25 2 Nuclear Transformations Chapter 25 Nuclear Chemistry










- Slides: 57

25. 2 Nuclear Transformations > Chapter 25 Nuclear Chemistry 25. 1 Nuclear Radiation 25. 2 Nuclear Transformations 25. 3 Fission and Fusion 25. 4 Radiation in Your Life 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > CHEMISTRY & YOU What is the source of radon in homes? Radon may accumulate in a basement that is not well ventilated. Test kits are available to measure the levels of radon in a building. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay Nuclear Stability and Decay What determines the type of decay a radioisotope undergoes? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay The nuclear force is an attractive force that acts between all nuclear particles that are extremely close together, such as protons and neutrons in a nucleus. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay The nuclear force is an attractive force that acts between all nuclear particles that are extremely close together, such as protons and neutrons in a nucleus. • At these short distances, the nuclear force dominates over electromagnetic repulsions and holds the nucleus together. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

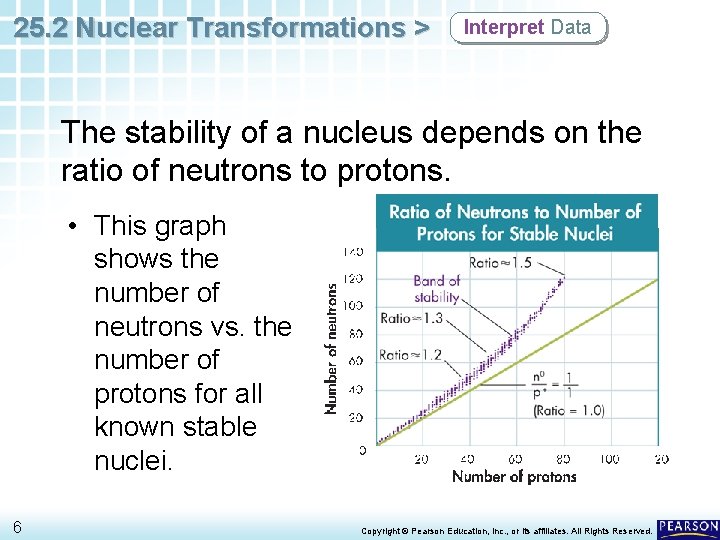
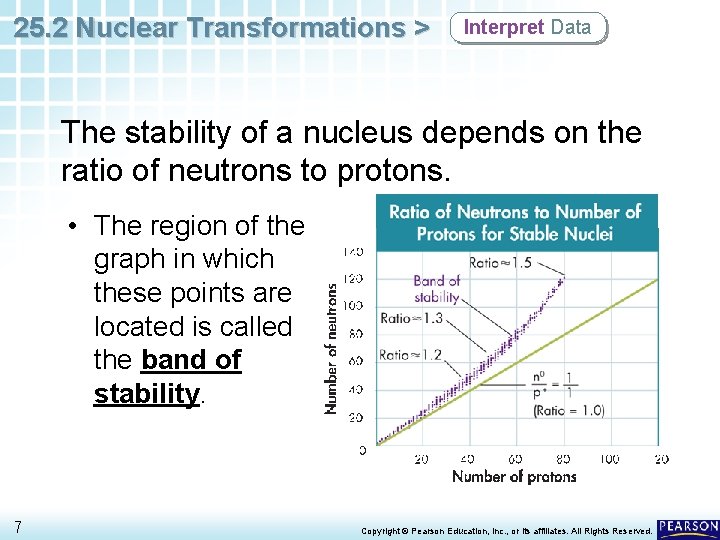
25. 2 Nuclear Transformations > Interpret Data The stability of a nucleus depends on the ratio of neutrons to protons. • This graph shows the number of neutrons vs. the number of protons for all known stable nuclei. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Interpret Data The stability of a nucleus depends on the ratio of neutrons to protons. • The region of the graph in which these points are located is called the band of stability. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

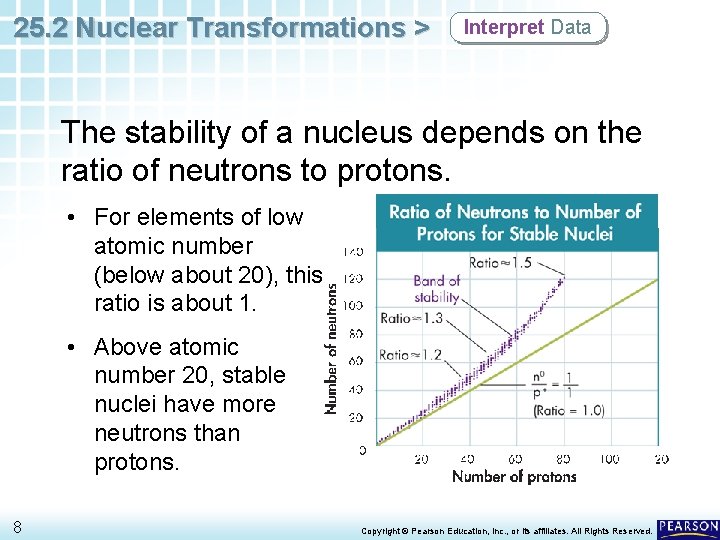
25. 2 Nuclear Transformations > Interpret Data The stability of a nucleus depends on the ratio of neutrons to protons. • For elements of low atomic number (below about 20), this ratio is about 1. • Above atomic number 20, stable nuclei have more neutrons than protons. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay A nucleus may be unstable and undergo spontaneous decay for different reasons. The neutron-to-proton ratio in a radioisotope determines the type of decay that occurs. 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

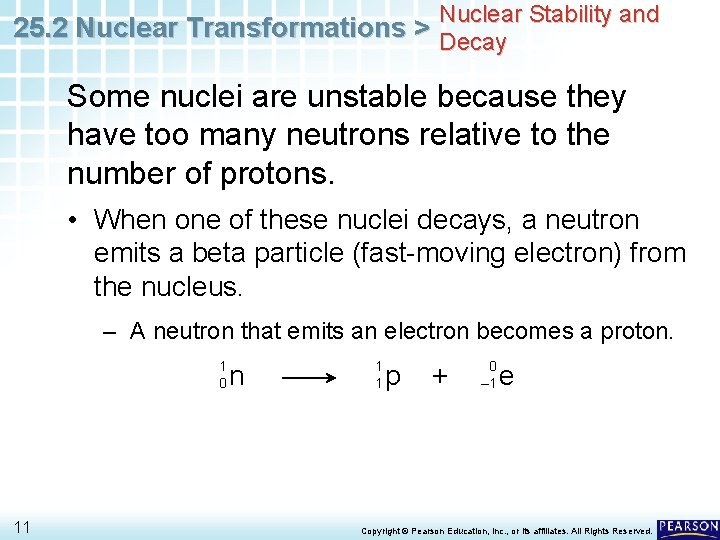
Nuclear Stability and 25. 2 Nuclear Transformations > Decay Some nuclei are unstable because they have too many neutrons relative to the number of protons. • When one of these nuclei decays, a neutron emits a beta particle (fast-moving electron) from the nucleus. 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

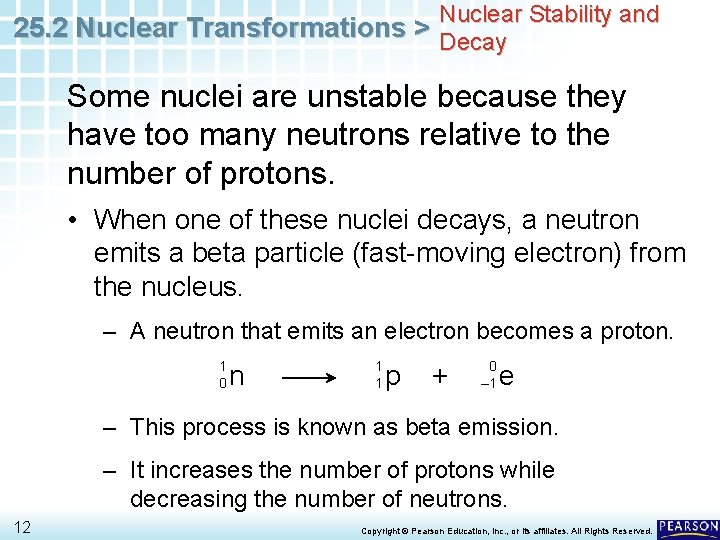
Nuclear Stability and 25. 2 Nuclear Transformations > Decay Some nuclei are unstable because they have too many neutrons relative to the number of protons. • When one of these nuclei decays, a neutron emits a beta particle (fast-moving electron) from the nucleus. – A neutron that emits an electron becomes a proton. 1 0 11 n 1 1 p + 0 – 1 e Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay Some nuclei are unstable because they have too many neutrons relative to the number of protons. • When one of these nuclei decays, a neutron emits a beta particle (fast-moving electron) from the nucleus. – A neutron that emits an electron becomes a proton. 1 0 n 1 1 p + 0 – 1 e – This process is known as beta emission. – It increases the number of protons while decreasing the number of neutrons. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

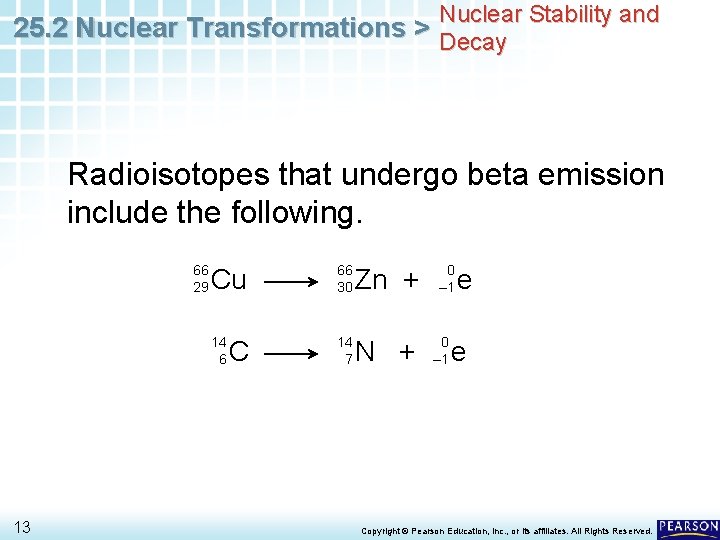
Nuclear Stability and 25. 2 Nuclear Transformations > Decay Radioisotopes that undergo beta emission include the following. 66 29 13 Cu 66 30 14 6 14 7 C Zn + N + 0 – 1 e e Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

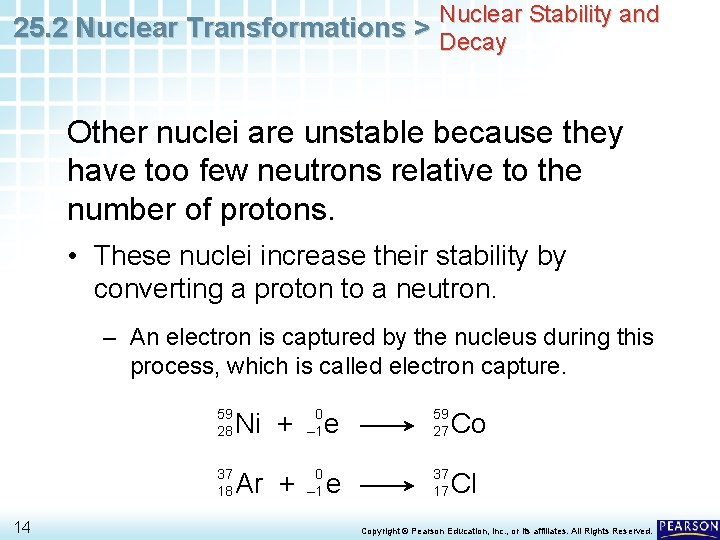
Nuclear Stability and 25. 2 Nuclear Transformations > Decay Other nuclei are unstable because they have too few neutrons relative to the number of protons. • These nuclei increase their stability by converting a proton to a neutron. – An electron is captured by the nucleus during this process, which is called electron capture. 59 28 37 18 14 Ni + 0 – 1 Ar + 0 – 1 e 59 27 Co e 37 17 Cl Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

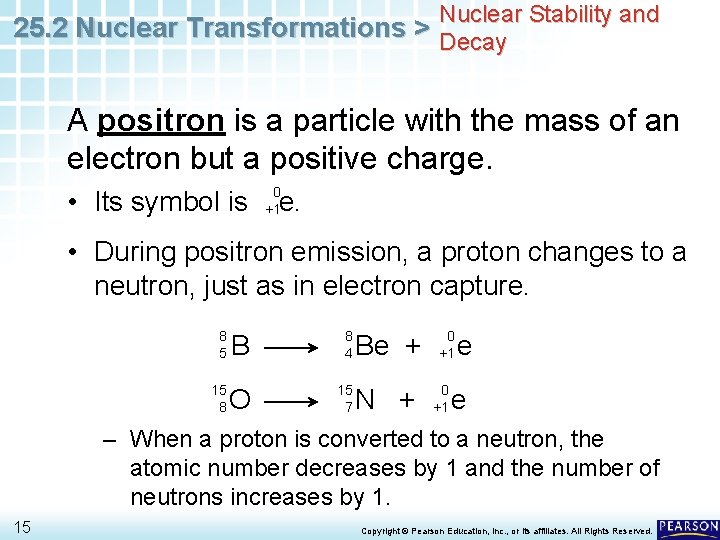
Nuclear Stability and 25. 2 Nuclear Transformations > Decay A positron is a particle with the mass of an electron but a positive charge. • Its symbol is 0 +1 e. • During positron emission, a proton changes to a neutron, just as in electron capture. 8 5 15 8 B 8 4 O 15 7 Be + N + 0 +1 e e – When a proton is converted to a neutron, the atomic number decreases by 1 and the number of neutrons increases by 1. 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

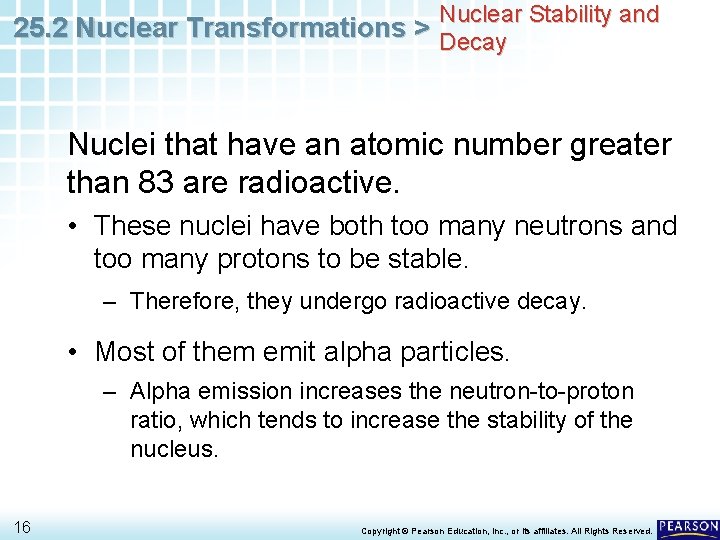
Nuclear Stability and 25. 2 Nuclear Transformations > Decay Nuclei that have an atomic number greater than 83 are radioactive. • These nuclei have both too many neutrons and too many protons to be stable. – Therefore, they undergo radioactive decay. • Most of them emit alpha particles. – Alpha emission increases the neutron-to-proton ratio, which tends to increase the stability of the nucleus. 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

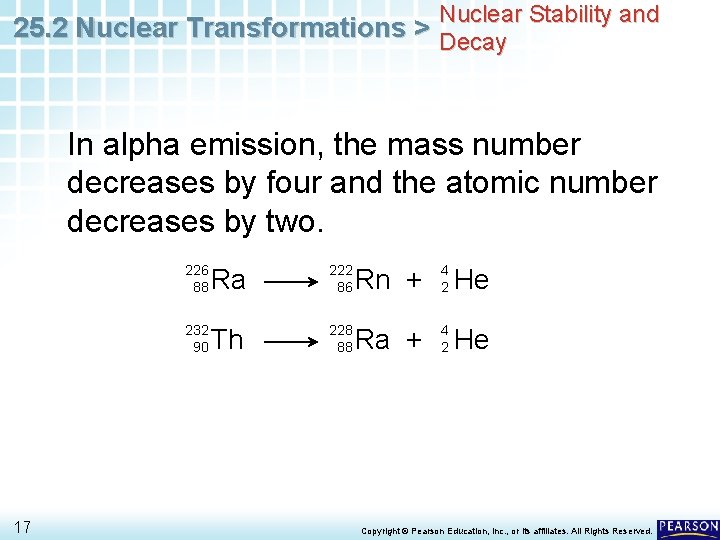
Nuclear Stability and 25. 2 Nuclear Transformations > Decay In alpha emission, the mass number decreases by four and the atomic number decreases by two. 226 88 232 90 17 Ra 222 86 Th 228 88 Rn + 4 2 He Ra + 4 2 He Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Nuclear Stability and 25. 2 Nuclear Transformations > Decay Recall that conservation of mass is an important property of chemical reactions. • In contrast, mass is not conserved during nuclear reactions. • An extremely small quantity of mass is converted into energy released during radioactive decay. 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > During nuclear decay, if the atomic number decreases by one but the mass number is unchanged, the radiation emitted is A. a positron. B. an alpha particle. C. a beta particle. D. a proton. 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > During nuclear decay, if the atomic number decreases by one but the mass number is unchanged, the radiation emitted is A. a positron. B. an alpha particle. C. a beta particle. D. a proton. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Half-Life How much of a radioactive sample remains after each half-life? 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

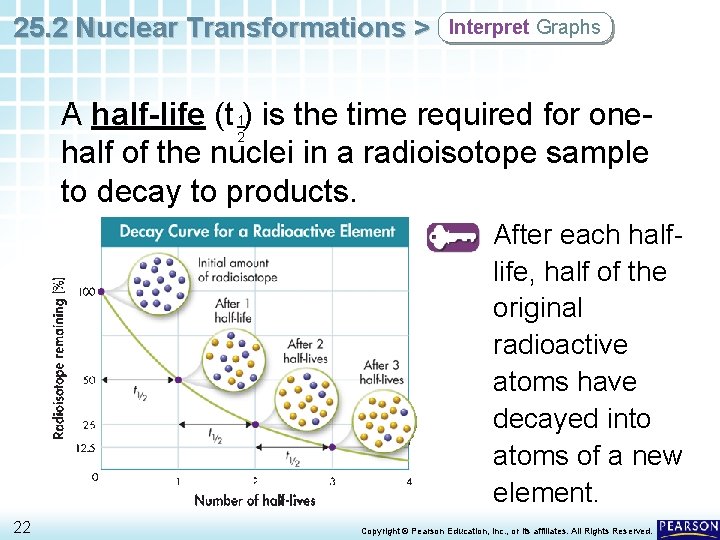
25. 2 Nuclear Transformations > Interpret Graphs A half-life (t 1) is the time required for one 2 half of the nuclei in a radioisotope sample to decay to products. After each halflife, half of the original radioactive atoms have decayed into atoms of a new element. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

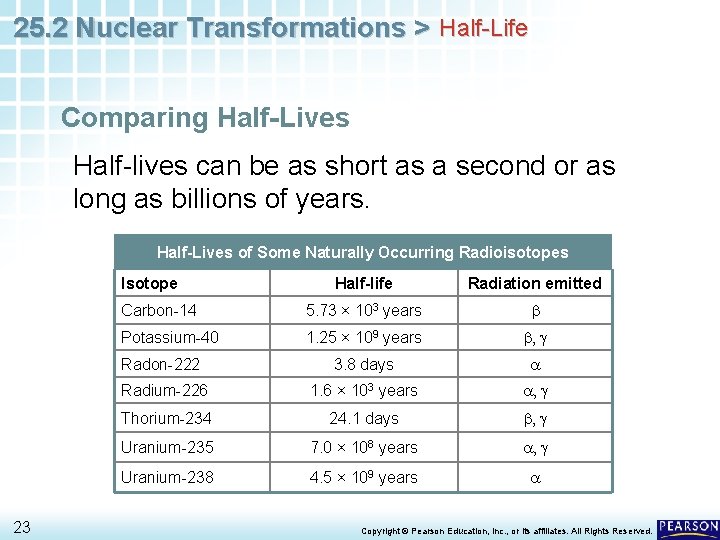
25. 2 Nuclear Transformations > Half-Life Comparing Half-Lives Half-lives can be as short as a second or as long as billions of years. Half-Lives of Some Naturally Occurring Radioisotopes Isotope 23 Half-life Radiation emitted Carbon-14 5. 73 × 103 years b Potassium-40 1. 25 × 109 years b, g Radon-222 3. 8 days a Radium-226 1. 6 × 103 years a, g Thorium-234 24. 1 days b, g Uranium-235 7. 0 × 108 years a, g Uranium-238 4. 5 × 109 years a Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Half-Life Comparing Half-Lives • Scientists use half-lives of some long-term radioisotopes to determine the age of ancient objects. • Many artificially produced radioisotopes have short half-lives, which makes them useful in nuclear medicine. – Short-lived isotopes are not a long-term radiation hazard for patients. 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

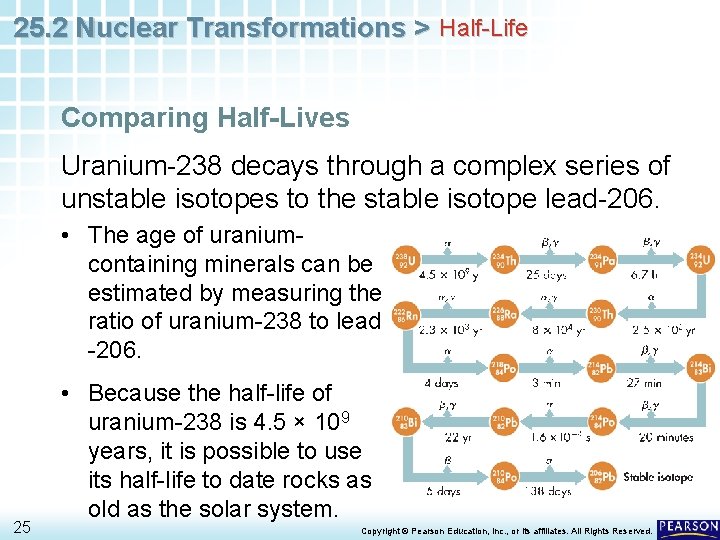
25. 2 Nuclear Transformations > Half-Life Comparing Half-Lives Uranium-238 decays through a complex series of unstable isotopes to the stable isotope lead-206. • The age of uraniumcontaining minerals can be estimated by measuring the ratio of uranium-238 to lead -206. 25 • Because the half-life of uranium-238 is 4. 5 × 109 years, it is possible to use its half-life to date rocks as old as the solar system. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > CHEMISTRY & YOU Uranium compounds are found in rocks and in soils that form from these rocks. How can these uranium compounds lead to a buildup of radon in homes and other buildings? 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > CHEMISTRY & YOU Uranium compounds are found in rocks and in soils that form from these rocks. How can these uranium compounds lead to a buildup of radon in homes and other buildings? Radon gas is a product of the decay of uranium. As the uranium compounds in the soil beneath homes and buildings decay, radon is produced and seeps into the structure. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Half-Life Radiocarbon Dating Plants use carbon dioxide to produce carbon compounds, such as glucose. • The ratio of carbon-14 to other carbon isotopes is constant during an organism’s life. • When an organism dies, it stops exchanging carbon with the environment and its radioactive 14 6 C atoms decay without being replaced. • Archaeologists can use this data to estimate when an organism died. 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

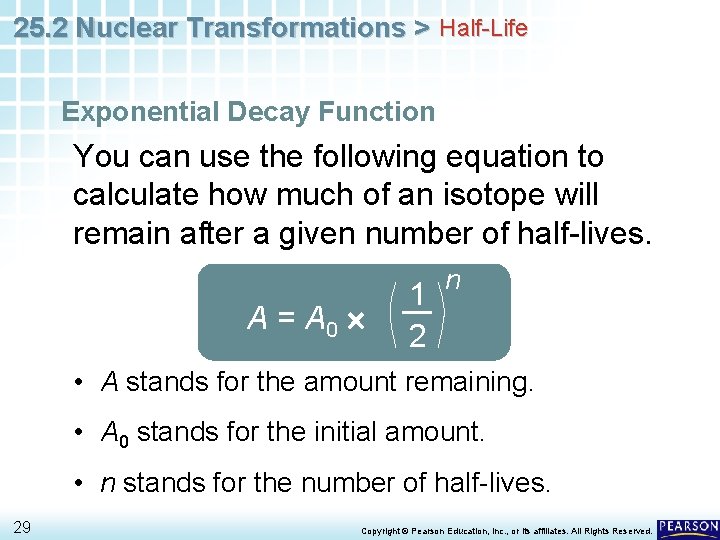
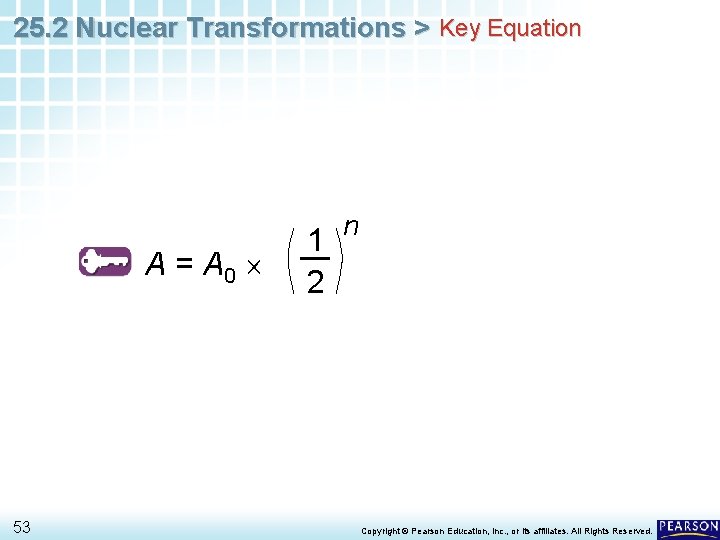
25. 2 Nuclear Transformations > Half-Life Exponential Decay Function You can use the following equation to calculate how much of an isotope will remain after a given number of half-lives. A = A 0 1 2 n • A stands for the amount remaining. • A 0 stands for the initial amount. • n stands for the number of half-lives. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

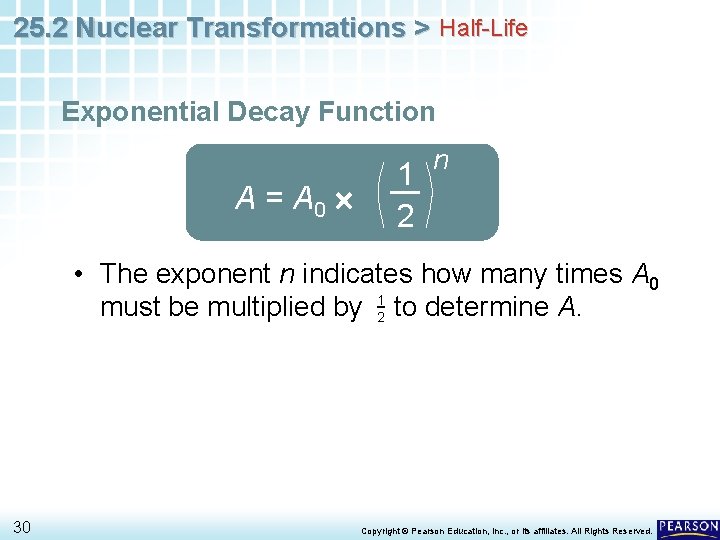
25. 2 Nuclear Transformations > Half-Life Exponential Decay Function A = A 0 1 2 n • The exponent n indicates how many times A 0 must be multiplied by 12 to determine A. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

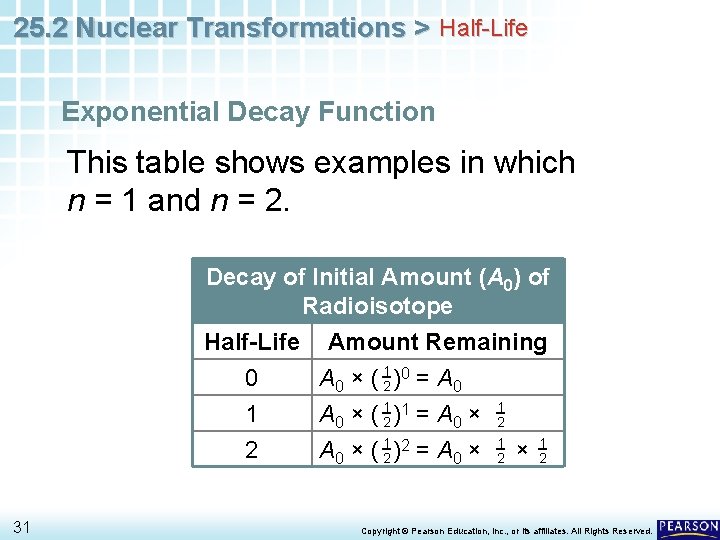
25. 2 Nuclear Transformations > Half-Life Exponential Decay Function This table shows examples in which n = 1 and n = 2. Decay of Initial Amount (A 0) of Radioisotope Half-Life Amount Remaining 0 A 0 × ( 12 )0 = A 0 1 A 0 × ( 12 )1 = A 0 × 12 2 A 0 × ( 12 )2 = A 0 × 12 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

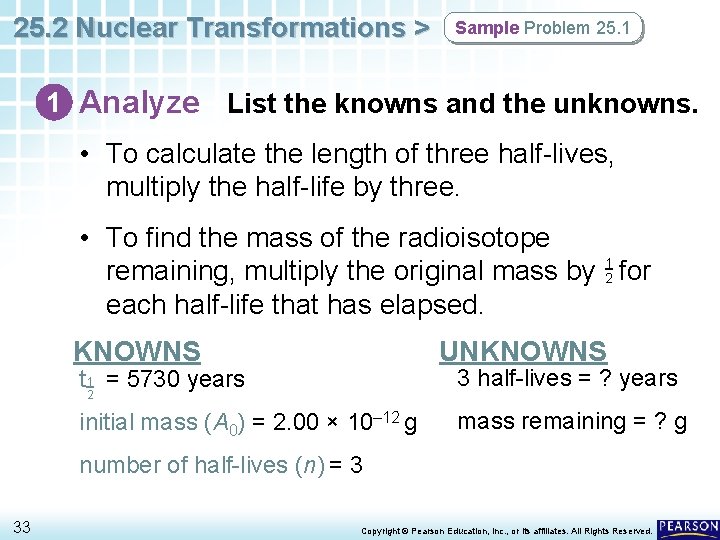
25. 2 Nuclear Transformations > Sample Problem 25. 1 Using Half-Lives in Calculations Carbon-14 emits beta radiation and decays with a half-life (t 12 ) of 5730 years. Assume that you start with a mass of 2. 00 × 10– 12 g of carbon-14. a. How long is three half-lives? b. How many grams of the isotope remain at the end of three half-lives? 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Sample Problem 25. 1 1 Analyze List the knowns and the unknowns. • To calculate the length of three half-lives, multiply the half-life by three. • To find the mass of the radioisotope 1 remaining, multiply the original mass by 2 for each half-life that has elapsed. KNOWNS UNKNOWNS 3 half-lives = ? years t 1 = 5730 years 2 initial mass (A 0) = 2. 00 × 10– 12 g mass remaining = ? g number of half-lives (n) = 3 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

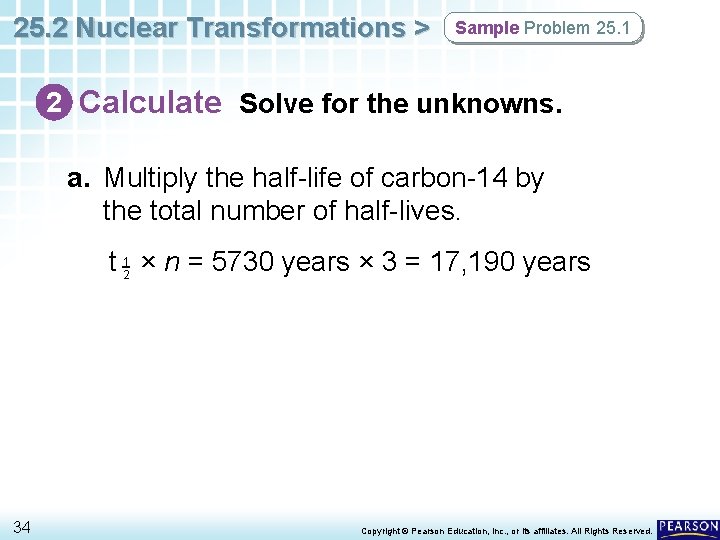
25. 2 Nuclear Transformations > Sample Problem 25. 1 2 Calculate Solve for the unknowns. a. Multiply the half-life of carbon-14 by the total number of half-lives. t 12 × n = 5730 years × 3 = 17, 190 years 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

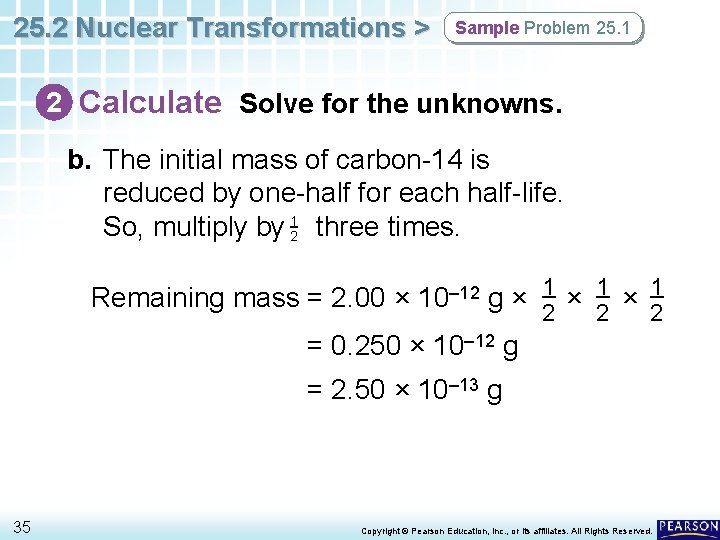
25. 2 Nuclear Transformations > Sample Problem 25. 1 2 Calculate Solve for the unknowns. b. The initial mass of carbon-14 is reduced by one-half for each half-life. So, multiply by 12 three times. Remaining mass = 2. 00 × 10– 12 g × 12 = 0. 250 × 10– 12 g = 2. 50 × 10– 13 g 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

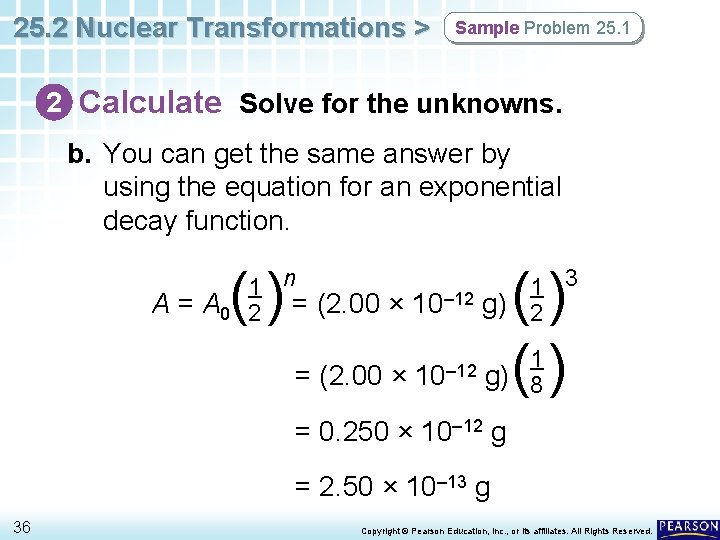
25. 2 Nuclear Transformations > Sample Problem 25. 1 2 Calculate Solve for the unknowns. b. You can get the same answer by using the equation for an exponential decay function. () A = A 0 () g) ( ) 3 1 n 1 – 12 g) = (2. 00 × 10 2 2 = (2. 00 × 10– 12 1 8 = 0. 250 × 10– 12 g = 2. 50 × 10– 13 g 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Sample Problem 25. 1 3 Evaluate Do the results make sense? • The mass of carbon-14 after three half-lives should be one-eighth of the original mass. • If you divide 2. 5 × 10– 13 g by 2. 00 × 10– 12 g, you will get 12. 5%, or 18. 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

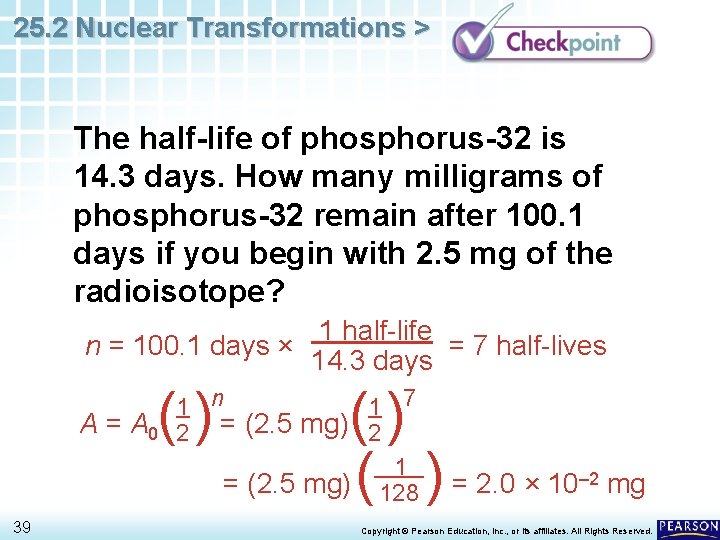
25. 2 Nuclear Transformations > The half-life of phosphorus-32 is 14. 3 days. How many milligrams of phosphorus-32 remain after 100. 1 days if you begin with 2. 5 mg of the radioisotope? 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > The half-life of phosphorus-32 is 14. 3 days. How many milligrams of phosphorus-32 remain after 100. 1 days if you begin with 2. 5 mg of the radioisotope? n = 100. 1 days × () A = A 0 39 1 half-life = 7 half-lives 14. 3 days () ( ) = 2. 0 × 10 1 n 1 7 2 = (2. 5 mg) 2 1 = (2. 5 mg) 128 – 2 mg Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Transmutation Reactions What are two ways in which transmutation can occur? 40 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Transmutation Reactions For thousands of years, alchemists tried to change lead into gold. • What they wanted to achieve is transmutation, or the conversion of an atom of one element into an atom of another element. 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Transmutation Reactions Transmutation can occur by radioactive decay, or when particles bombard the nucleus of an atom. • The particles may be protons, neutrons, alpha particles, or small atoms. 42 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

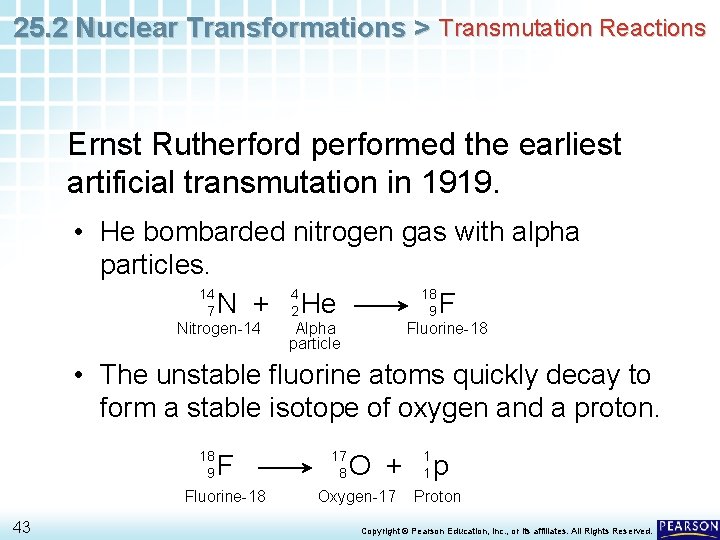
25. 2 Nuclear Transformations > Transmutation Reactions Ernst Rutherford performed the earliest artificial transmutation in 1919. • He bombarded nitrogen gas with alpha particles. 14 4 18 7 N + 2 He 9 F Nitrogen-14 Alpha particle Fluorine-18 • The unstable fluorine atoms quickly decay to form a stable isotope of oxygen and a proton. 18 9 F Fluorine-18 43 17 8 O + Oxygen-17 1 1 p Proton Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

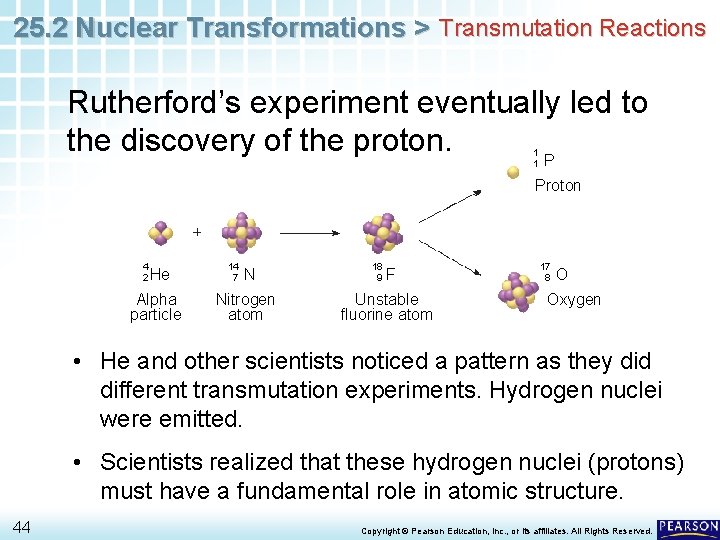
25. 2 Nuclear Transformations > Transmutation Reactions Rutherford’s experiment eventually led to the discovery of the proton. P 1 1 Proton 4 2 He Alpha particle 14 7 N Nitrogen atom 18 9 F Unstable fluorine atom 17 8 O Oxygen • He and other scientists noticed a pattern as they did different transmutation experiments. Hydrogen nuclei were emitted. • Scientists realized that these hydrogen nuclei (protons) must have a fundamental role in atomic structure. 44 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

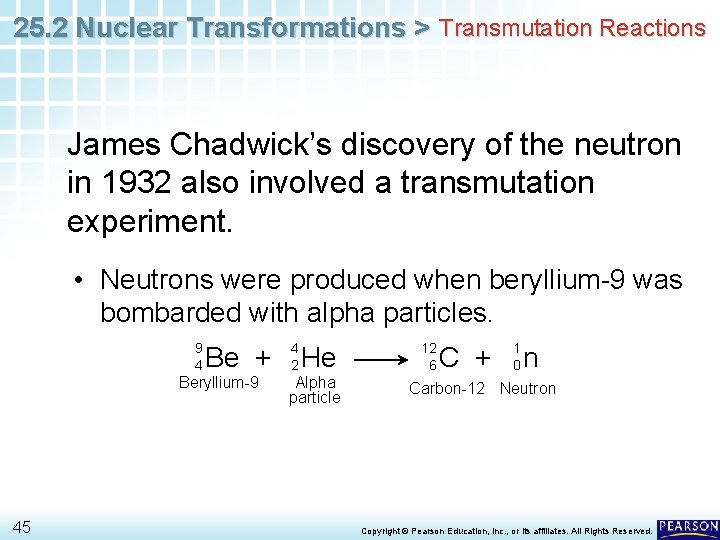
25. 2 Nuclear Transformations > Transmutation Reactions James Chadwick’s discovery of the neutron in 1932 also involved a transmutation experiment. • Neutrons were produced when beryllium-9 was bombarded with alpha particles. 9 4 Be + Beryllium-9 45 4 2 He Alpha particle 12 6 C + 1 0 n Carbon-12 Neutron Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

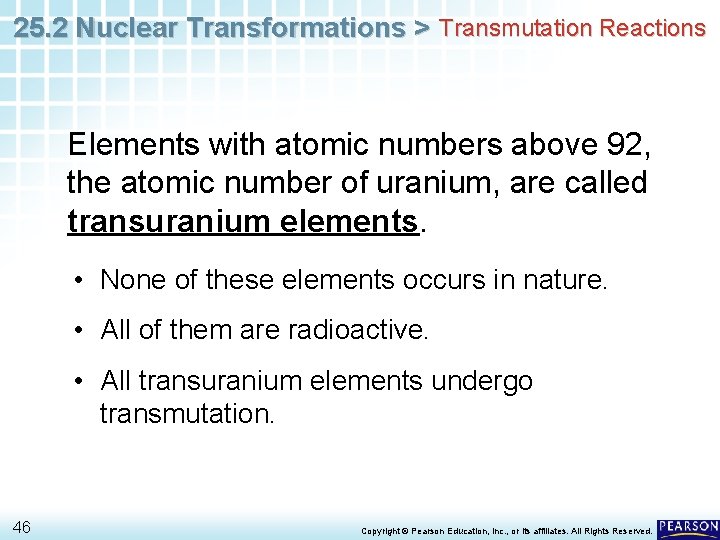
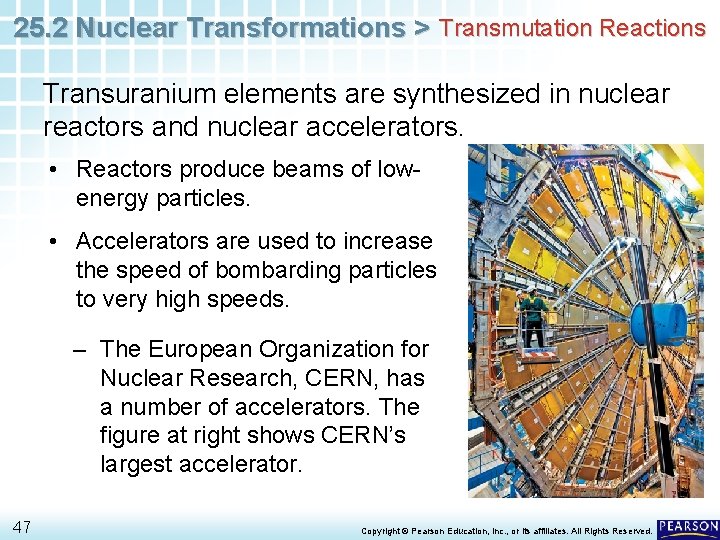
25. 2 Nuclear Transformations > Transmutation Reactions Elements with atomic numbers above 92, the atomic number of uranium, are called transuranium elements. • None of these elements occurs in nature. • All of them are radioactive. • All transuranium elements undergo transmutation. 46 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Transmutation Reactions Transuranium elements are synthesized in nuclear reactors and nuclear accelerators. • Reactors produce beams of lowenergy particles. • Accelerators are used to increase the speed of bombarding particles to very high speeds. – The European Organization for Nuclear Research, CERN, has a number of accelerators. The figure at right shows CERN’s largest accelerator. 47 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

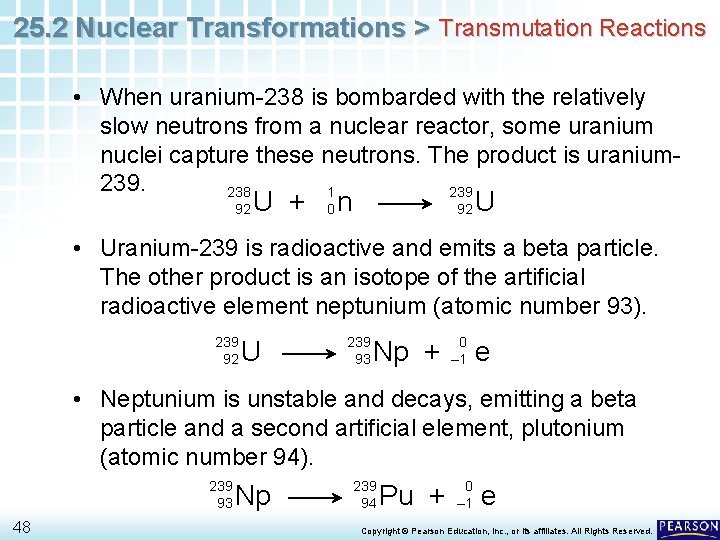
25. 2 Nuclear Transformations > Transmutation Reactions • When uranium-238 is bombarded with the relatively slow neutrons from a nuclear reactor, some uranium nuclei capture these neutrons. The product is uranium 239. 238 1 239 92 U + 0 n 92 U • Uranium-239 is radioactive and emits a beta particle. The other product is an isotope of the artificial radioactive element neptunium (atomic number 93). 239 92 U 239 93 Np + 0 – 1 e • Neptunium is unstable and decays, emitting a beta particle and a second artificial element, plutonium (atomic number 94). 239 93 48 Np 239 94 Pu + 0 – 1 e Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Transmutation Reactions Scientists in Berkeley, California, synthesized the first two artificial elements in 1940. • Since that time, more than 20 additional transuranium elements have been produced artificially. 49 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Which of the following always changes when transmutation occurs? A. The number of electrons B. The mass number C. The atomic number D. The number of neutrons 50 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Which of the following always changes when transmutation occurs? A. The number of electrons B. The mass number C. The atomic number D. The number of neutrons 51 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Key Concepts The neutron-to-proton ratio in a radioisotope determines the type of decay that occurs. After each half-life, half of the original radioactive atoms have decayed into atoms of a new element. Transmutation can occur by radioactive decay, or when particles bombard the nucleus of an atom. 52 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Key Equation A = A 0 53 1 2 n Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Glossary Terms • nuclear force: an attractive force that acts between all nuclear particles that are extremely close together, like protons and neutrons in a nucleus • band of stability: the location of stable nuclei on a neutron-vs. -proton plot • positron: a particle with the mass of an electron but a positive charge 54 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > Glossary Terms • half-life: the time required for one-half of the nuclei of a radioisotope sample to decay to products • transmutation: the conversion of an atom of one element to an atom of another element • transuranium elements: any elements in the periodic table with atomic number above 92, the atomic number of uranium 55 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > BIG IDEA Electrons and the Structure of Atoms • Unstable atomic nuclei decay by emitting alpha or beta particles. • Often gamma rays are emitted. 56 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

25. 2 Nuclear Transformations > END OF 25. 2 57 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.