Nuclear Chemistry rev 111908 Topic 12 Nuclear Chemistry

























- Slides: 86

Nuclear Chemistry rev. 11/19/08 Topic 12 Nuclear Chemistry Courtesy I. Markic

2 Nuclear Chemistry rev. 11/19/08 Nuclear Chemistry • The study of nuclear reactions and their uses in chemistry • When nuclei change spontaneously, spontaneously emitting radiation, they are said to be radioactive

3 Nuclear Chemistry rev. 11/19/08 Radioactive elements are used in medicine as… i. diagnostic tools ii. treatment for cancer iii. determine mechanisms for chemical reactions iv. trace movements of atoms in biological systems Radioactive elements are used in archeology… • To date important historical artifacts Uses of nuclear reactions… i. generate electricity ii. create WMDs of property and/or life

4 Radioactivity Nuclear Chemistry rev. 11/19/08 • Radioactive nuclei are called radionuclides • Atoms containing radioactive nuclei are called radioisotopes • Atoms with the same atomic number but different mass numbers are called isotopes • Different isotopes of an element are distinguished by their mass numbers • Different nuclei have different stabilities • The nuclear properties of an atom depend on the ratio of neutrons to protons in its nucleus

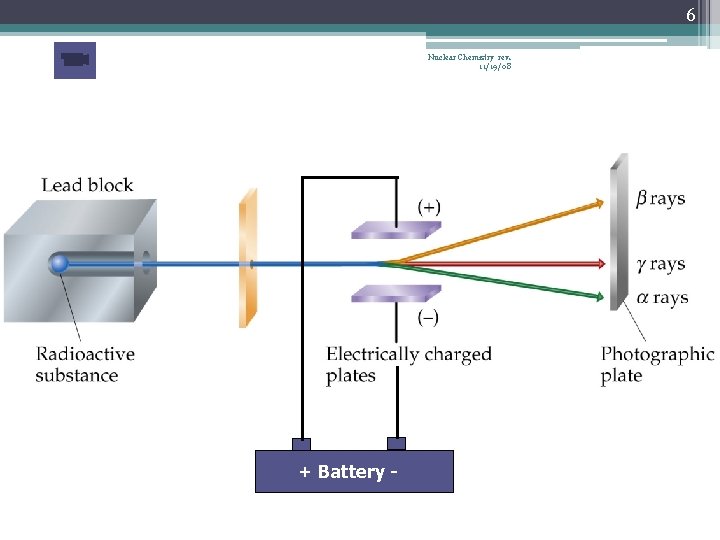
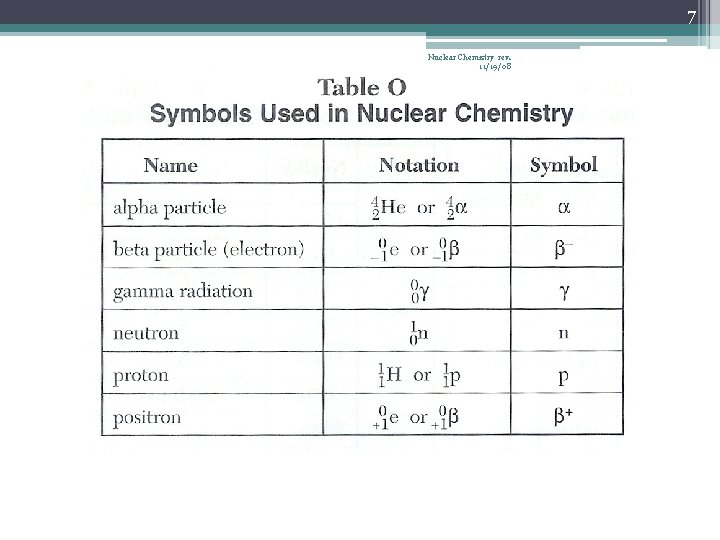
5 Nuclear Chemistry rev. 11/19/08 Types of Radioactive Decay • The most common types… 1. alpha decay α 2 He 4 2. beta decay β -1 e 0 or -1β 0 3. gamma radiation 0 0 or just

6 Nuclear Chemistry rev. 11/19/08 + Battery -

7 Nuclear Chemistry rev. 11/19/08

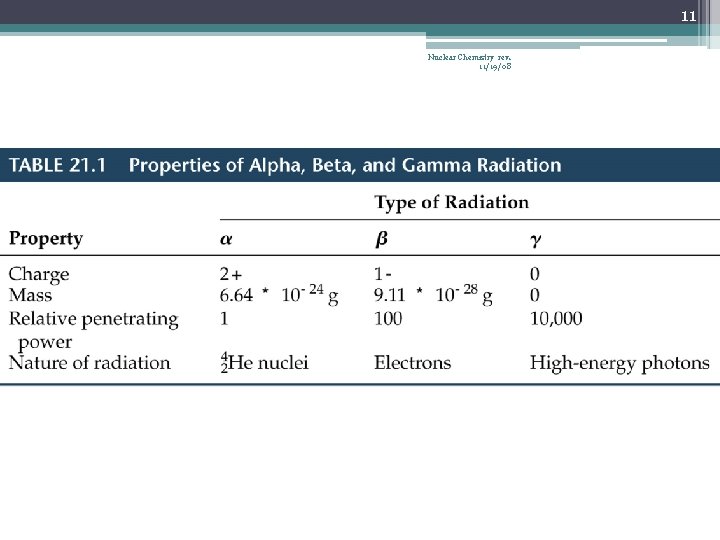
8 Nuclear Chemistry rev. 11/19/08 Alpha Radiation • α 2 He 4 • Contains 2 protons and 2 neutrons • Has heavy mass, does not travel far…| …stopped by a thin sheet of paper or skin • Dangerous if ingested 238 234 + He 4 α emission U Th 90 2 • 92 238 + He 4 242 U Pu α absorption 2 94 • 92

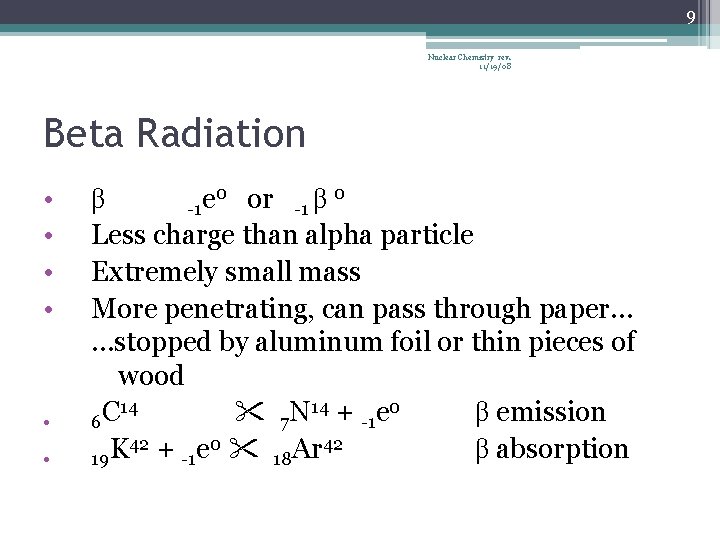
9 Nuclear Chemistry rev. 11/19/08 Beta Radiation • • • 0 or 0 β e β -1 -1 Less charge than alpha particle Extremely small mass More penetrating, can pass through paper… …stopped by aluminum foil or thin pieces of wood 14 14 + e 0 C N β emission 6 7 -1 42 + e 0 42 K Ar β absorption 19 -1 18

10 Nuclear Chemistry rev. 11/19/08 Gamma Radiation • • 0 0 or just No mass, no charge High energy photon Often emitted with α or β particles during decay Can pass though paper, wood, and our bodies Can mostly be stopped by several meters of concrete or several cm of lead 230 222 + 2 He 4 + 0 Th Ra 90 86 2 0

11 Nuclear Chemistry rev. 11/19/08

12 Nuclear Chemistry rev. 11/19/08

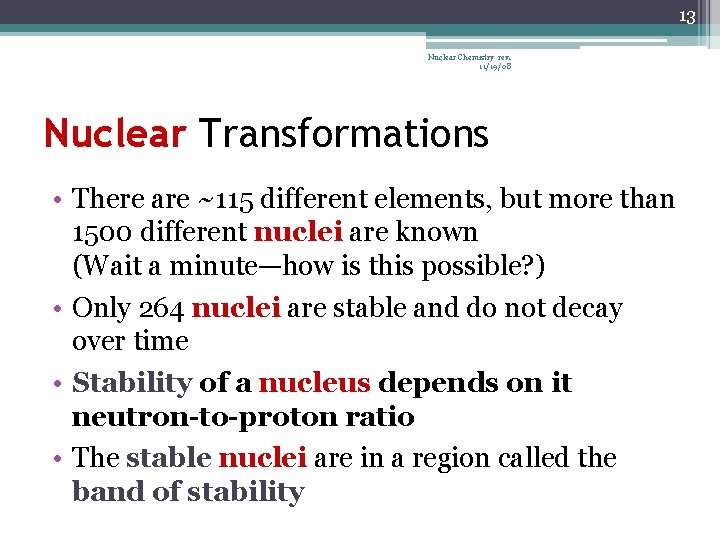
13 Nuclear Chemistry rev. 11/19/08 Nuclear Transformations • There are ~115 different elements, but more than 1500 different nuclei are known (Wait a minute—how is this possible? ) • Only 264 nuclei are stable and do not decay over time • Stability of a nucleus depends on it neutron-to-proton ratio • The stable nuclei are in a region called the band of stability

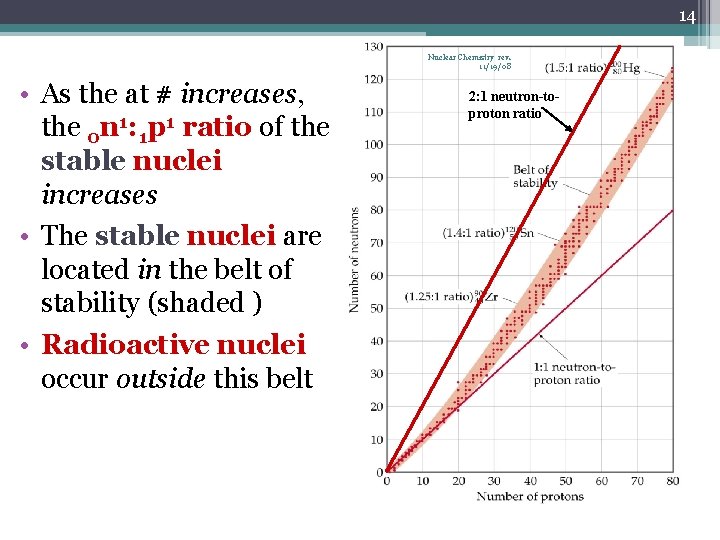
14 Nuclear Chemistry rev. 11/19/08 • As the at # increases, the 0 n 1: 1 p 1 ratio of the stable nuclei increases • The stable nuclei are located in the belt of stability (shaded ) • Radioactive nuclei occur outside this belt 2: 1 neutron-toproton ratio

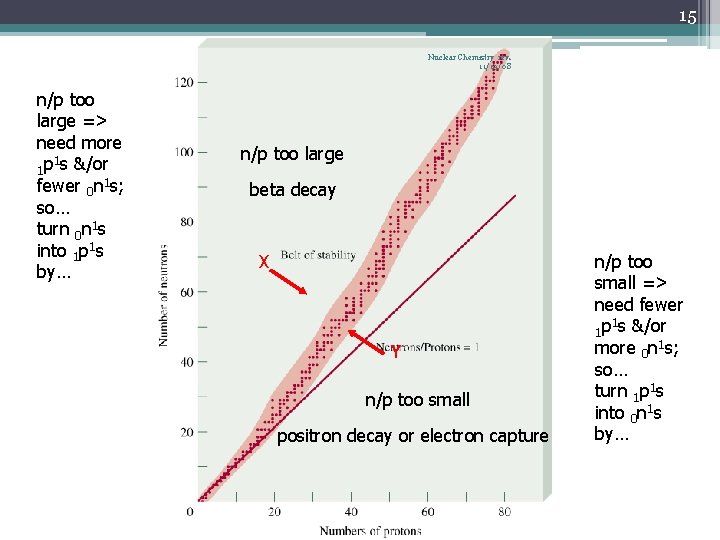
15 Nuclear Chemistry rev. 11/19/08 n/p too large => need more 1 1 p s &/or fewer 0 n 1 s; so… turn 0 n 1 s into 1 p 1 s by… n/p too large beta decay X Y n/p too small positron decay or electron capture n/p too small => need fewer 1 1 p s &/or more 0 n 1 s; so… turn 1 p 1 s into 0 n 1 s by…

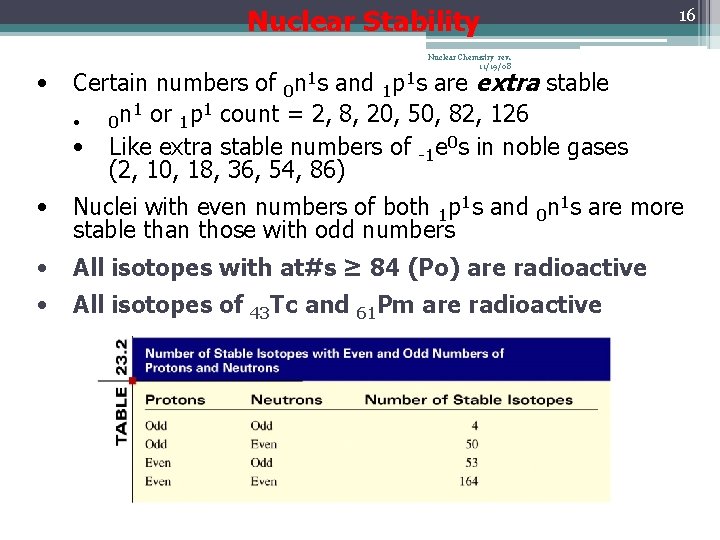
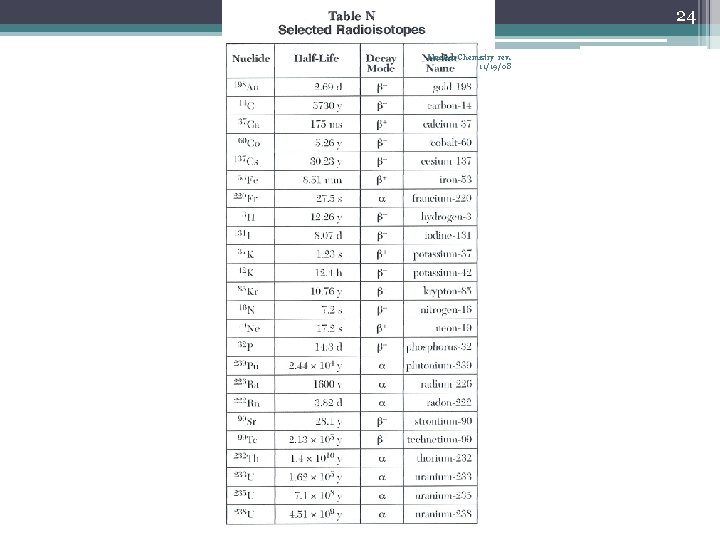
Nuclear Stability 16 Nuclear Chemistry rev. 11/19/08 • Certain numbers of 0 n 1 s and 1 p 1 s are extra stable 1 1 0 n or 1 p count = 2, 8, 20, 50, 82, 126 • • Like extra stable numbers of -1 e 0 s in noble gases (2, 10, 18, 36, 54, 86) • Nuclei with even numbers of both 1 p 1 s and 0 n 1 s are more stable than those with odd numbers • All isotopes with at#s ≥ 84 (Po) are radioactive • All isotopes of 43 Tc and 61 Pm are radioactive

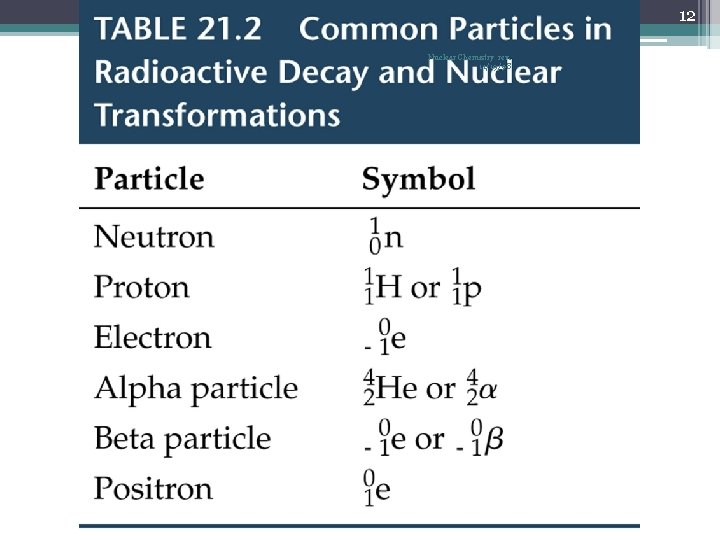
17 Nuclear Chemistry rev. 11/19/08 Transmutation Reactions • Transmutation - the conversion of an atom of one element into an atom of a different element • Can occur by radioactive decay • Can occur when particles (1 p 1 -s, 0 n 1 -s, or 2 He 4 -s) bombard the nucleus of an atom 15 + H 1 C 12 + He 4 1 6 2 • 7 N 238 + n 1 239 + β 0 U Np 0 93 -1 • 92 9 + He 4 C 12 + n 1 Be 2 6 0 • 4

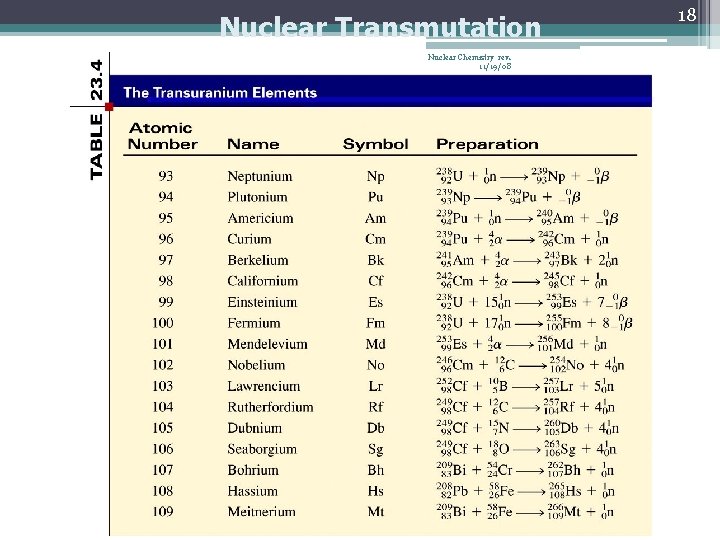
Nuclear Transmutation Nuclear Chemistry rev. 11/19/08 18

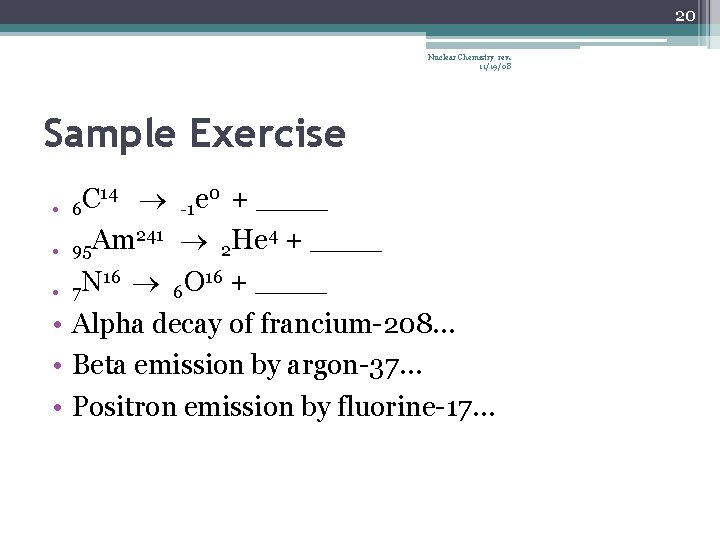
19 Nuclear Chemistry rev. 11/19/08 Sample Exercise • What product is formed when radium-226 undergoes alpha decay? • What element undergoes alpha decay to form lead-208? 27 decays by beta emission to form… Mg • 12 3 __ + e 0 H -1 • 1 9 Be 9 + __ Li 4 • 3

20 Nuclear Chemistry rev. 11/19/08 Sample Exercise 14 0 + ____ C e -1 • 6 241 He 4 + ____ Am 2 • 95 16 O 16 + ____ N 6 • 7 • Alpha decay of francium-208… • Beta emission by argon-37… • Positron emission by fluorine-17…

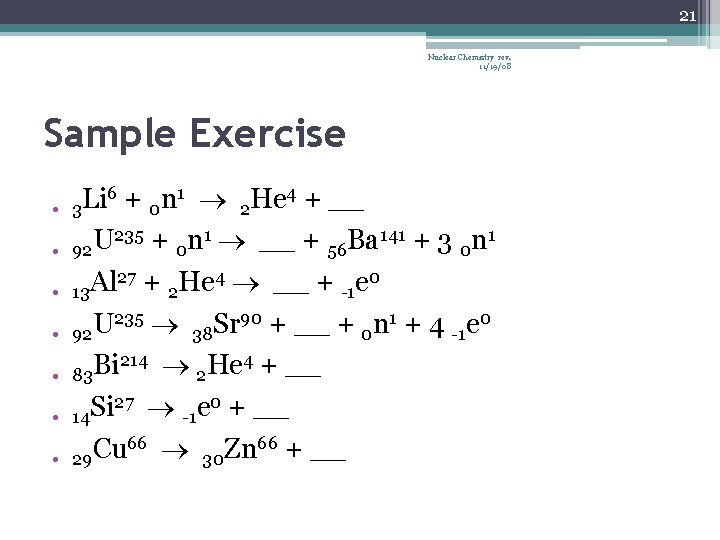
21 Nuclear Chemistry rev. 11/19/08 Sample Exercise • • 6 + n 1 He 4 + __ Li 3 0 2 235 + n 1 __ + Ba 141 + 3 n 1 U 92 0 56 0 27 + He 4 __ + e 0 Al 13 2 -1 235 90 + __ + n 1 + 4 e 0 92 U 38 Sr 0 -1 214 He 4 + __ Bi 83 2 27 e 0 + __ Si 14 -1 66 + __ Cu Zn 29 30

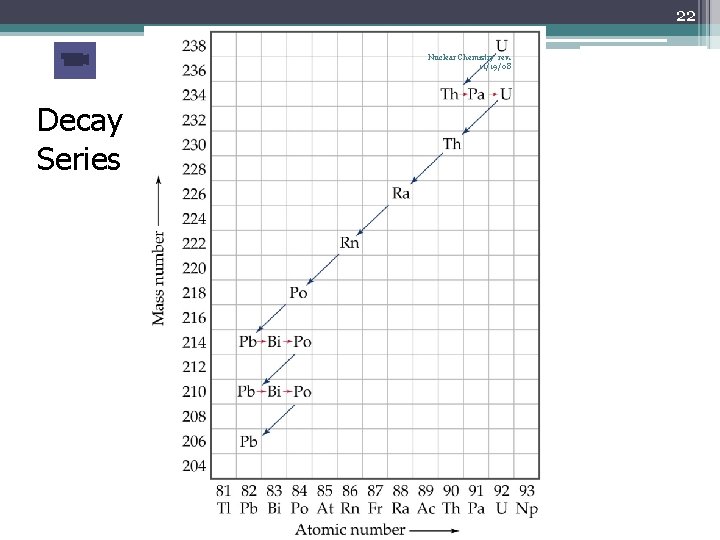
22 Nuclear Chemistry rev. 11/19/08 Decay Series

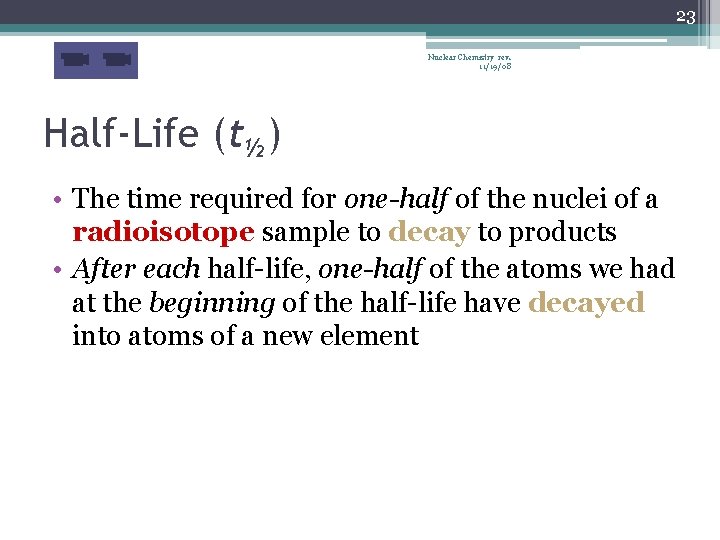
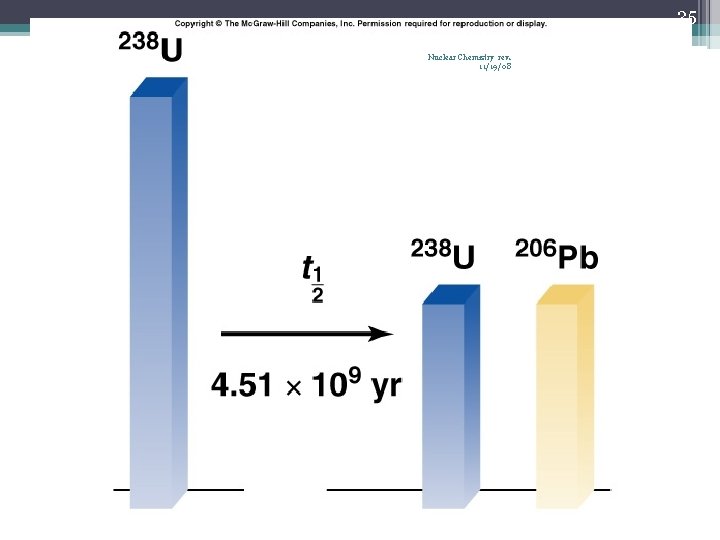
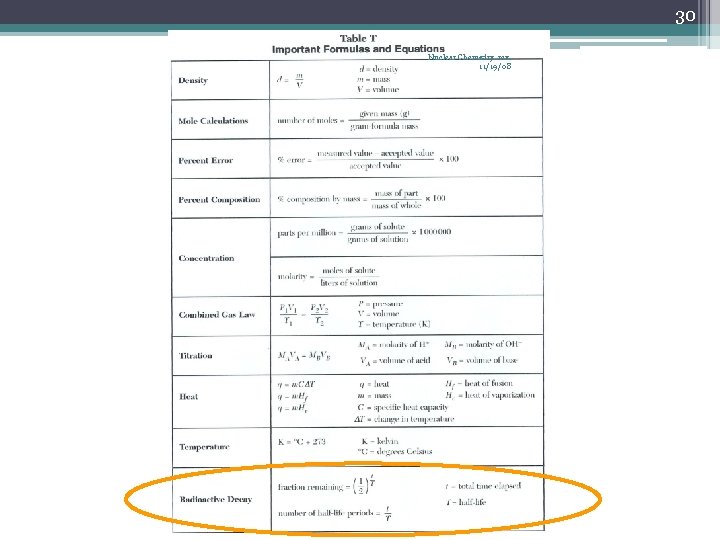
23 Nuclear Chemistry rev. 11/19/08 Half-Life (t½) • The time required for one-half of the nuclei of a radioisotope sample to decay to products • After each half-life, one-half of the atoms we had at the beginning of the half-life have decayed into atoms of a new element

24 Nuclear Chemistry rev. 11/19/08

25 Nuclear Chemistry rev. 11/19/08 Fig. 23. 3

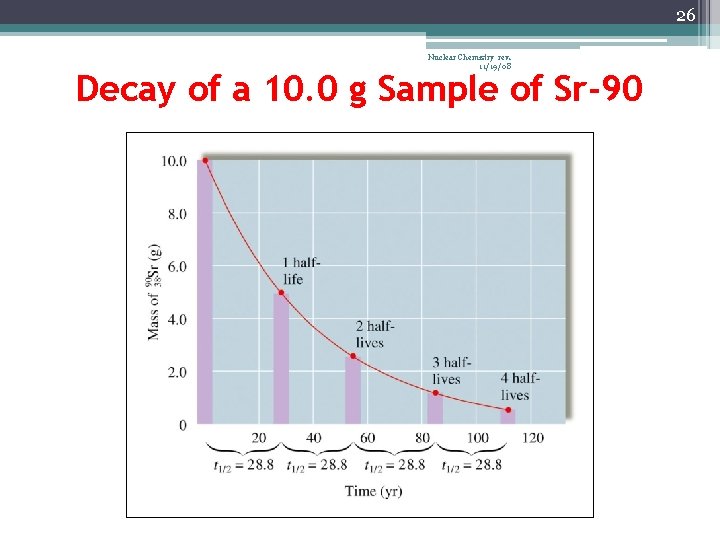
26 Nuclear Chemistry rev. 11/19/08 Decay of a 10. 0 g Sample of Sr-90

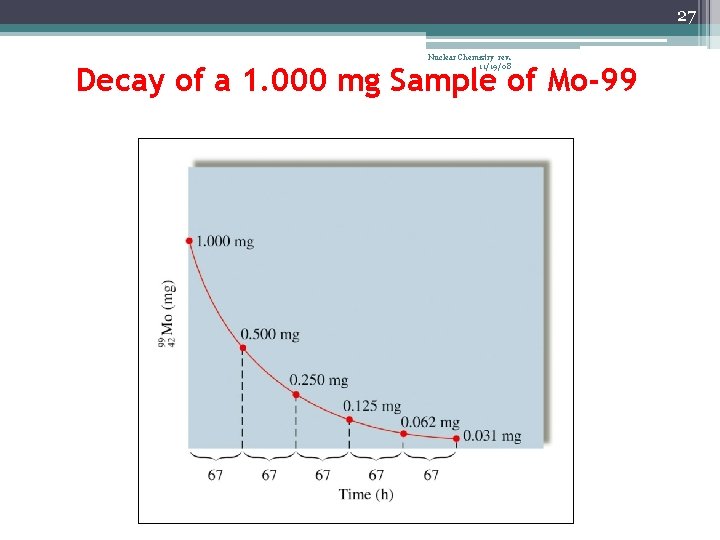
27 Nuclear Chemistry rev. 11/19/08 Decay of a 1. 000 mg Sample of Mo-99

28 Nuclear Chemistry rev. 11/19/08 Brigham Young Researcher Scott Woodward Taking a Bone Sample

29 A Dendrochronologist Cutting a Section from a Dead Tree Nuclear Chemistry rev. 11/19/08

30 Nuclear Chemistry rev. 11/19/08

31 Nuclear Chemistry rev. 11/19/08 Sample Exercise • Po-214 has a relatively short t½ of 164 sec. How many seconds would it take for 8. 0 g of this isotope to decay to 0. 25 g? • How many days does it take for 16 g of Pa-107 to decay to 1. 0 g? t½ of Pa-107 is 17 days.

32 Nuclear Chemistry rev. 11/19/08 Sample Exercise • In 5. 49 seconds, 1. 20 g of Ar-35 decay to leave only 0. 15 g. What is t½ of Ar-35? • Na-24 has a t½ of 15 hours. How much Na-24 remains in an 18. 0 g sample after 60 hours?

33 Sample Exercise Nuclear Chemistry rev. 11/19/08 • In 6. 20 h, a 100. g sample of Ag-112 decays to 25. 0 g. What is t½ of Ag-112? • Mn-56 is a β emitter with t½ of 2. 6 h. What is the mass of Mn-56 in a 1. 0 mg sample of the isotope at the end of 10. 4 h? • C-14 emits beta radiation and decays with t½ of 5730 years. Assume you start with 2. 00 x 10 -12 g C-14. ▫ How long is 3 t½s? ▫ How many grams of the isotope remain at the end of 3 t½s?

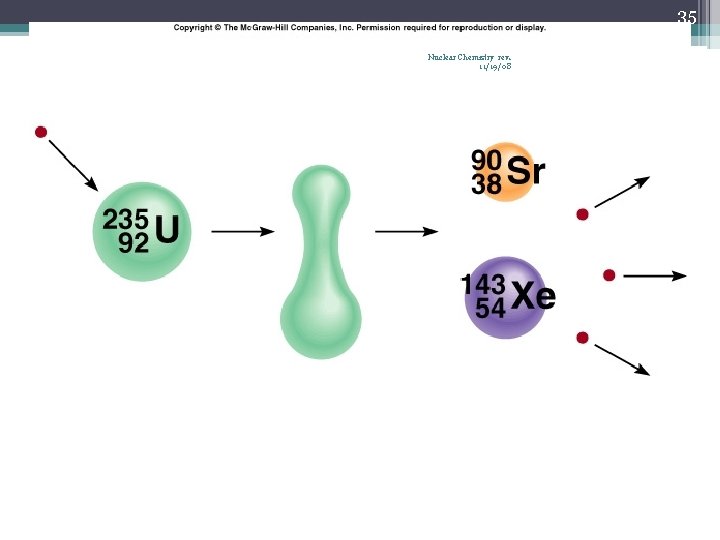
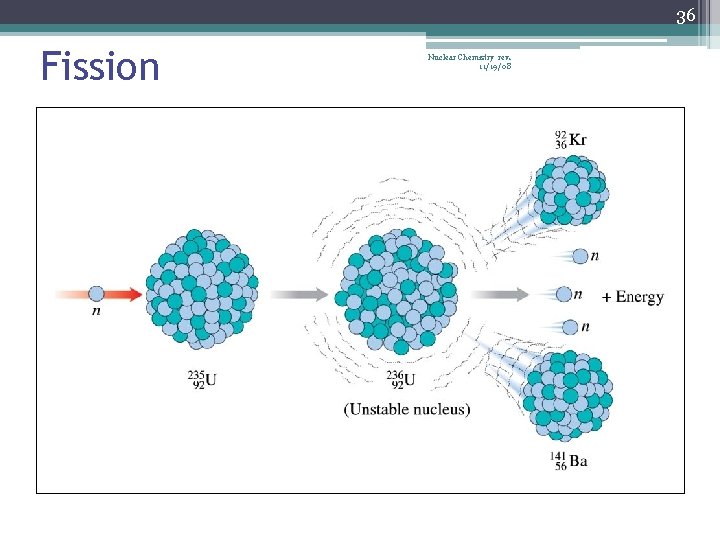
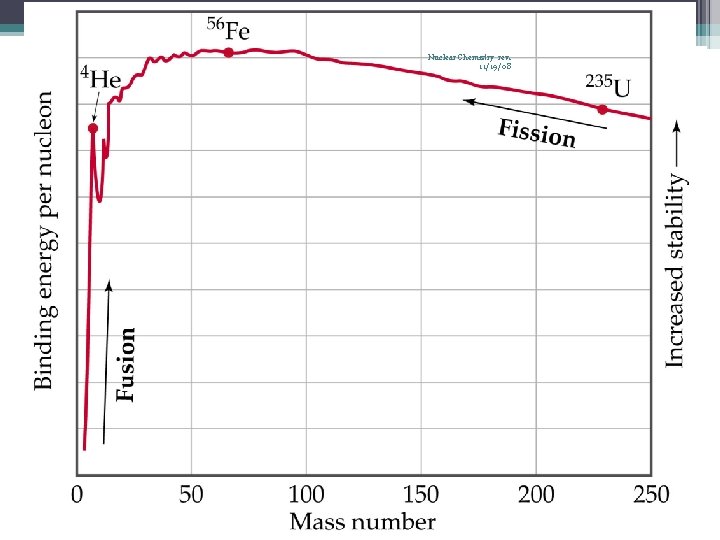
34 Nuclear Fission Nuclear Chemistry rev. 11/19/08 • Occurs when the nuclei of certain isotopes are bombarded with neutrons… • …and the nucleus splits into smaller fragments • In a chain reaction, some of the neutrons produced react with other fissionable atoms, producing more neutrons with will react with still more fissionable atoms • Can release enormous amounts of energy (atomic bombs, nuclear reactors) • Exothermic processes • Undergoes fission when struck by a slow-moving neutron • U-235 or Pu-239

35 Nuclear Chemistry rev. 11/19/08 Fig. 23. 6

36 Fission Nuclear Chemistry rev. 11/19/08

37 Nuclear Chemistry rev. 11/19/08 Uranium Oxide (refined uranium)

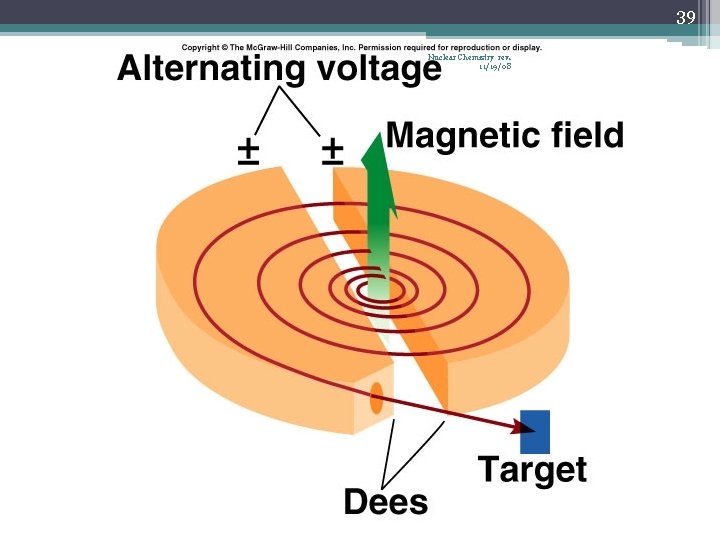
38 Nuclear Chemistry rev. 11/19/08 A PHYSICIST WORKS WITH A SMALL CYCLOTRON AT THE UNIVERSITY OF CALIFORNIA BERKLEY

39 Nuclear Chemistry rev. 11/19/08

40 An Aerial View of the Fermilab High Energy Particle Accelerator Nuclear Chemistry rev. 11/19/08

41 Nuclear Chemistry rev. 11/19/08 The Accelerator Tunnel at Fermilab

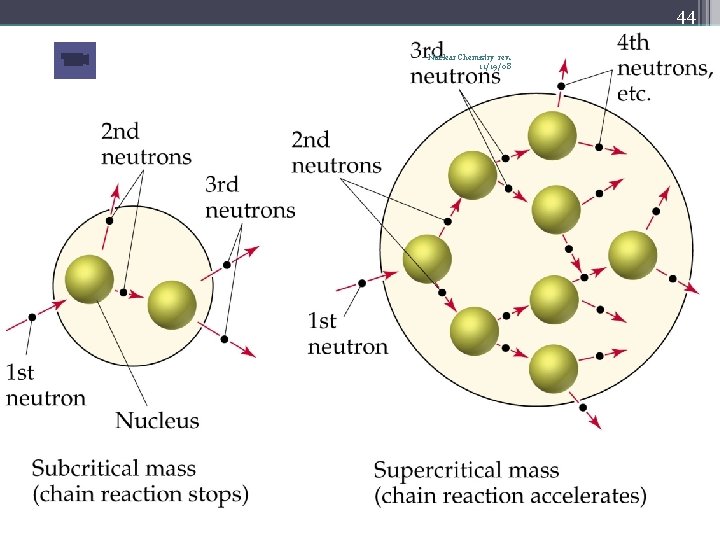
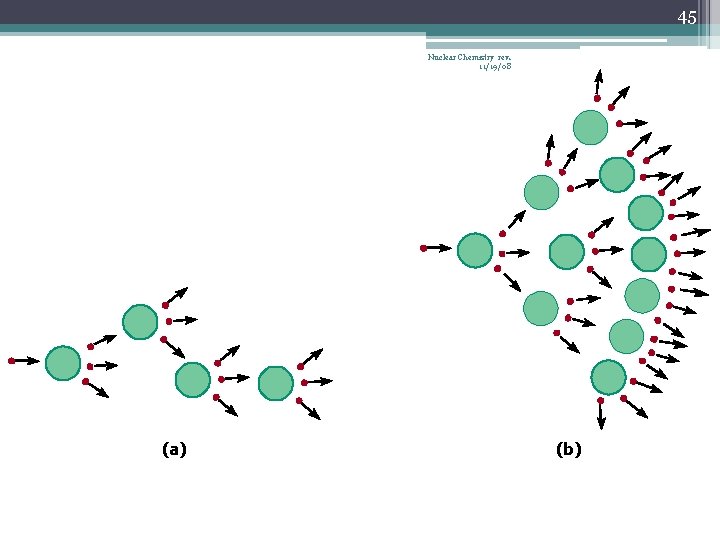
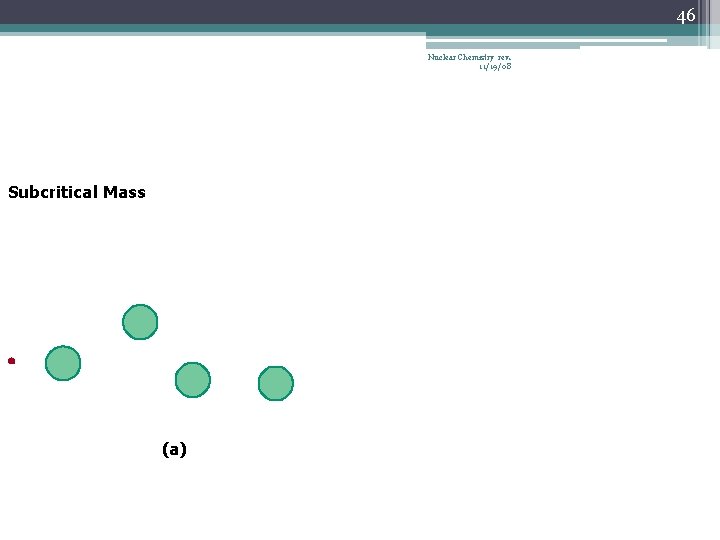
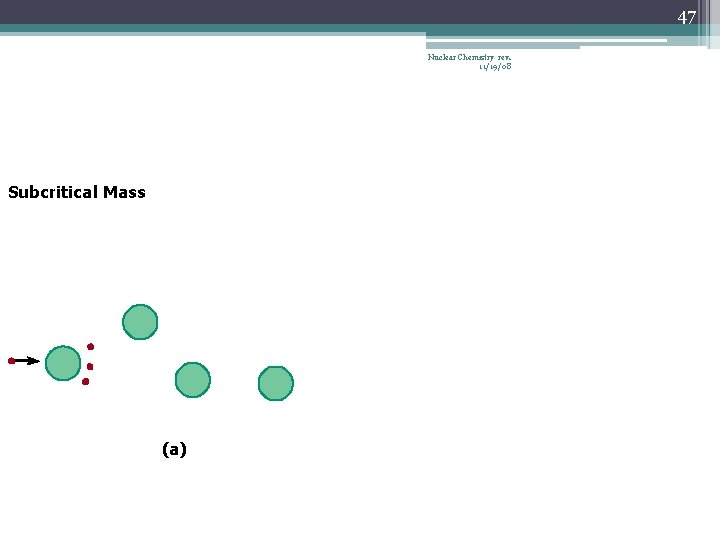
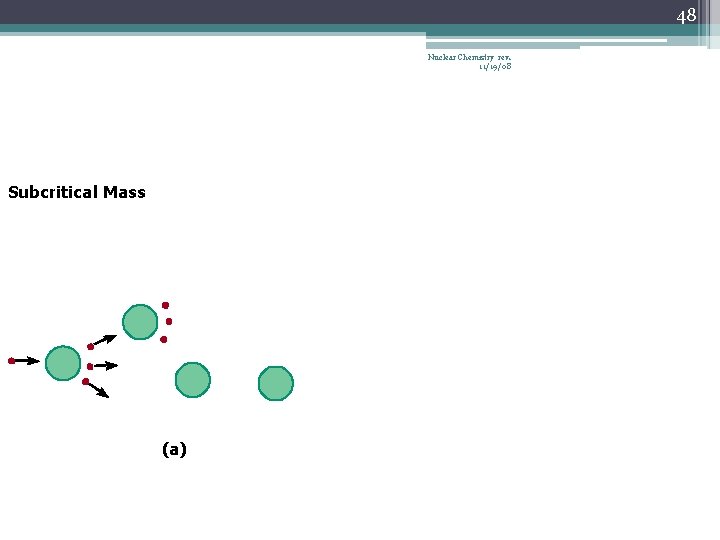
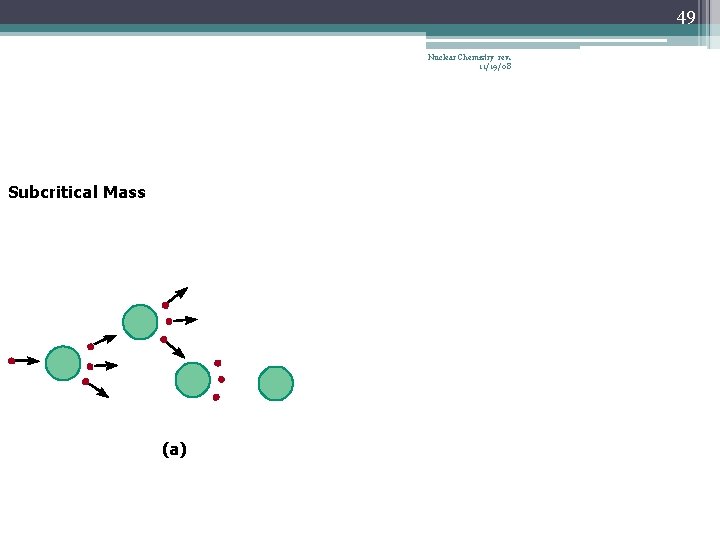
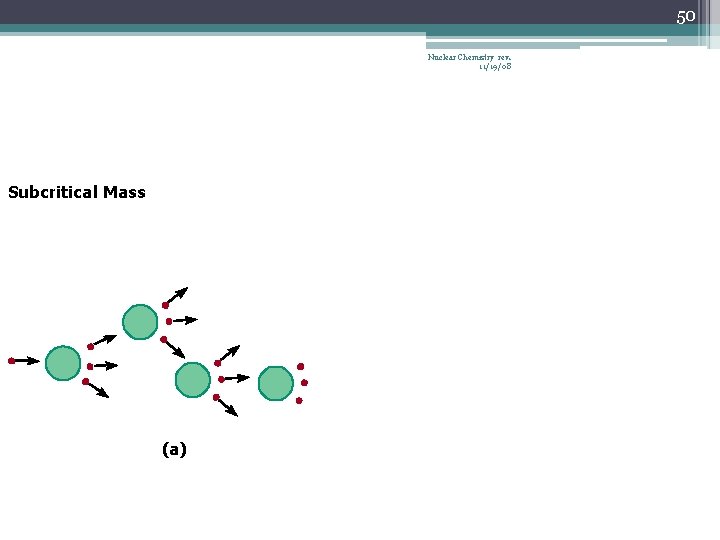
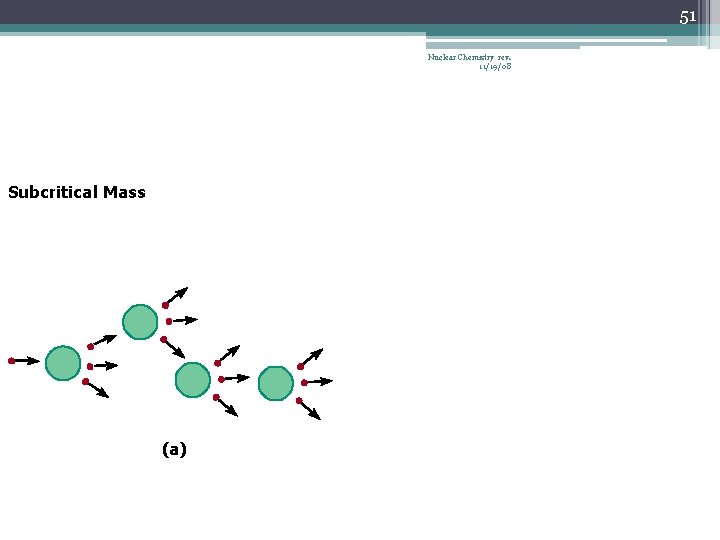
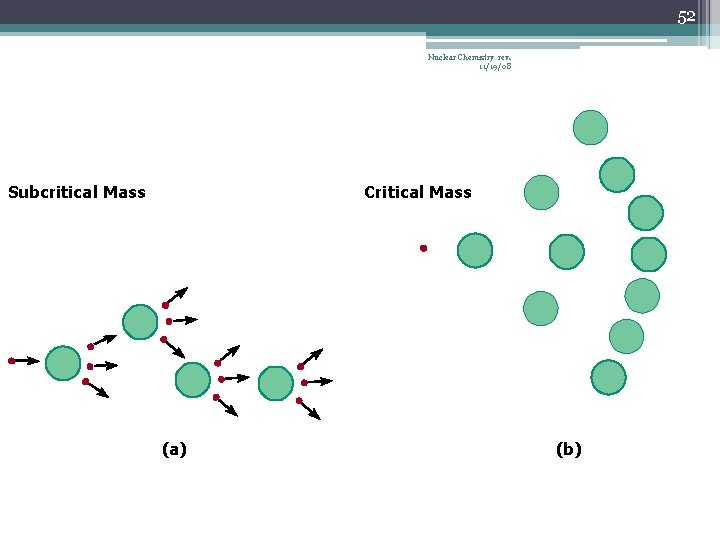
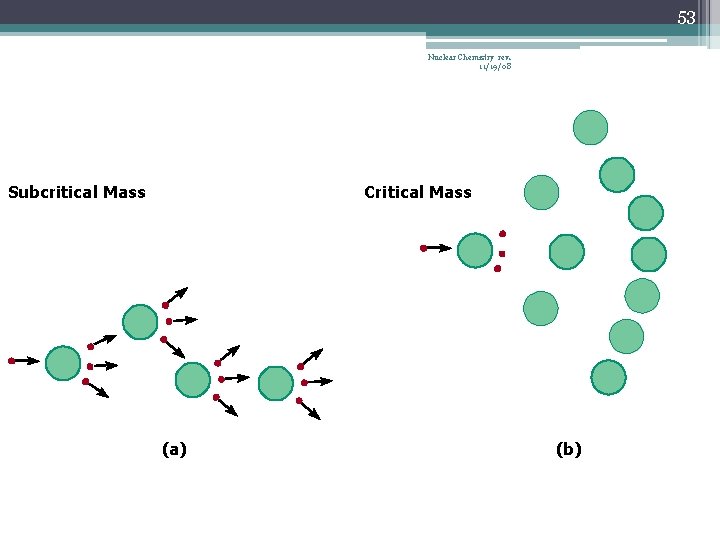
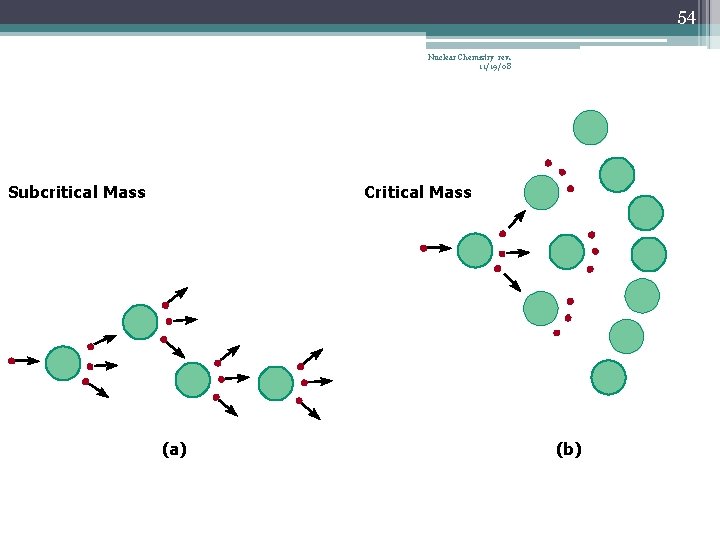
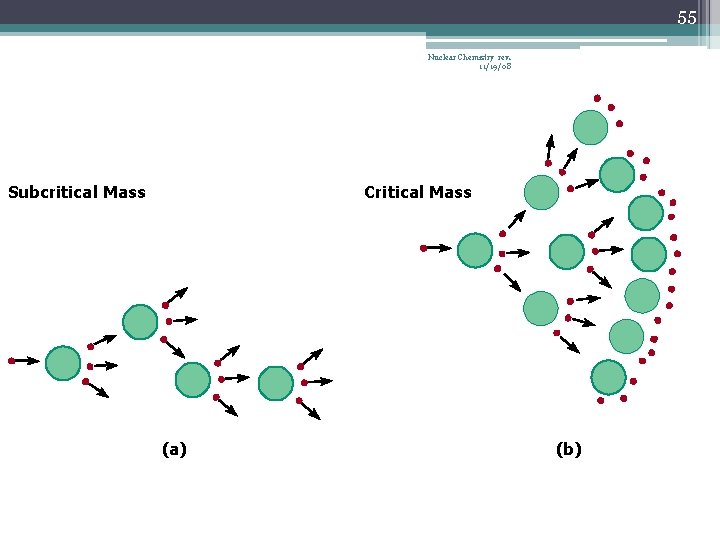
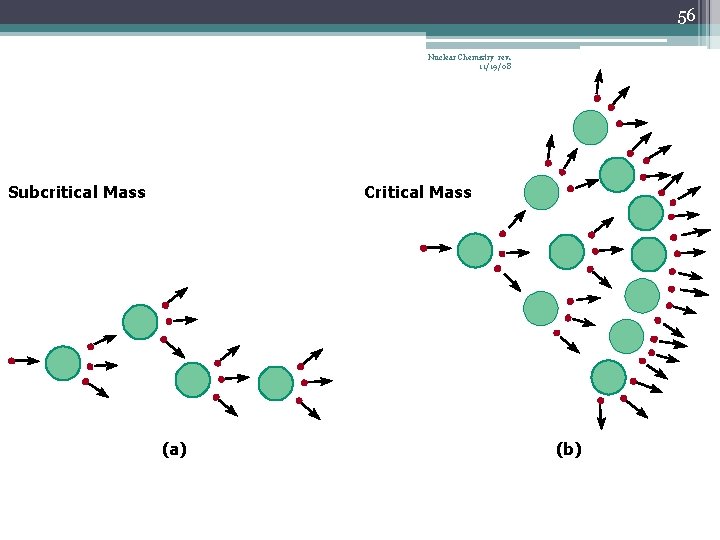
42 Chain Reaction Nuclear Chemistry rev. 11/19/08 • If the fission process continues and the energy is unchecked, the result is a violent explosion • The sample of fissionable material must have a minimum ‘critical mass’ – if not, 0 n 1 -s escape from the sample before they strike another nucleus and cause additional fission • The chain Rx stops if enough 0 n 1 -s are lost

43 Nuclear Chemistry rev. 11/19/08 Critical Mass • The amount of fissionable material large enough to maintain the chain reaction with a constant rate of fission • When critical mass is present, only one 0 n 1 from each fission is subsequently effective in producing another fission

44 Nuclear Chemistry rev. 11/19/08

45 Nuclear Chemistry rev. 11/19/08 (a) (b)

46 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

47 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

48 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

49 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

50 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

51 Nuclear Chemistry rev. 11/19/08 Subcritical Mass (a)

52 Nuclear Chemistry rev. 11/19/08 Subcritical Mass Critical Mass (a) (b)

53 Nuclear Chemistry rev. 11/19/08 Subcritical Mass Critical Mass (a) (b)

54 Nuclear Chemistry rev. 11/19/08 Subcritical Mass Critical Mass (a) (b)

55 Nuclear Chemistry rev. 11/19/08 Subcritical Mass Critical Mass (a) (b)

56 Nuclear Chemistry rev. 11/19/08 Subcritical Mass Critical Mass (a) (b)

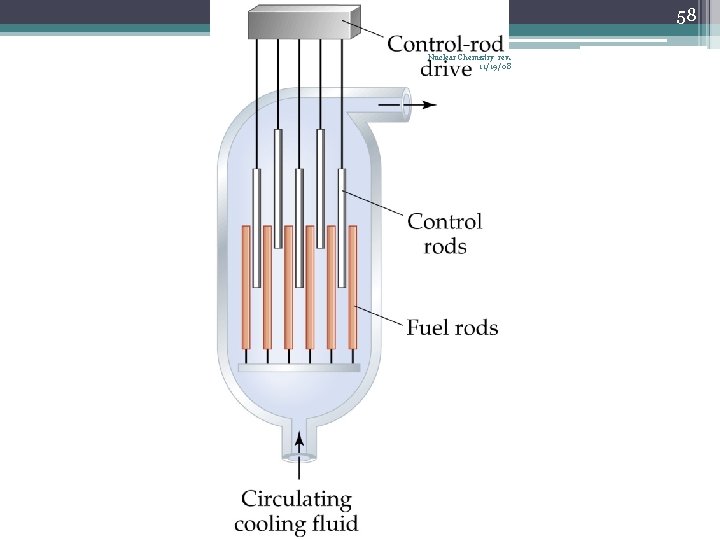
57 Nuclear Chemistry rev. 11/19/08

58 Nuclear Chemistry rev. 11/19/08

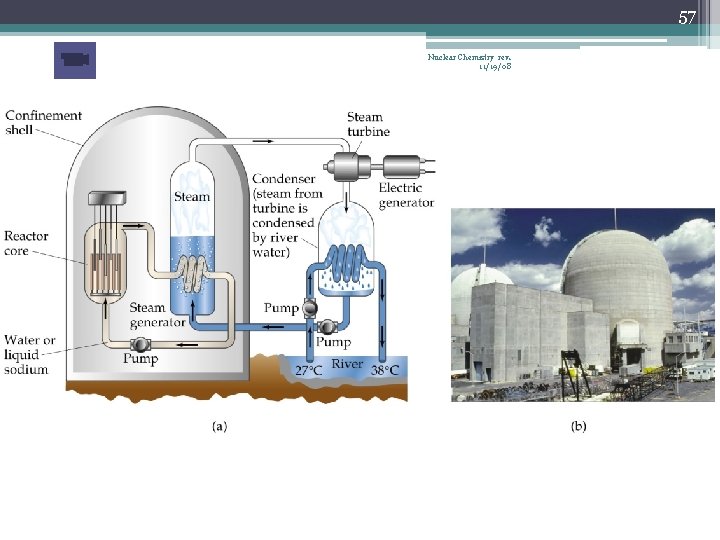
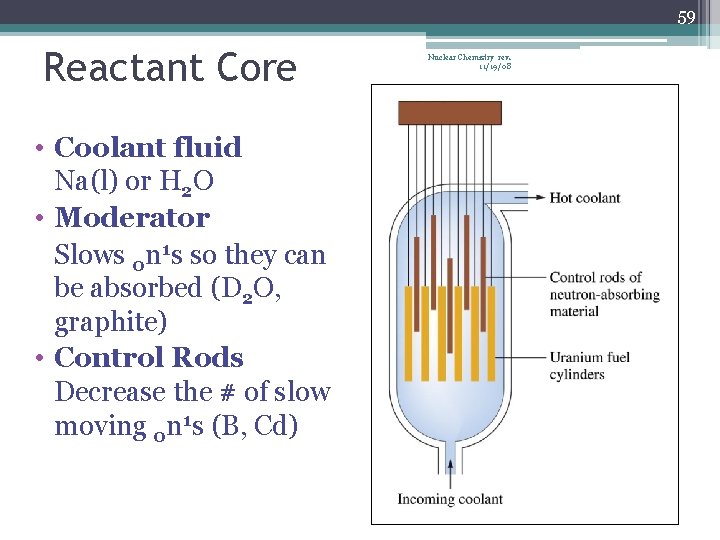
59 Reactant Core • Coolant fluid Na(l) or H 2 O • Moderator Slows 0 n 1 s so they can be absorbed (D 2 O, graphite) • Control Rods Decrease the # of slow moving 0 n 1 s (B, Cd) Nuclear Chemistry rev. 11/19/08

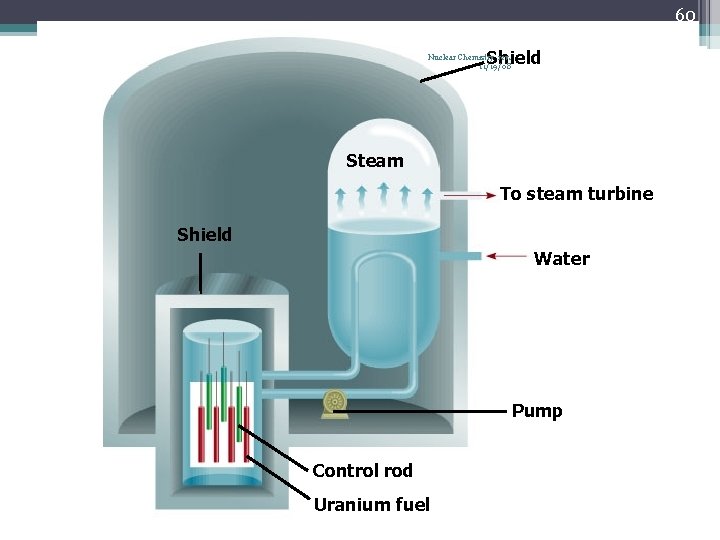
60 Shield Nuclear Chemistry rev. 11/19/08 Steam To steam turbine Shield Water Pump Control rod Uranium fuel

61 Nuclear Waste Nuclear Chemistry rev. 11/19/08 • Fuel rods are one major source of waste • Fuel rods are made of fissionable isotope U-235 or Pu-239 • Isotope-depleted (spent) fuel rods are removed and replaced with new fuel rods • All nuclear power plants have holding tanks, or “swimming pools, ” for spent fuel rods • Water cools the spent rods, and acts as a radiation shield to reduce the radiation levels (12 m deep) • The rods continue to produce heat for years after their removal from the core • Spent fuel rods spend a decade or more in a holding tank

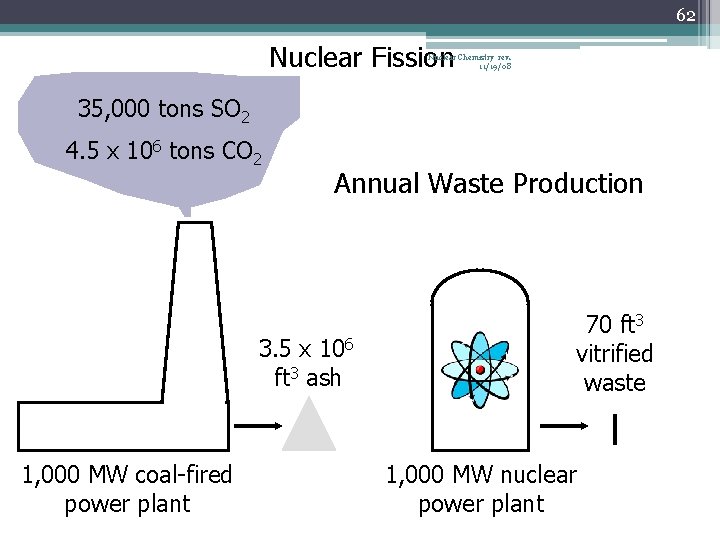
62 Nuclear Fission Nuclear Chemistry rev. 11/19/08 35, 000 tons SO 2 4. 5 x 106 tons CO 2 Annual Waste Production 3. 5 x 106 ft 3 ash 1, 000 MW coal-fired power plant 70 ft 3 vitrified waste 1, 000 MW nuclear power plant

63 Nuclear Chemistry rev. 11/19/08 Fig. 23. p 961 bottom

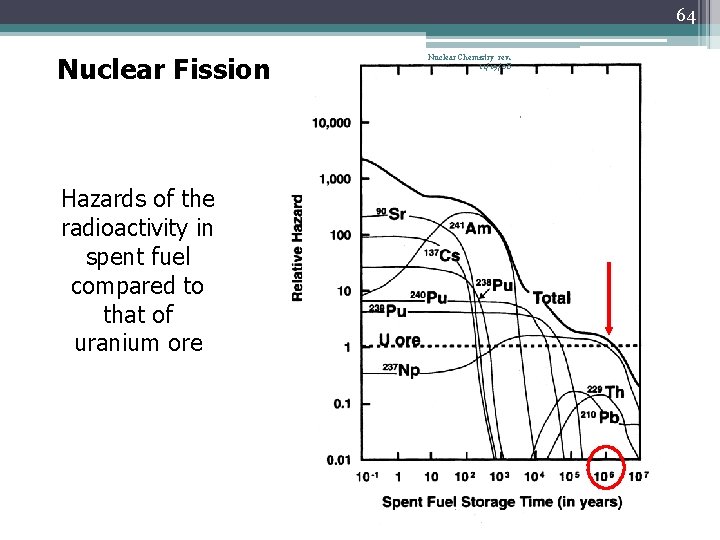
64 Nuclear Fission Hazards of the radioactivity in spent fuel compared to that of uranium ore Nuclear Chemistry rev. 11/19/08

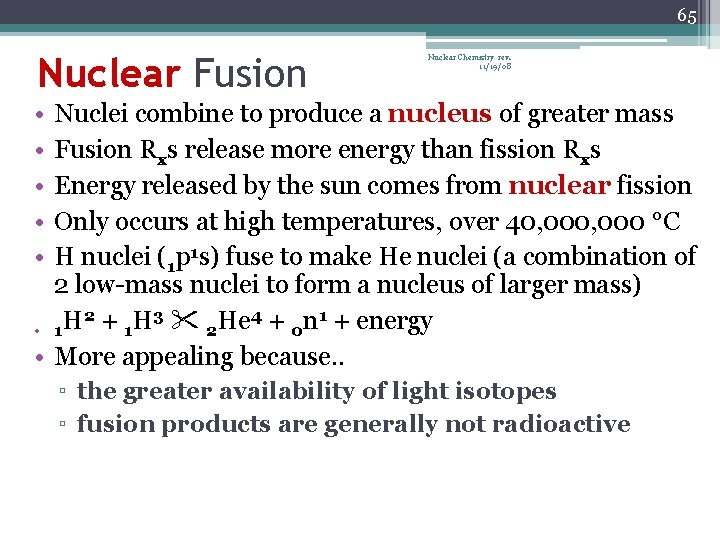
65 Nuclear Fusion Nuclear Chemistry rev. 11/19/08 • • • Nuclei combine to produce a nucleus of greater mass Fusion Rxs release more energy than fission Rxs Energy released by the sun comes from nuclear fission Only occurs at high temperatures, over 40, 000 °C H nuclei (1 p 1 s) fuse to make He nuclei (a combination of 2 low-mass nuclei to form a nucleus of larger mass) 2 3 4 1 • 1 H + 1 H 2 He + 0 n + energy • More appealing because. . ▫ the greater availability of light isotopes ▫ fusion products are generally not radioactive

66 Nuclear Chemistry rev. 11/19/08

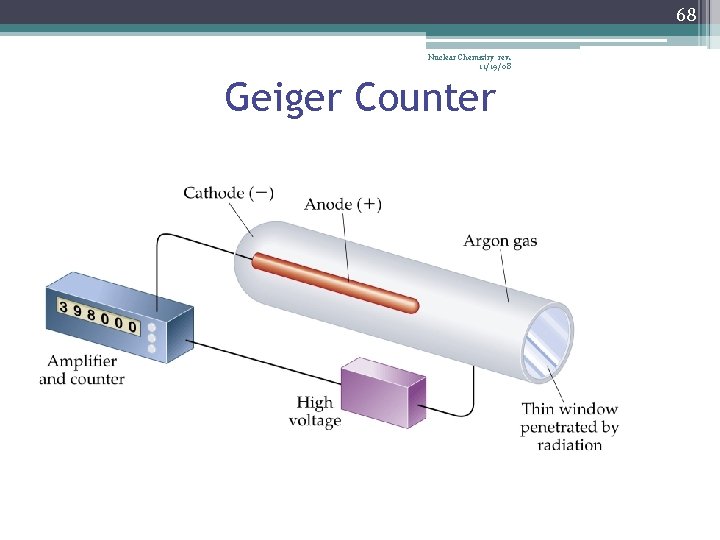
67 Nuclear Chemistry rev. 11/19/08 Detecting Radiation • Radiation cannot be heard, felt, nor smelled… • …but, radiation produces ions, which can be detected by instruments… • … such as Geiger counters, scintillation counters, and film badges

68 Nuclear Chemistry rev. 11/19/08 Geiger Counter

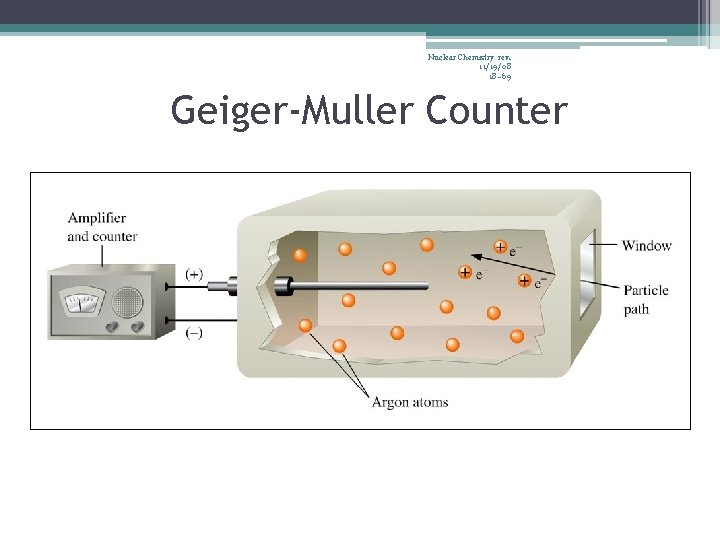
Nuclear Chemistry rev. 11/19/08 18– 69 Geiger-Muller Counter

70 Nuclear Chemistry rev. 11/19/08 Geiger-Muller Counter

71 Nuclear Chemistry rev. 11/19/08 Scintillator

72 Nuclear Chemistry rev. 11/19/08 Film Badge

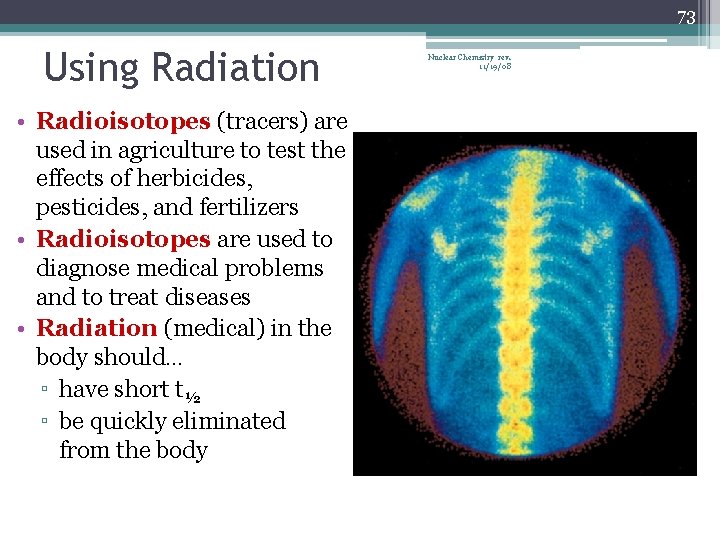
73 Using Radiation • Radioisotopes (tracers) are used in agriculture to test the effects of herbicides, pesticides, and fertilizers • Radioisotopes are used to diagnose medical problems and to treat diseases • Radiation (medical) in the body should… ▫ have short t½ ▫ be quickly eliminated from the body Nuclear Chemistry rev. 11/19/08

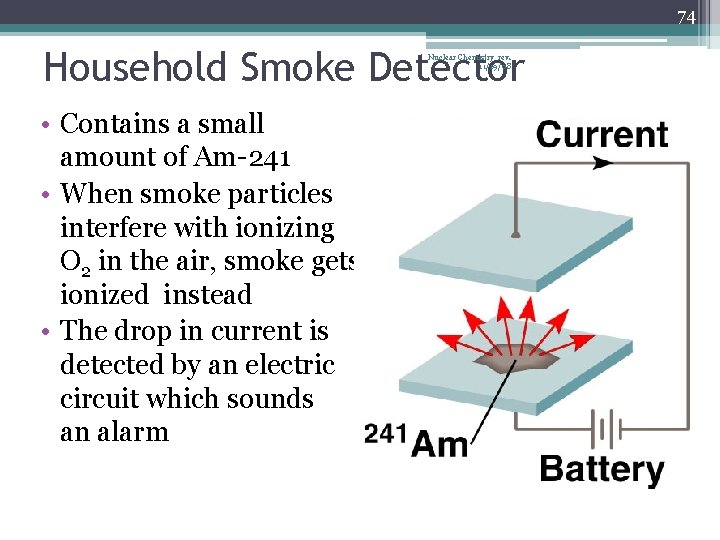
74 Household Smoke Detector Nuclear Chemistry rev. 11/19/08 • Contains a small amount of Am-241 • When smoke particles interfere with ionizing O 2 in the air, smoke gets ionized instead • The drop in current is detected by an electric circuit which sounds an alarm

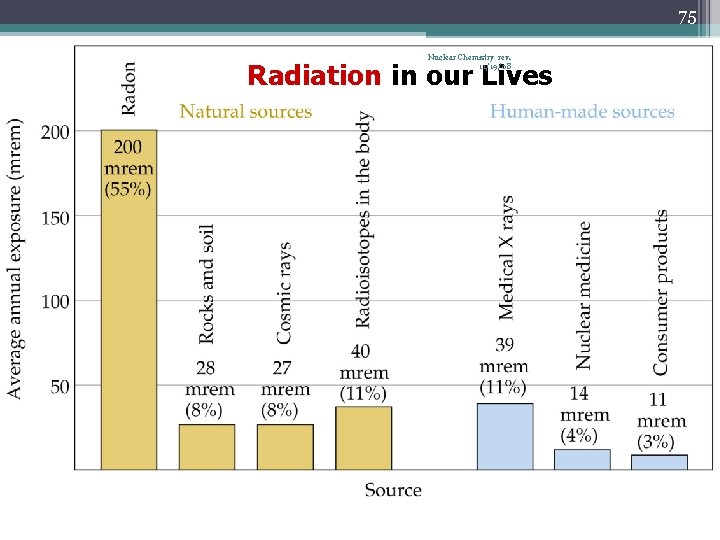
75 Nuclear Chemistry rev. 11/19/08 Radiation in our Lives

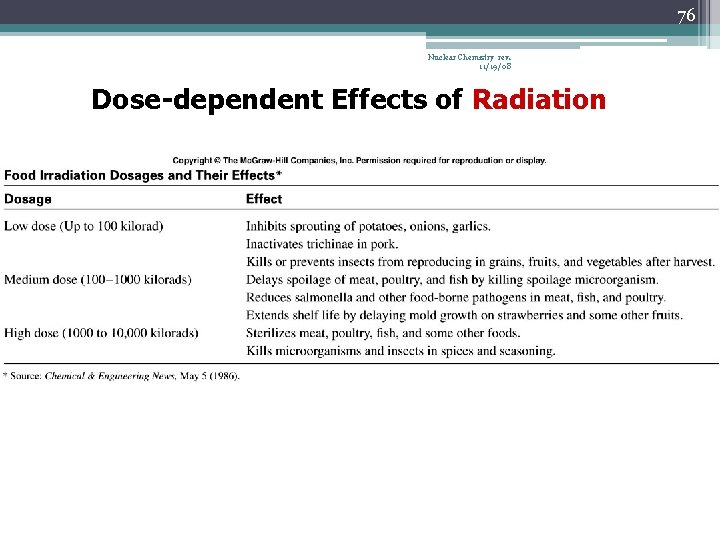
76 Nuclear Chemistry rev. 11/19/08 Dose-dependent Effects of Radiation

77 Nuclear Chemistry rev. 11/19/08

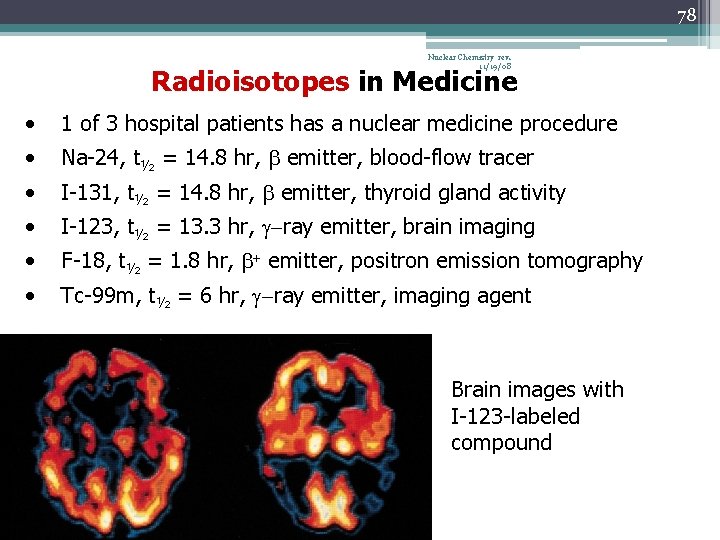
78 Nuclear Chemistry rev. 11/19/08 Radioisotopes in Medicine • 1 of 3 hospital patients has a nuclear medicine procedure • Na-24, t½ = 14. 8 hr, b emitter, blood-flow tracer • I-131, t½ = 14. 8 hr, b emitter, thyroid gland activity • I-123, t½ = 13. 3 hr, g-ray emitter, brain imaging • F-18, t½ = 1. 8 hr, b+ emitter, positron emission tomography • Tc-99 m, t½ = 6 hr, g-ray emitter, imaging agent Brain images with I-123 -labeled compound

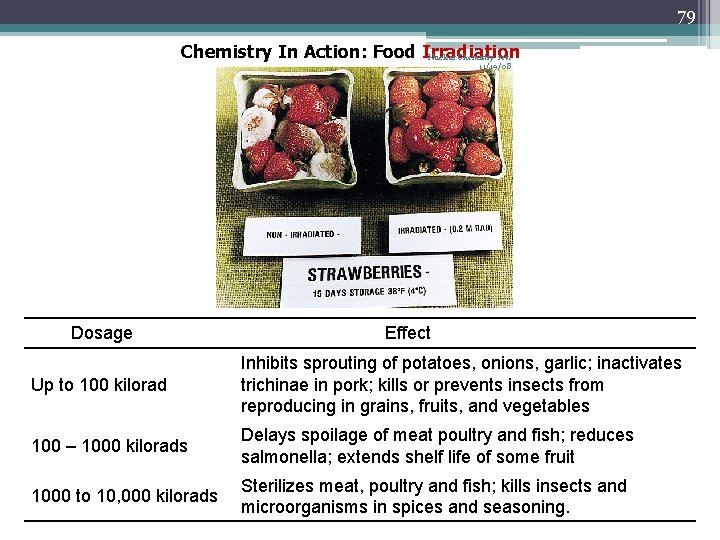
79 Chemistry In Action: Food Irradiation Nuclear Chemistry rev. 11/19/08 Dosage Effect Up to 100 kilorad Inhibits sprouting of potatoes, onions, garlic; inactivates trichinae in pork; kills or prevents insects from reproducing in grains, fruits, and vegetables 100 – 1000 kilorads Delays spoilage of meat poultry and fish; reduces salmonella; extends shelf life of some fruit 1000 to 10, 000 kilorads Sterilizes meat, poultry and fish; kills insects and microorganisms in spices and seasoning.

80 Nuclear Chemistry rev. 11/19/08 Fig. 23. p 969 top

81 Nuclear Chemistry rev. 11/19/08 Image of a portion of the Cygnus Loop supernova remnant

82 Nuclear Chemistry rev. 11/19/08 A pellet containing radioactive I-131

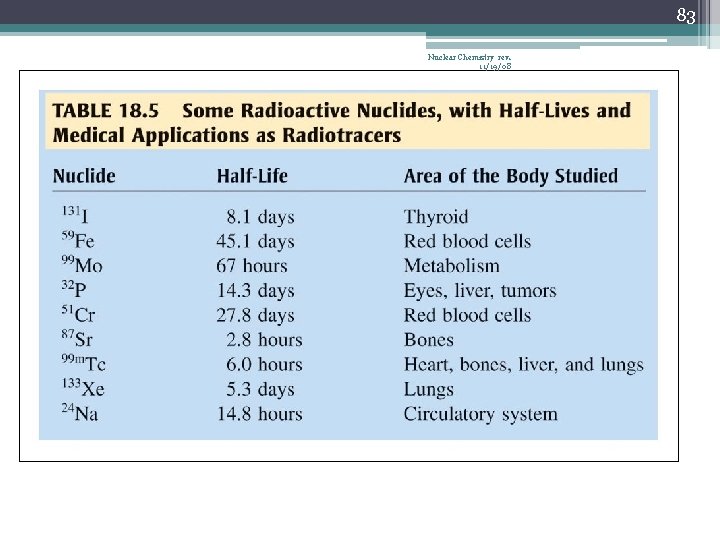
83 Nuclear Chemistry rev. 11/19/08

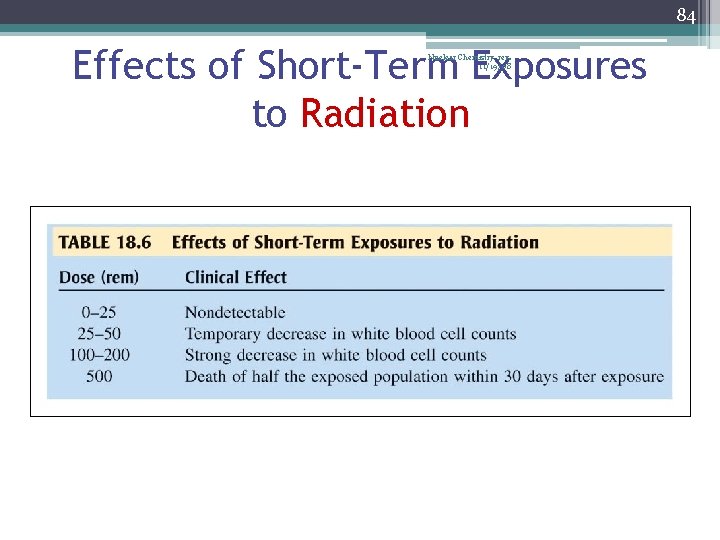
84 Effects of Short-Term Exposures to Radiation Nuclear Chemistry rev. 11/19/08

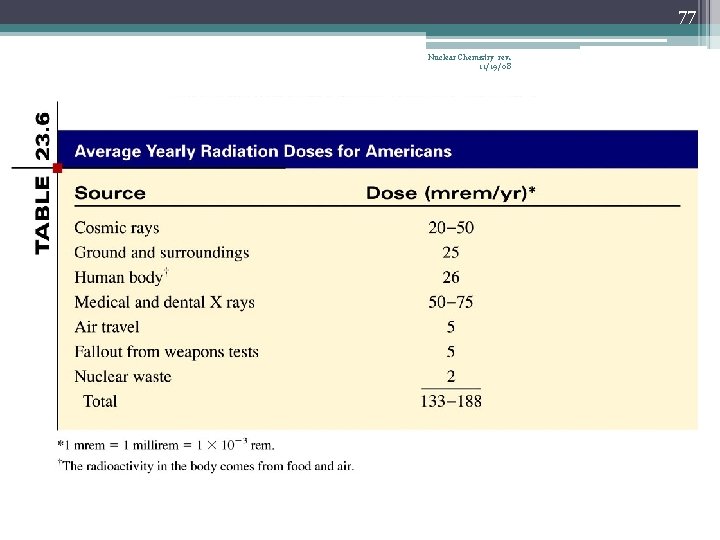
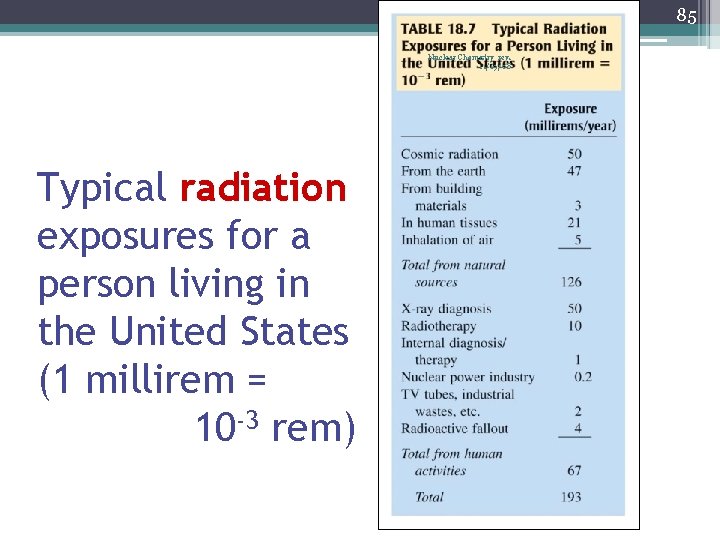
85 Nuclear Chemistry rev. 11/19/08 Typical radiation exposures for a person living in the United States (1 millirem = 10 -3 rem)

86 Nuclear Chemistry rev. 11/19/08 • End