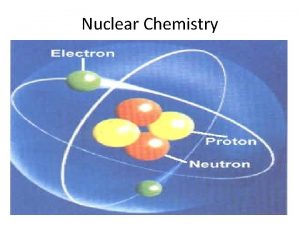
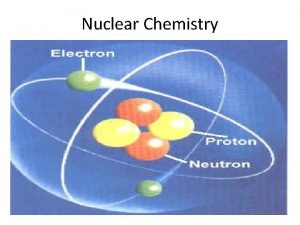
Nuclear Chemistry Nuclear chemistry is the study of




















- Slides: 37


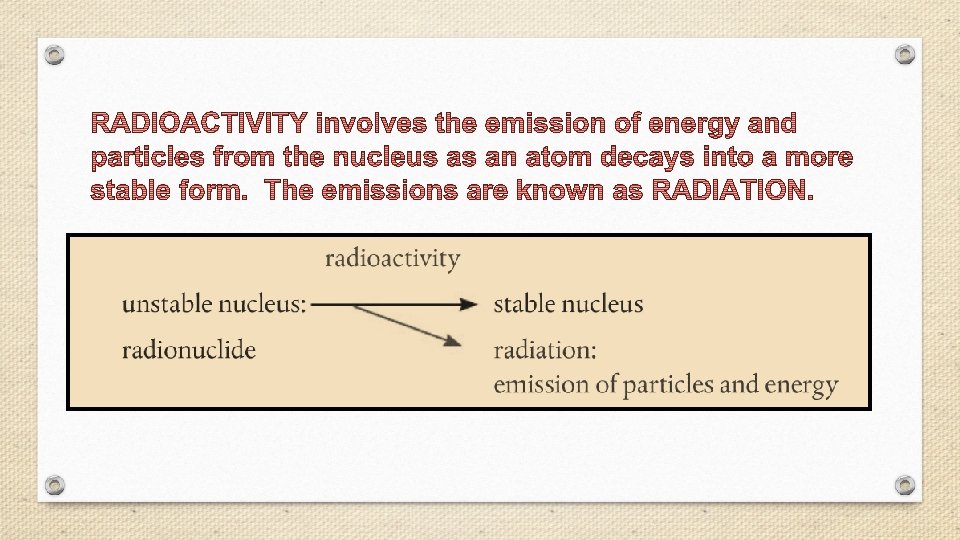
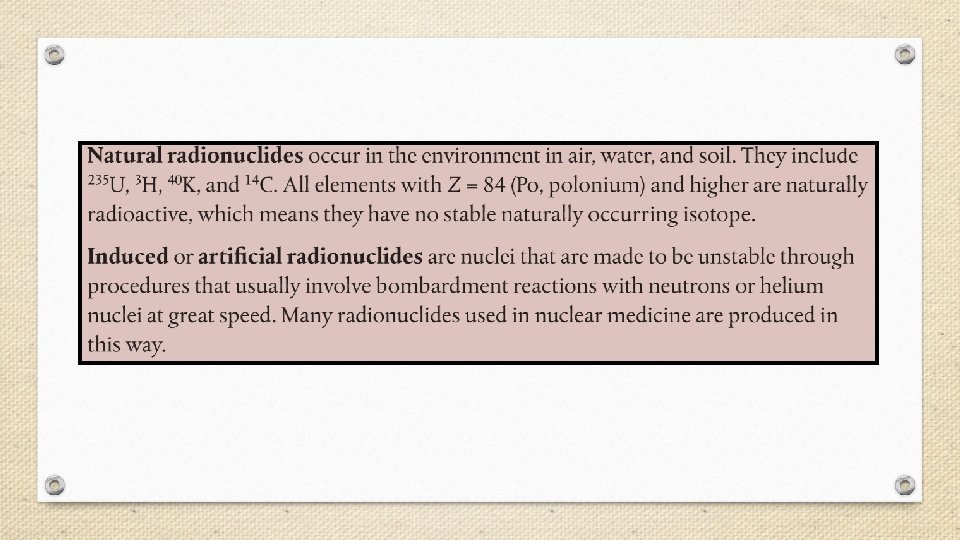
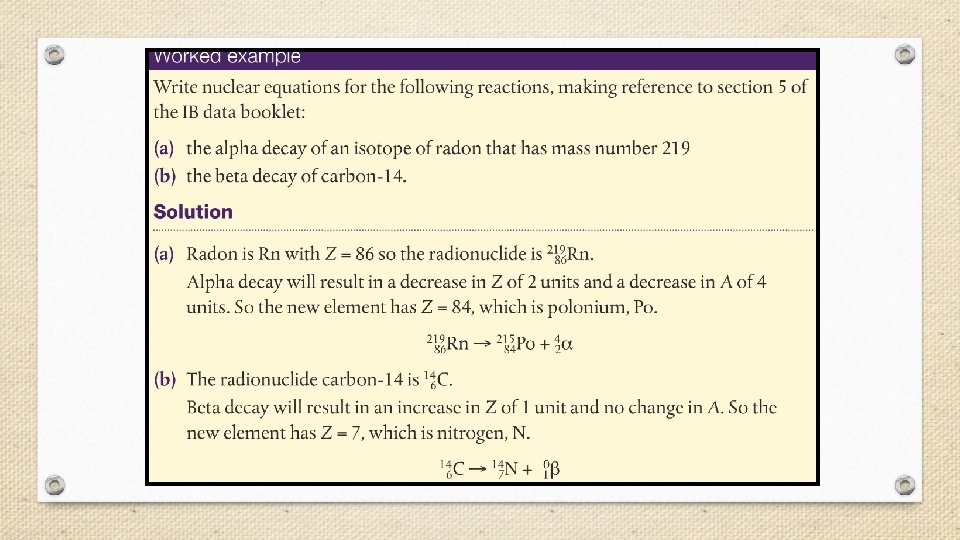
Nuclear Chemistry • Nuclear chemistry is the study of the properties and reactions of atomic nuclei. • Radioactivity- the spontaneous emission of radiation from a nucleus. • Every element in the periodic table has at least one radioactive isotope, or radioisotope. • Radioactivity is the result of atoms having unstable nuclei.

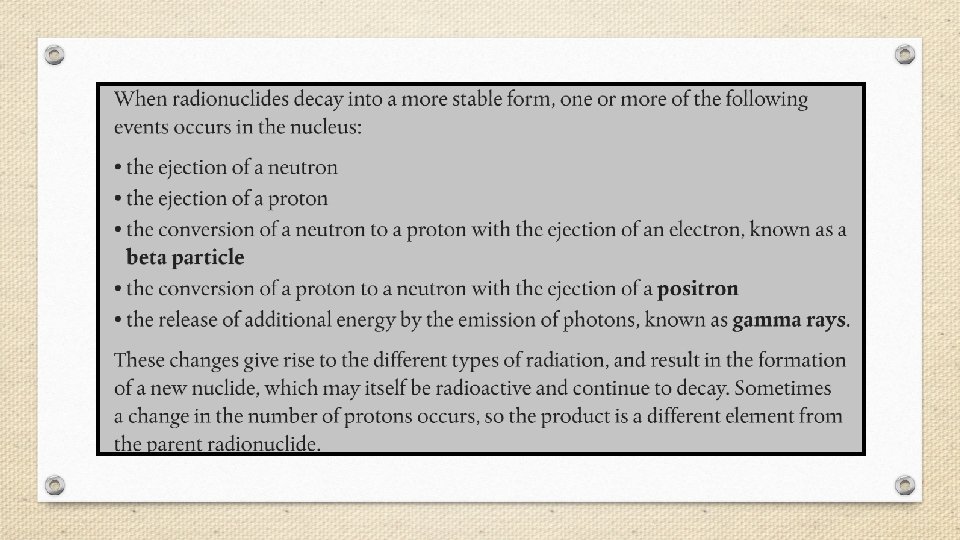
§ Radiation is emitted when an unstable radioactive nucleus, or radionuclide spontaneously changes into a more stable one. § The stability of an atom’s nucleus depends on the number and type of nuclear particles, called nucleons (nucleon is a general term for both protons and neutrons).




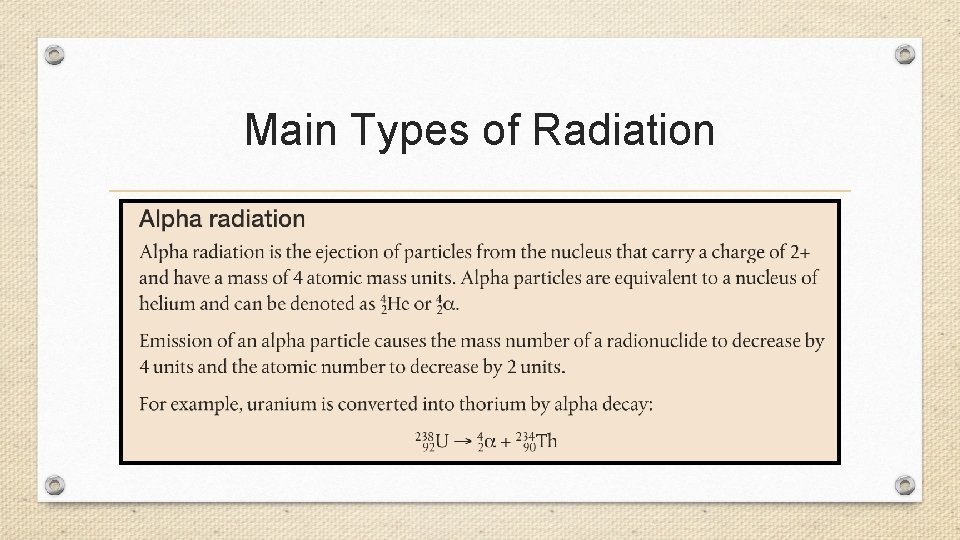
Main Types of Radiation



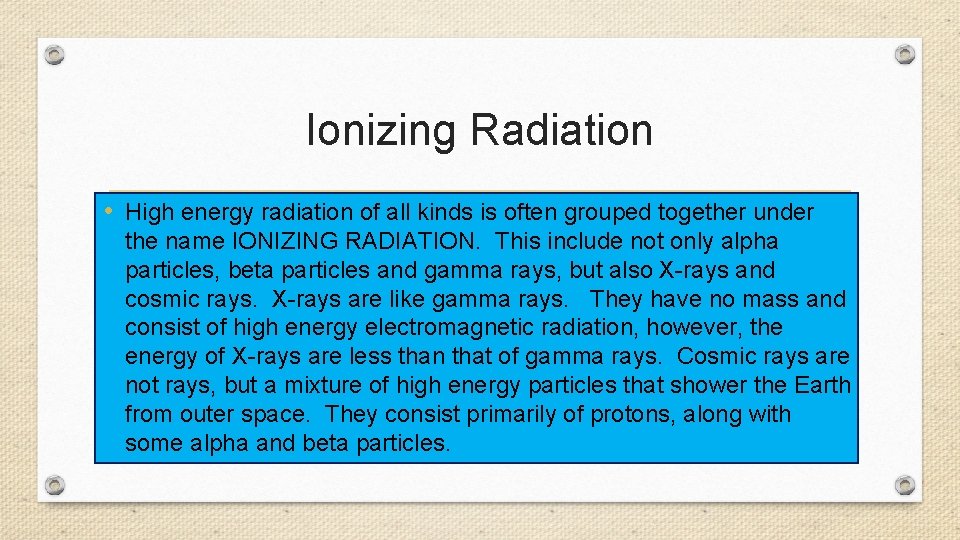
Ionizing Radiation • High energy radiation of all kinds is often grouped together under the name IONIZING RADIATION. This include not only alpha particles, beta particles and gamma rays, but also X-rays and cosmic rays. X-rays are like gamma rays. They have no mass and consist of high energy electromagnetic radiation, however, the energy of X-rays are less than that of gamma rays. Cosmic rays are not rays, but a mixture of high energy particles that shower the Earth from outer space. They consist primarily of protons, along with some alpha and beta particles.

The interaction of any kind of ionizing radiation with a molecule knocks out an electron, converting the atom or molecule into an extremely reactive ion (a highly unstable radical). This reactive ion can react with other molecules nearby, creating still other fragments that can in turn cause further reactions.


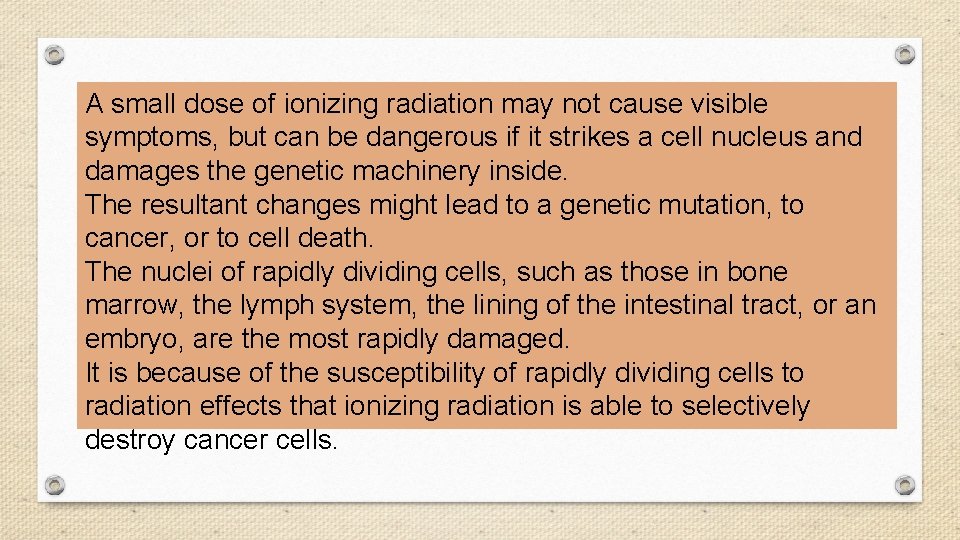
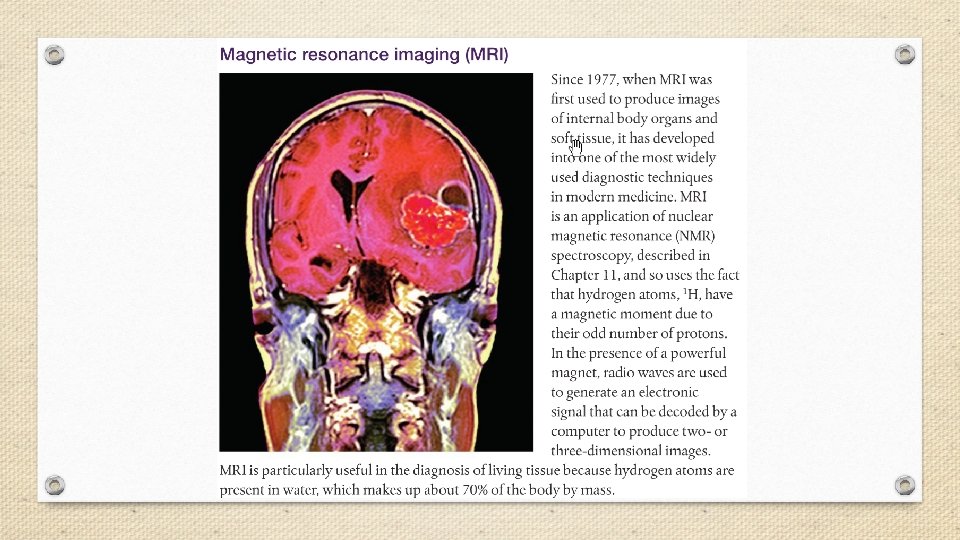
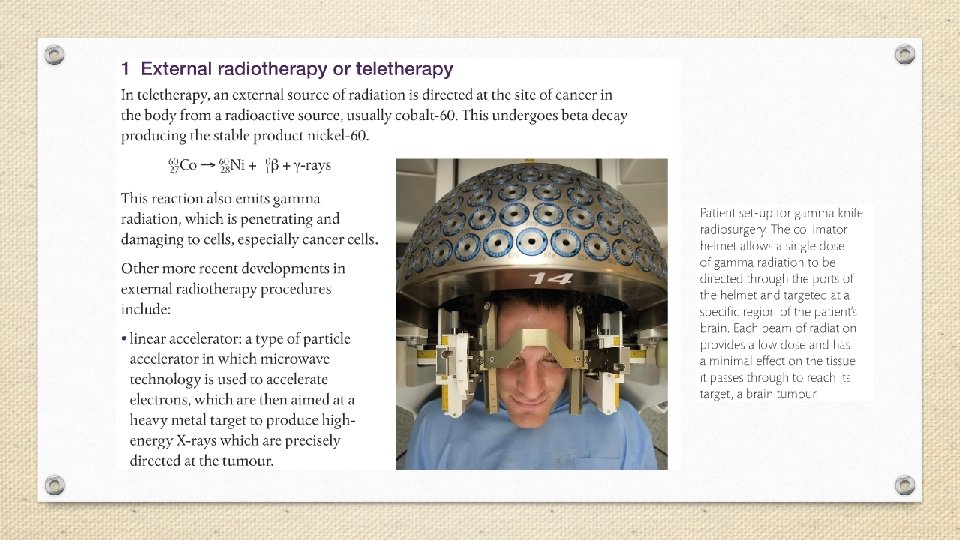
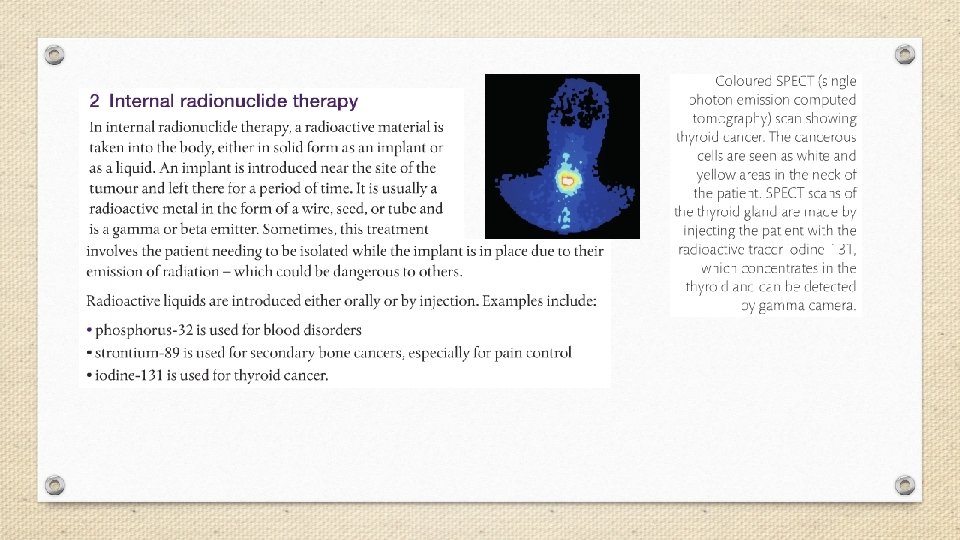
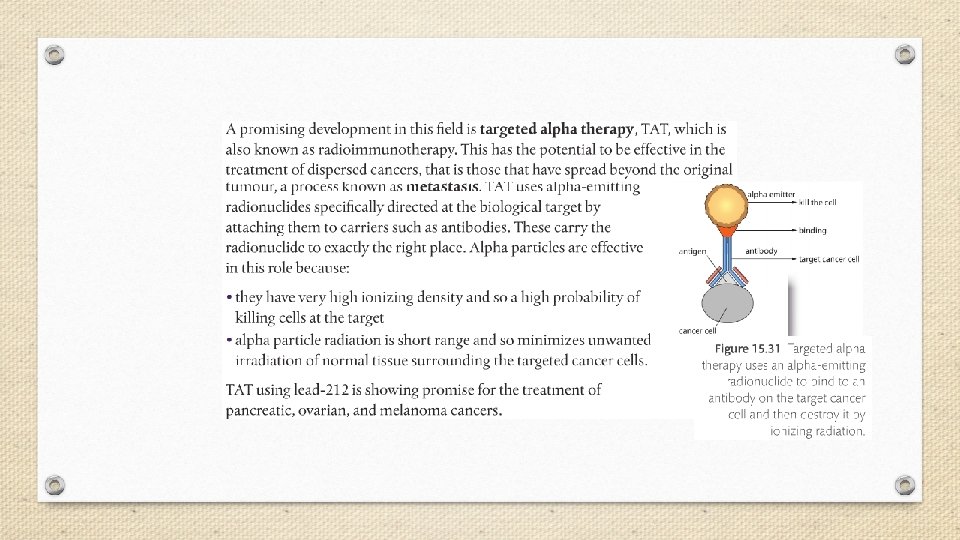
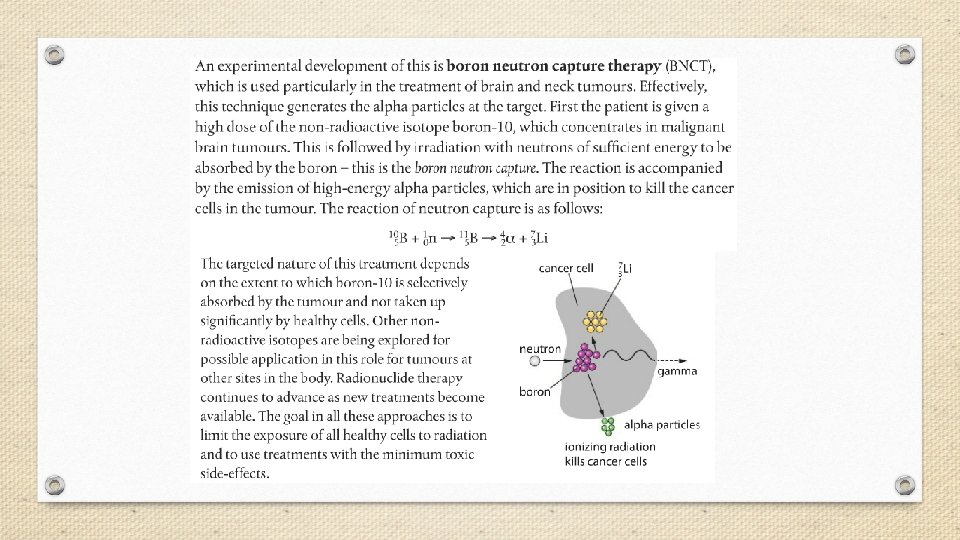
A small dose of ionizing radiation may not cause visible symptoms, but can be dangerous if it strikes a cell nucleus and damages the genetic machinery inside. The resultant changes might lead to a genetic mutation, to cancer, or to cell death. The nuclei of rapidly dividing cells, such as those in bone marrow, the lymph system, the lining of the intestinal tract, or an embryo, are the most rapidly damaged. It is because of the susceptibility of rapidly dividing cells to radiation effects that ionizing radiation is able to selectively destroy cancer cells.

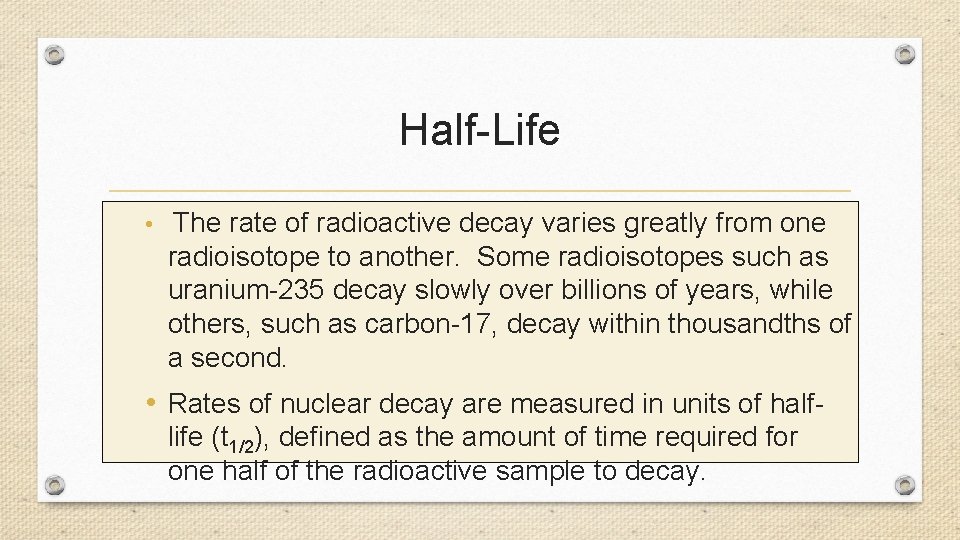
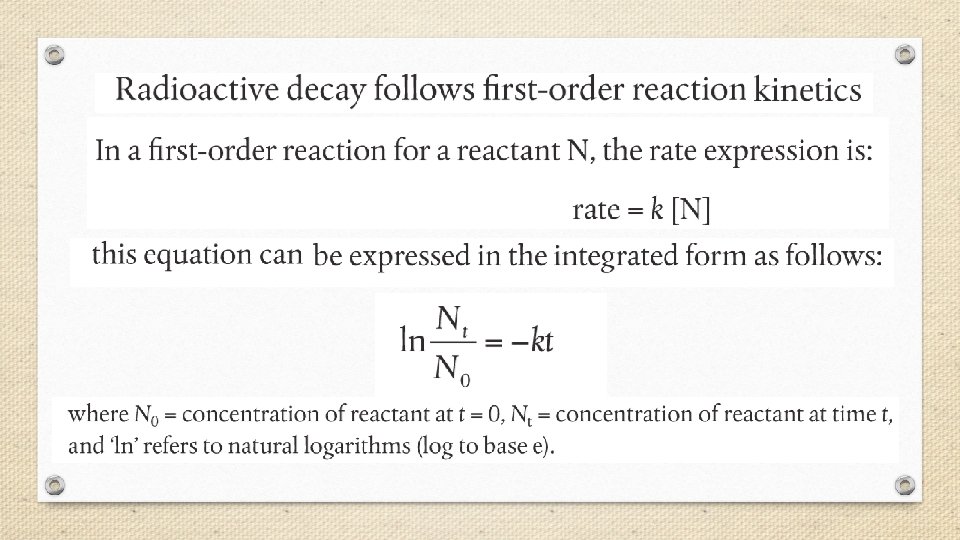
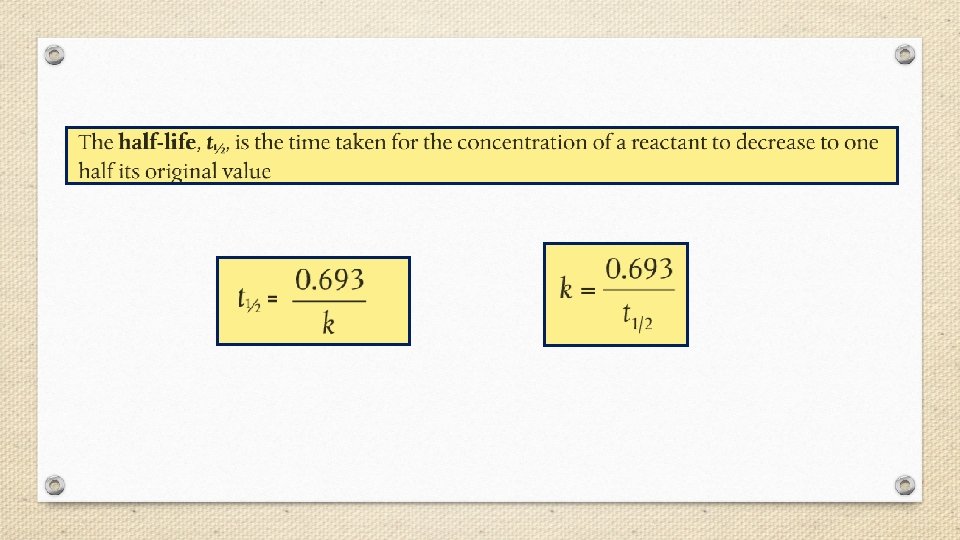
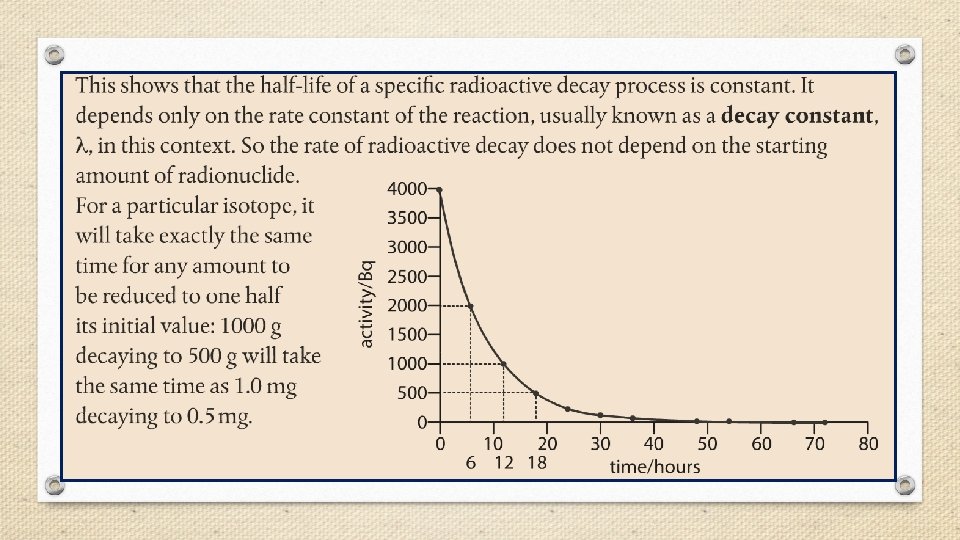
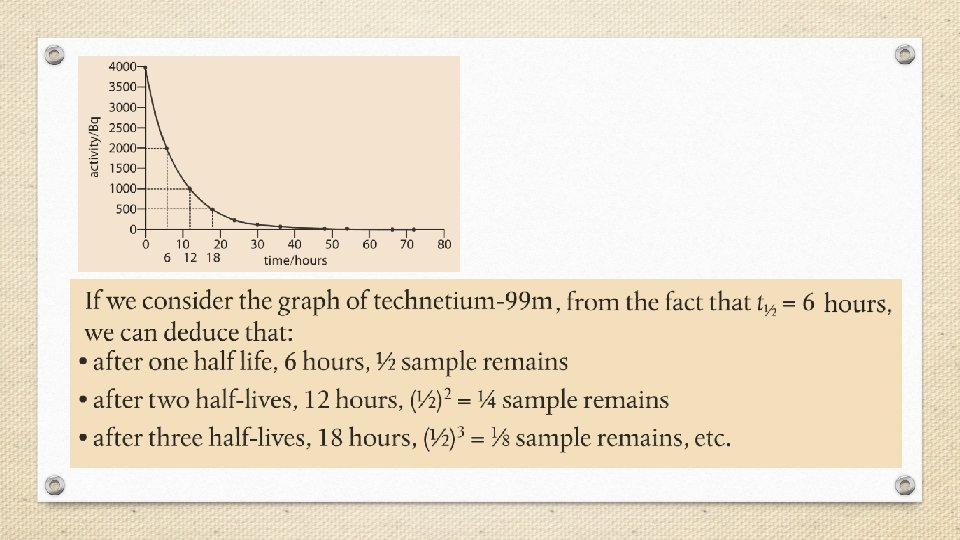
Half-Life • The rate of radioactive decay varies greatly from one radioisotope to another. Some radioisotopes such as uranium-235 decay slowly over billions of years, while others, such as carbon-17, decay within thousandths of a second. • Rates of nuclear decay are measured in units of halflife (t 1/2), defined as the amount of time required for one half of the radioactive sample to decay.






Fraction of original sample remaining = (1/2)n where n = the number of half-lives.

















 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave 25 m/s
25 m/s Nuclear chemistry
Nuclear chemistry Nuclear fusion in real life
Nuclear fusion in real life Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nitrogen-13 decay equation
Nitrogen-13 decay equation Applications of nuclear chemistry
Applications of nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Case study conclusion
Case study conclusion Nuclear pe exam
Nuclear pe exam Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan