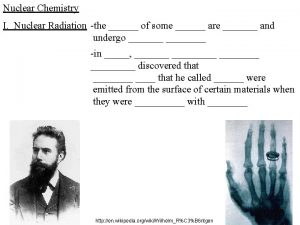
Nuclear Chemistry The study of nuclear change Some








- Slides: 20

Nuclear Chemistry The study of nuclear change. Some elements undergo chemical changes in their nucleus that alters their number of protons or neutrons.

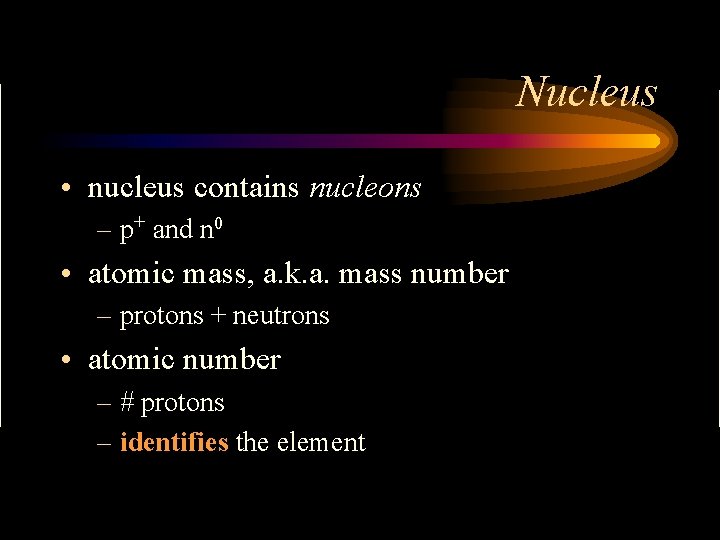
Nucleus • nucleus contains nucleons – p+ and n 0 • atomic mass, a. k. a. mass number – protons + neutrons • atomic number – # protons – identifies the element

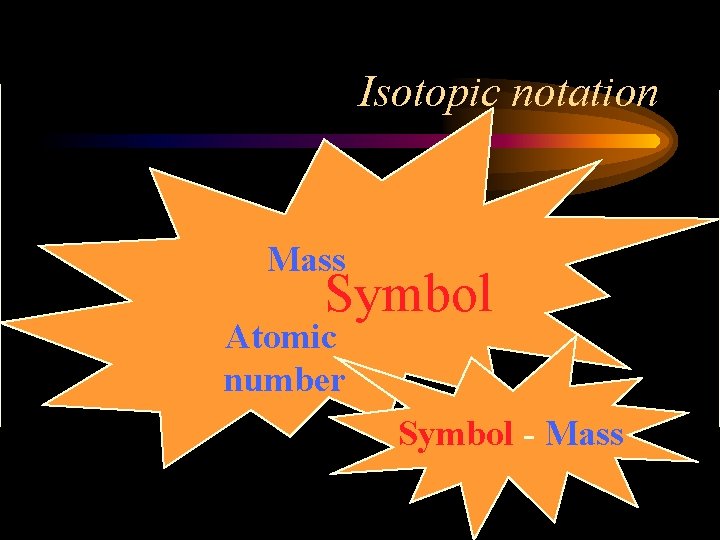
Isotopic notation Mass Symbol Atomic number Symbol - Mass

U-242 U-232 U 238 U-234 U 235 U-240 U 234 U-236 U-239 U 226 U 230 U-238 U-229 U 230 U-235 U 228

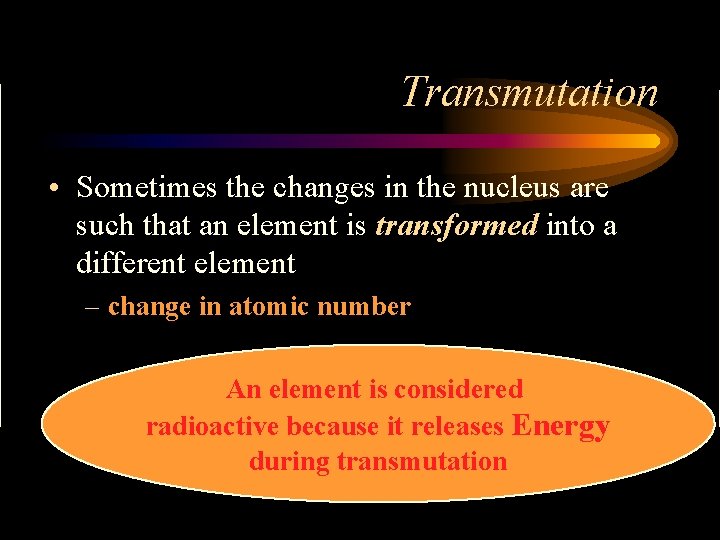
Transmutation • Sometimes the changes in the nucleus are such that an element is transformed into a different element – change in atomic number An element is considered radioactive because it releases Energy during transmutation

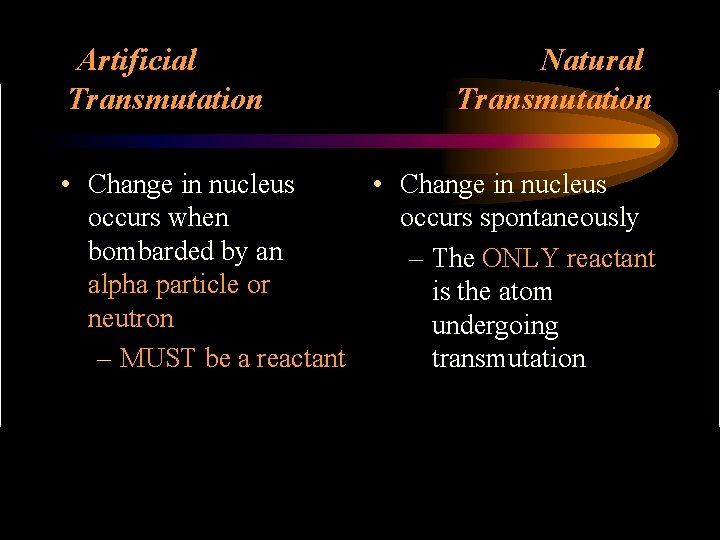
Artificial Transmutation Natural Transmutation • Change in nucleus occurs when occurs spontaneously bombarded by an – The ONLY reactant alpha particle or is the atom neutron undergoing – MUST be a reactant transmutation

FYI. . . • Since 1940, 23 transuranium elements have been formed • All elements with atomic numbers > 93 were synthesized by nuclear reactions • Glenn Seaborg created 10 of these elements – atomic # 94 - 103 • Element #106 was named for him – Seaborgium

Stability of Nuclei • Most nuclei are stable – some elements have unstable isotopes • Radioisotopes / nuclide – Ex. C-12 stable and won’t transmutate C-14 unstable and will transmutate

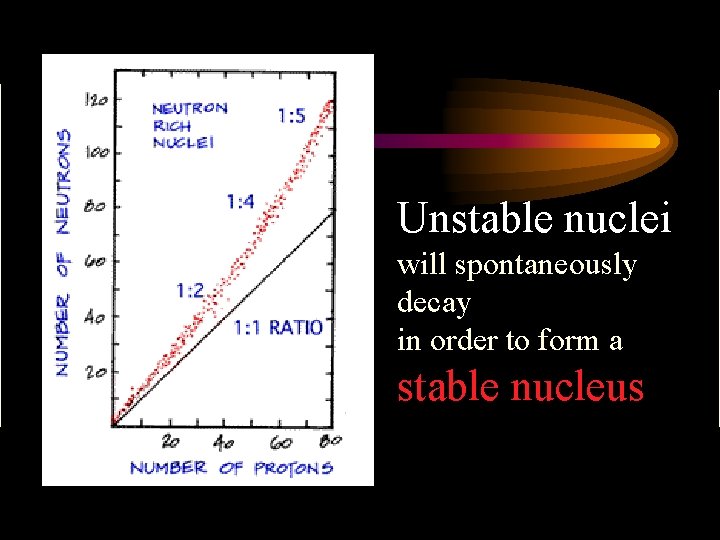
What causes instability? • As atoms increase in size the ratio of neutrons to protons also increases – the larger the ratio the more unstable the atom – Ex. compare C-14 to U-238 • All nuclei with atomic numbers > 83 are unstable and radioactive

Unstable nuclei will spontaneously decay in order to form a stable nucleus

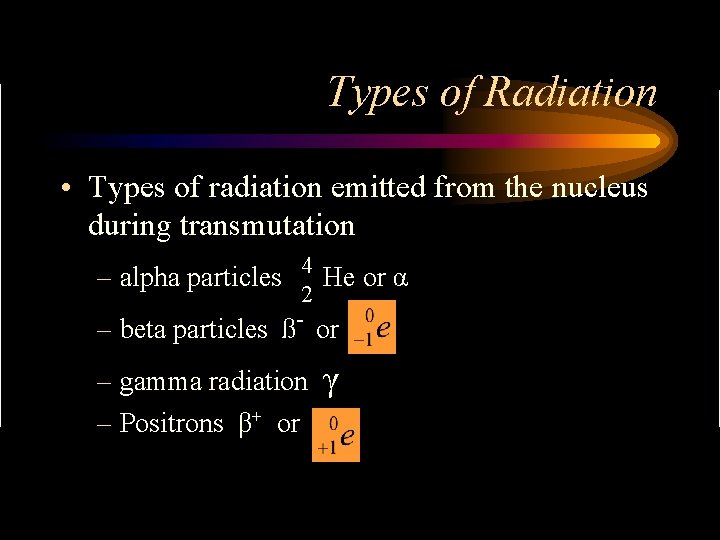
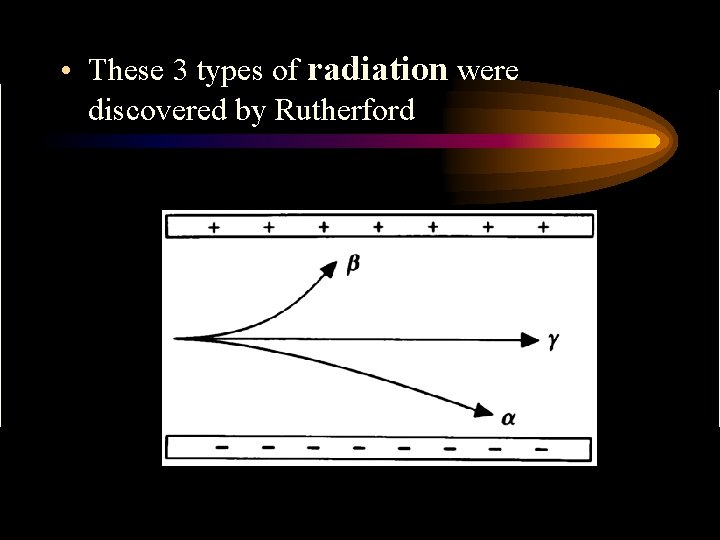
Types of Radiation • Types of radiation emitted from the nucleus during transmutation – alpha particles 4 He or α 2 – beta particles ß- or – gamma radiation – Positrons β+ or γ

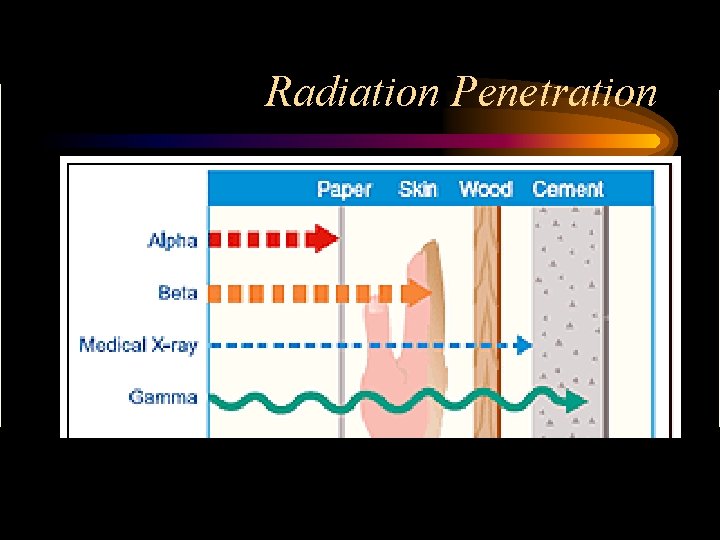
Radiation Penetration

How does Radiation affect cells? • Radiation has the ability to ionize atoms – Ionizing radiation causes damage to tissues by damaging molecules • Most damaging is alpha; least is gamma – Damage to proteins & DNA are most damaging – If radiation exposure is low the body may repair itself – If exposure is high, cells may die or cellular instructions might get “scrambled”

• Radioisotopes are used in diagnosing and treating cancer – I-131 • diagnose thyroid disorders – Co-60 • treat cancer Short half lives decay quickly

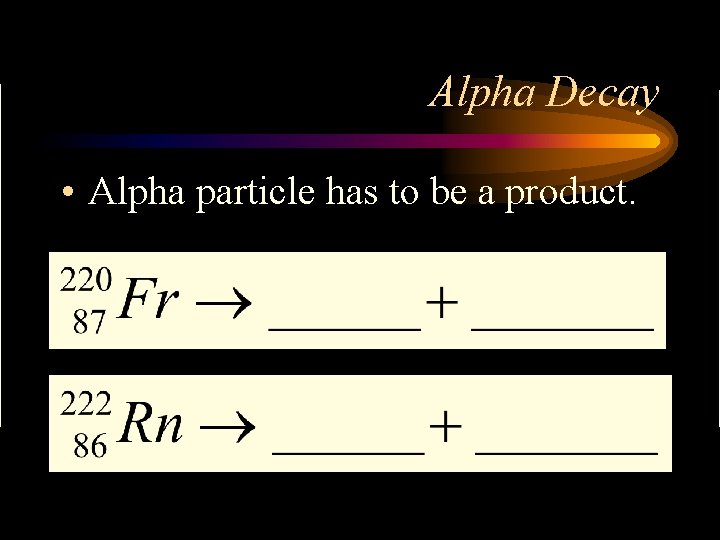
Alpha Decay • Alpha particle has to be a product.

Beta Decay • A Beta particle has to be a product

Gamma Radiation • Type of electromagnetic radiation like an x-ray • No charge, no mass, no equations!

• These 3 types of radiation were discovered by Rutherford

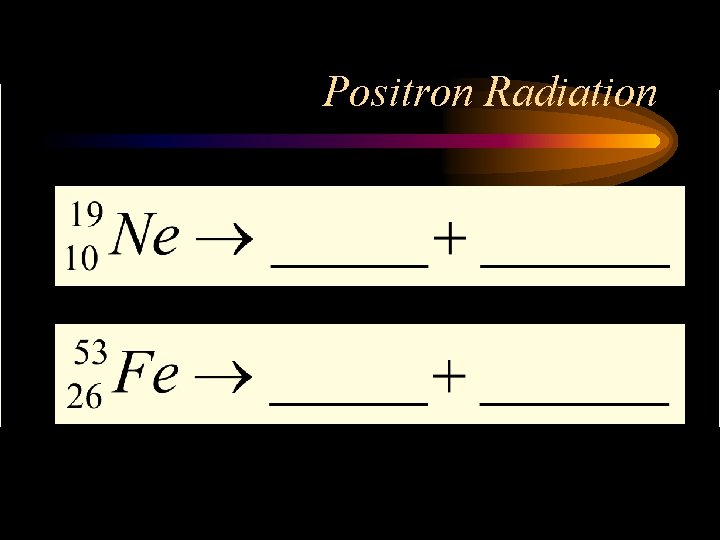
Positron Radiation

The End
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave 25 m/s
25 m/s T half life formula
T half life formula Types of decay
Types of decay Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Nuclear fusion
Nuclear fusion Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry