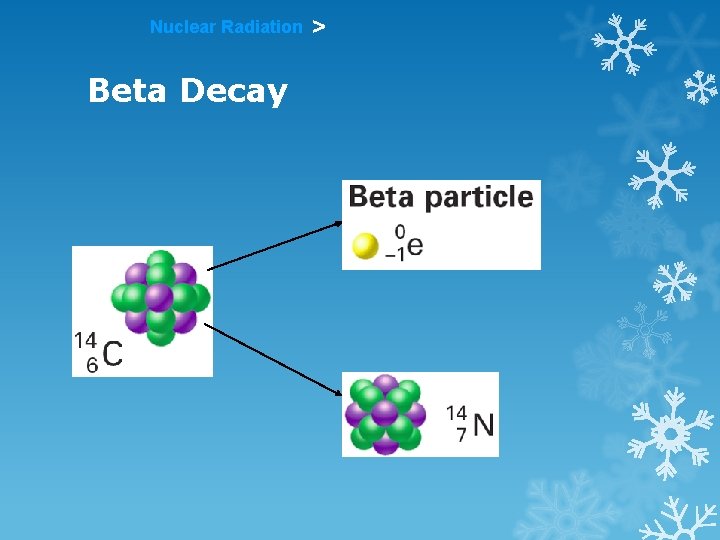
Nuclear Radiation > Beta Radiation An electron resulting from the breaking apart of a neutron in an atom is called a beta particle.

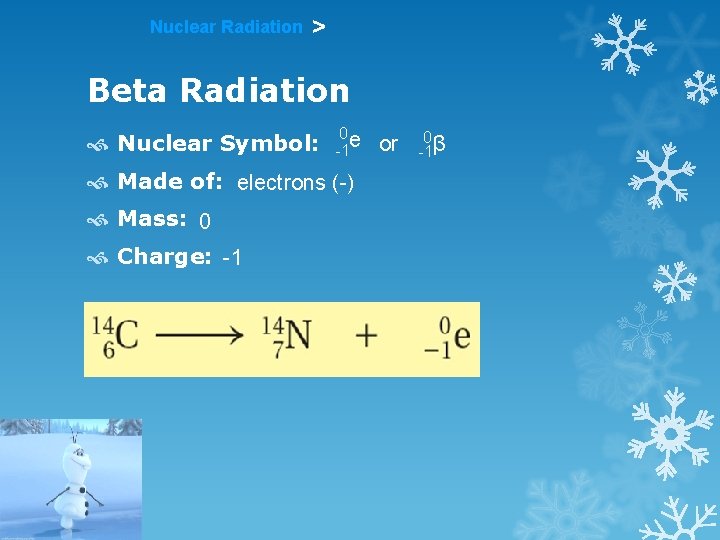
Nuclear Radiation > Beta Radiation Nuclear Symbol: 0 e -1 Made of: electrons (-) Mass: 0 Charge: -1 or 0 -1β

Nuclear Radiation Beta Decay >

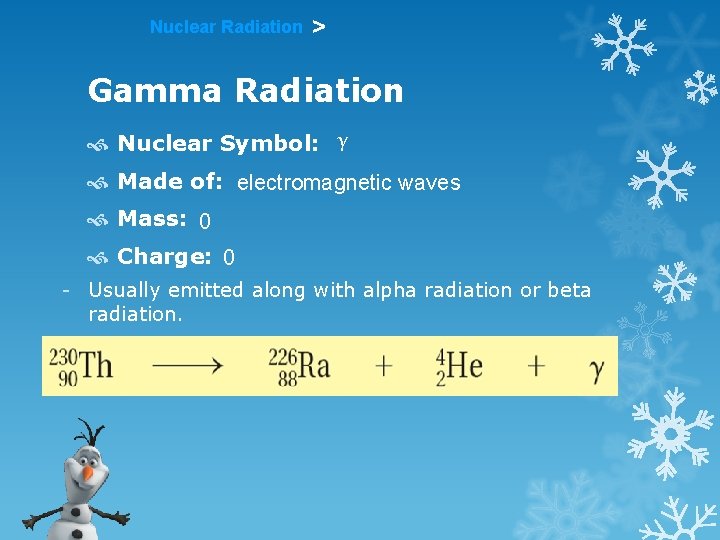
Nuclear Radiation > Gamma Radiation Nuclear Symbol: γ Made of: electromagnetic waves Mass: 0 Charge: 0 - Usually emitted along with alpha radiation or beta radiation.

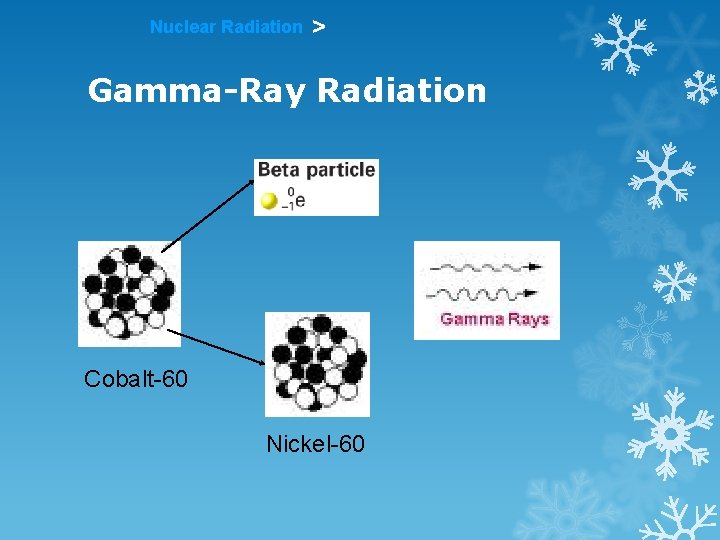
Nuclear Radiation > Gamma-Ray Radiation Cobalt-60 Nickel-60

Nuclear Radiation > What stops alpha, beta, and gamma radiation? Alpha particles are the least penetrating. Gamma rays are the most penetrating.

Nuclear Radiation > Nuclear Stability and Decay The stability of the nucleus determines the type of decay (alpha, beta, gamma) a radioisotope undergoes. The protons in a nucleus want to repel against each other. There is a strong force that holds the protons and neutrons together in the nucleus

Nuclear Radiation > What makes an atom radioactive? When the strong nuclear force cannot hold the protons and neutrons together.

Nuclear Radiation > Which elements are unstable (radioactive)? All nuclei with more than 83 protons (shade these in on the small periodic table) However, many other nuclei with only a few protons are also radioactive

Nuclear Radiation > Transmutation Reactions The conversion of an atom of one element to an atom of another element is called transmutation. Transmutation can occur by radioactive decay. Transmutation can also occur when particles bombard the nucleus of an atom.

Nuclear Radiation > Fission & Fusion BOTH: release A LOT of energy

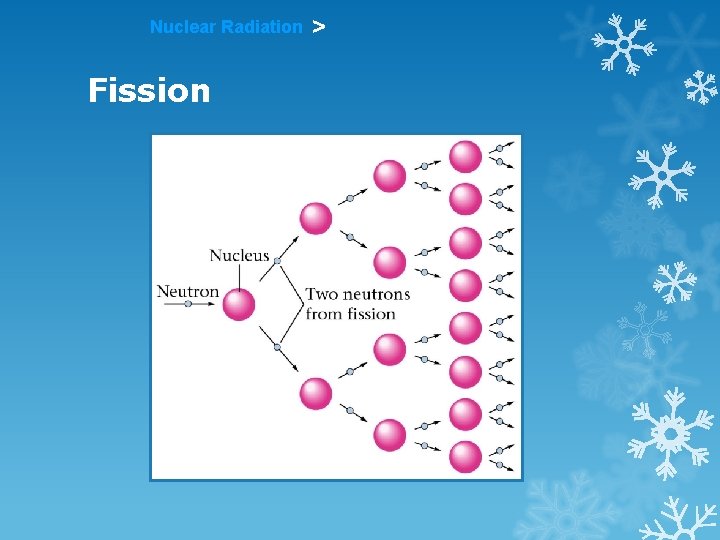
Nuclear Radiation > Fission A large nucleus is split apart. Usually releases particles that perpetuate a chain reaction

Nuclear Radiation Fission >

Nuclear Radiation > Fission A large nucleus is split apart. Usually releases particles that perpetuate a chain reaction Used to generate electricity and in nuclear weapons

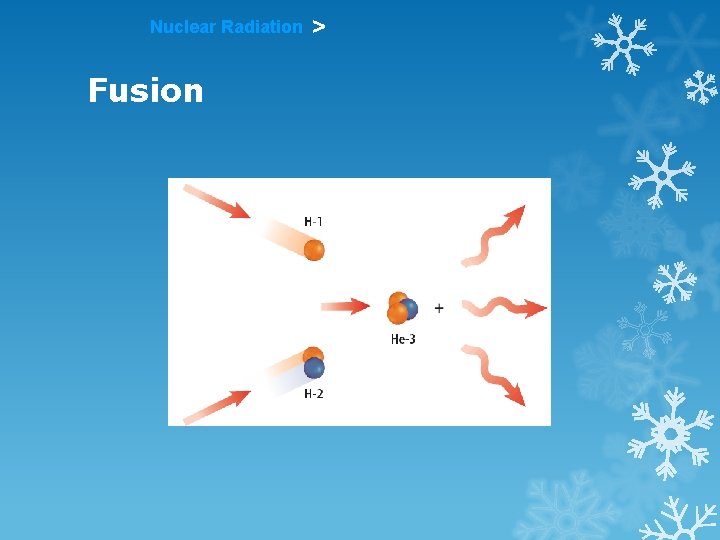
Nuclear Radiation > Fusion 2 small nuclei combine (fuse together) Occurs at very high temperature and pressure Occurs in the sun & other stars

Nuclear Radiation Fusion >

Nuclear Radiation > Geiger Counter Used to detect radioactive substances

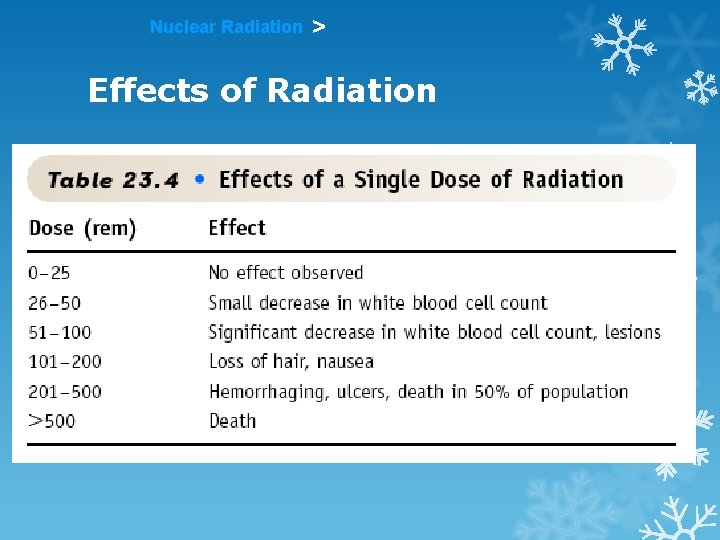
Nuclear Radiation > Effects of Radiation

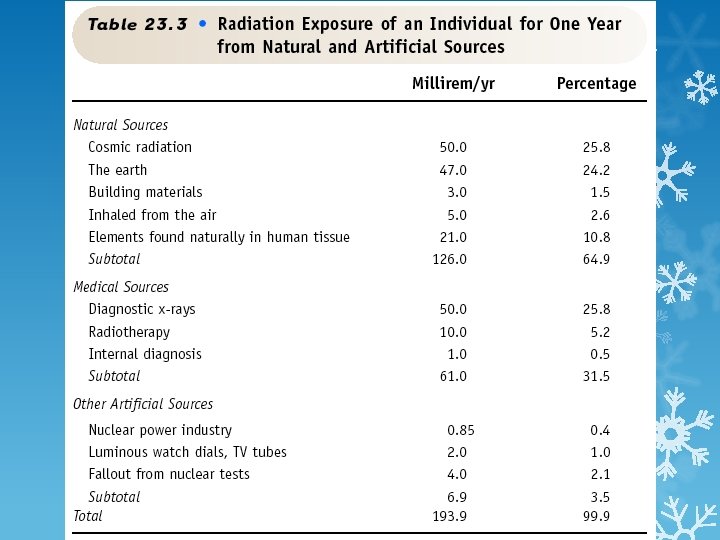
Nuclear Radiation >

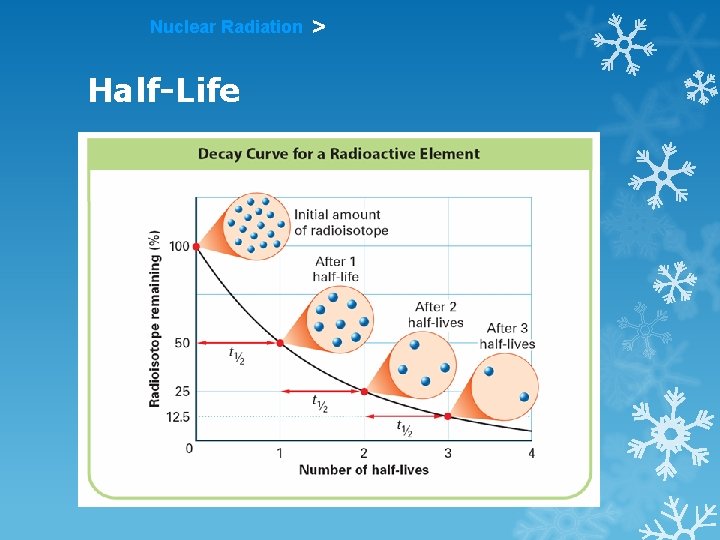
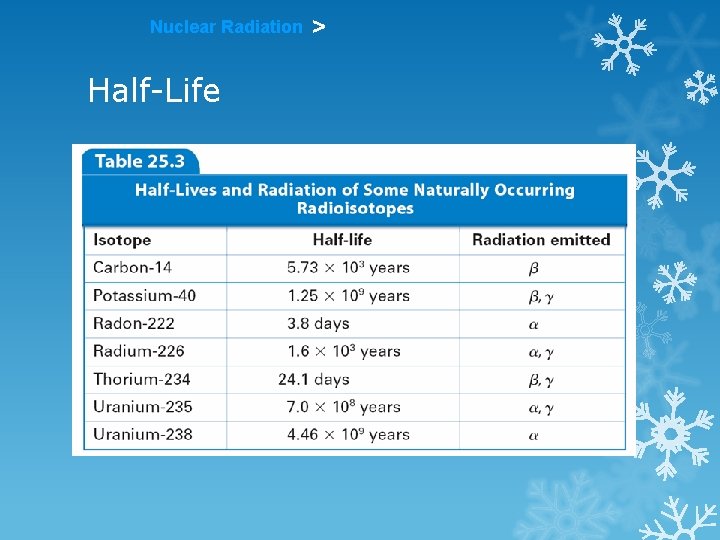
Nuclear Radiation > Half-Life HALF-LIFE is the time that it takes for 1/2 a sample to decay. The rate of a nuclear transformation depends only on the “reactant” concentration.

Nuclear Radiation Half-Life >

Nuclear Radiation Half-Life >

Nuclear Radiation > Half-Life The ratio of Carbon-14 to stable carbon in the remains of an organism changes in a predictable way that enables the archaeologist to obtain an estimate of its age. Radiocarbon dating is used to determine the approximate age of a sample of matter.