The Evolving Role of Immunotherapy as a Component













- Slides: 59

The Evolving Role of Immunotherapy as a Component of Multimodal Therapy in Earlier Stages of Lung Cancer Rationale, Current Evidence, Key Trials, and Implications for Multidisciplinary Care This event will take place at the AATS International Thoracic Surgical Oncology Summit.

Disclosures Naiyer Rizvi, MD, has a financial interest/relationship or affiliation in the form of: Benny Weksler, MBA, MD, has a financial interest/relationship or affiliation in the form of: Consultant for Astra. Zeneca; Bristol-Myers Squibb; Eli Lilly and Company; F. Hoffmann-La Roche; Merck & Co. , Inc. ; Novartis Pharmaceuticals Corporation; and Pfizer Inc. Other Financial or Material Support from Dr. Weksler is a proctor for Intuitive Surgical Inc. Stock Shareholder in Gritstone Oncology where Dr. Rizvi is a Co-Founder. Naiyer Rizvi, MD, does intend to discuss either non– FDA-approved or investigational use for the following products/devices: Immune checkpoint inhibitors as monotherapies or as part of combinations. Benny Weksler, MBA, MD, does intend to discuss either non–FDA-approved or investigational use for the following products/devices: Immune checkpoint inhibitors as monotherapies or as part of combinations. This CME/MOC/CE activity is jointly provided by Medical Learning Institute, Inc. and PVI, Peer. View Institute for Medical Education. This activity is supported by an independent educational grant from Astra. Zeneca.

Disclosures CME Reviewer Medical Director Siyang Leng, MD, has no financial interests/relationships or affiliations in relation to this activity. Kadrin Wilfong, MD CE Reviewer PVI, Peer. View Institute for Medical Education Kadrin Wilfong, MD, has no financial interests/relationships or affiliations in relation to this activity. Janice Trainor-Tellier, MSN, RN, has no financial interests/relationships or affiliations in relation to this activity. The associates of Medical Learning Institute, Inc. , the accredited provider for this activity, and PVI, Peer. View Institute for Medical Education do not have any financial relationships or relationships to products or devices with any commercial interest related to the content of this CME/MOC/CE activity during the past 12 months.

Visit us at www. peerview. com/Lung 18 • Watch for the on. Demand version in the coming weeks • Download the slides and Practice Aids • Apply for CME/MOC/CE credit Need more information? Send an email to live@peerview. com Join the conversation on Twitter @Peer. View

Master. Class Immunotherapy as a Component of Multimodal Therapy of Lung Cancer Where Are We Now and Where Are We Going Next?

Module 1 Evolving Role of Immunotherapy in Unresectable Stage III NSCLC Naiyer Rizvi, MD Price Family Chair Professor of Medicine Director, Thoracic Oncology Co-Director, Cancer Immunotherapy Columbia University Medical Center New York, New York

• How does cancer immunotherapy work? • What are the immune checkpoint inhibitors?

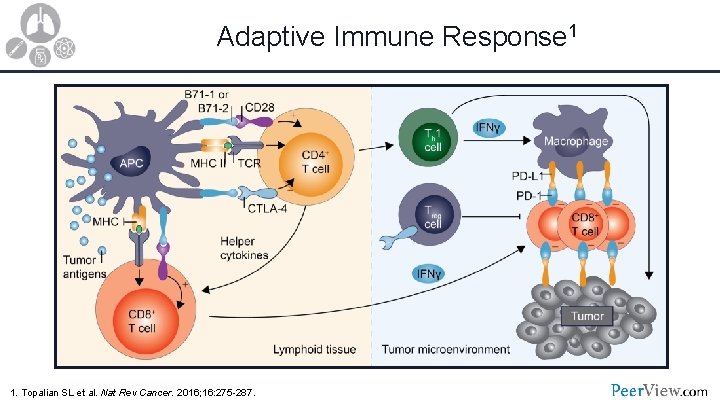
Adaptive Immune Response 1 1. Topalian SL et al. Nat Rev Cancer. 2016; 16: 275 -287.

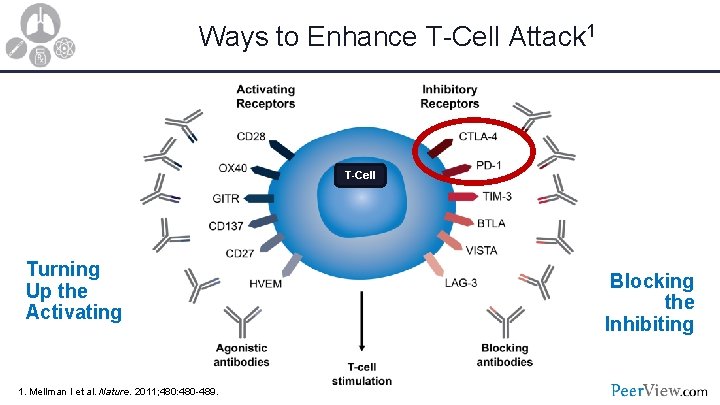
Ways to Enhance T-Cell Attack 1 T-Cell Turning Up the Activating 1. Mellman I et al. Nature. 2011; 480: 480 -489. Blocking the Inhibiting

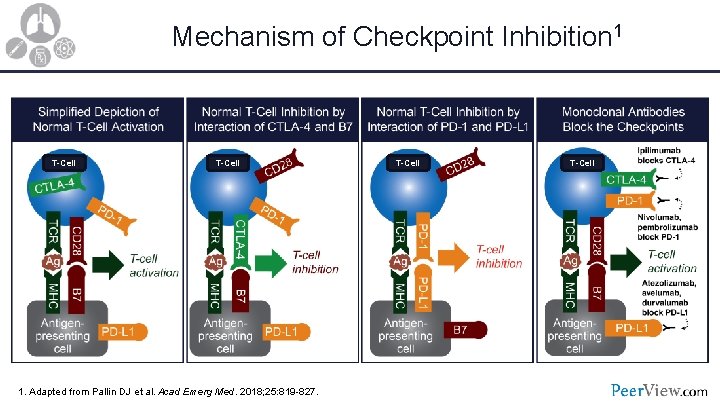
Mechanism of Checkpoint Inhibition 1 T-Cell 1. Adapted from Pallin DJ et al. Acad Emerg Med. 2018; 25: 819 -827. T-Cell

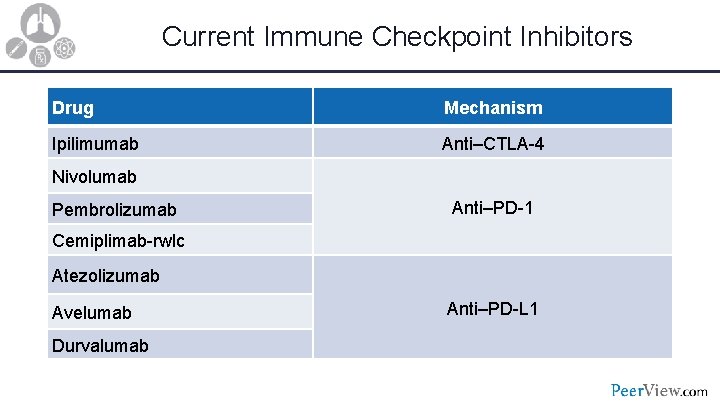
Current Immune Checkpoint Inhibitors Drug Mechanism Ipilimumab Anti–CTLA-4 Nivolumab Pembrolizumab Anti–PD-1 Cemiplimab-rwlc Atezolizumab Avelumab Durvalumab Anti–PD-L 1

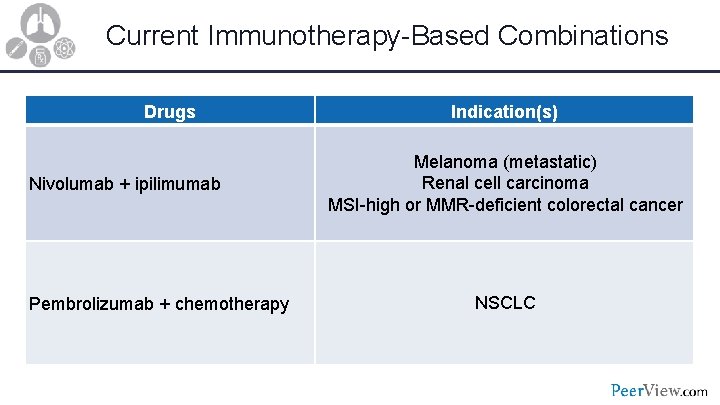
Current Immunotherapy-Based Combinations Drugs Nivolumab + ipilimumab Pembrolizumab + chemotherapy Indication(s) Melanoma (metastatic) Renal cell carcinoma MSI-high or MMR-deficient colorectal cancer NSCLC

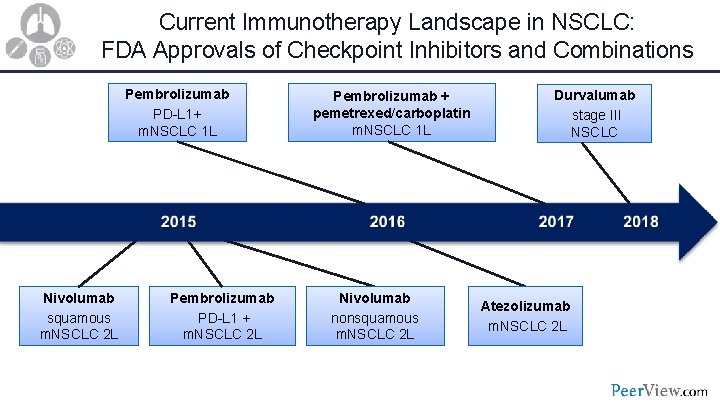
Current Immunotherapy Landscape in NSCLC: FDA Approvals of Checkpoint Inhibitors and Combinations Pembrolizumab PD-L 1+ m. NSCLC 1 L Nivolumab squamous m. NSCLC 2 L Pembrolizumab PD-L 1 + m. NSCLC 2 L Pembrolizumab + pemetrexed/carboplatin m. NSCLC 1 L Nivolumab nonsquamous m. NSCLC 2 L Durvalumab stage III NSCLC Atezolizumab m. NSCLC 2 L

• What is the rationale for using the immune checkpoint inhibitors in locally advanced, unresectable, stage III NSCLC?

PACIFIC—Setting > Unresectable Stage III NSCLC

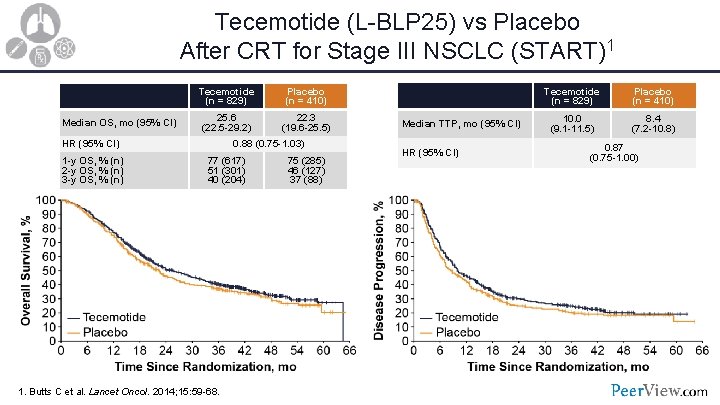
Tecemotide (L-BLP 25) vs Placebo After CRT for Stage III NSCLC (START)1 Median OS, mo (95% CI) Tecemotide (n = 829) Placebo (n = 410) 25. 6 (22. 5 -29. 2) 22. 3 (19. 6 -25. 5) HR (95% CI) 1 -y OS, % (n) 2 -y OS, % (n) 3 -y OS, % (n) 0. 88 (0. 75 -1. 03) 77 (617) 51 (301) 40 (204) 1. Butts C et al. Lancet Oncol. 2014; 15: 59 -68. 75 (285) 46 (127) 37 (88) Median TTP, mo (95% CI) HR (95% CI) Tecemotide (n = 829) Placebo (n = 410) 10. 0 (9. 1 -11. 5) 8. 4 (7. 2 -10. 8) 0. 87 (0. 75 -1. 00)

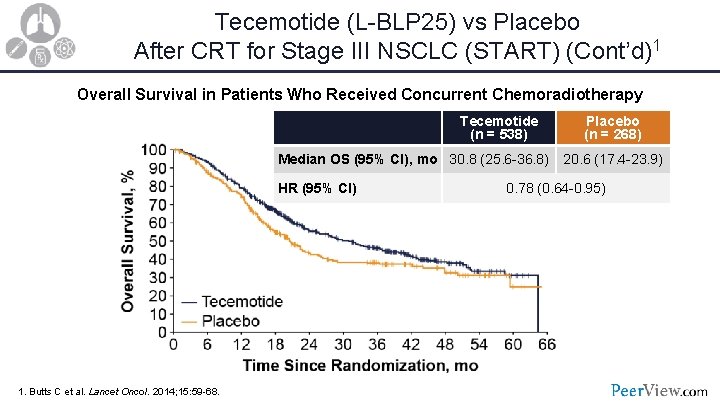
Tecemotide (L-BLP 25) vs Placebo After CRT for Stage III NSCLC (START) (Cont’d)1 Overall Survival in Patients Who Received Concurrent Chemoradiotherapy Tecemotide (n = 538) Median OS (95% CI), mo 30. 8 (25. 6 -36. 8) HR (95% CI) 1. Butts C et al. Lancet Oncol. 2014; 15: 59 -68. Placebo (n = 268) 20. 6 (17. 4 -23. 9) 0. 78 (0. 64 -0. 95)

Addressing Unmet Needs: Emergence of Immunotherapy in Stage III NSCLC q Median PFS with standard SOC c. CRT ≈8 -10 months and only 15% are alive at 5 years 1 -6 q No major advances in locally advanced NSCLC for many years significant unmet need exists for novel therapeutic approaches to improve outcomes beyond those achieved with c. CRT Immunotherapy is providing new hope and changing the management of stage III NSCLC 1. Aupérin A et al. J Clin Oncol. 2010; 28: 2181 -2190. 2. Yoon SM et al. World J Clin Oncol. 2017; 8: 1 -20. 3. Ahn JS et al. J Clin Oncol. 2015; 33: 2660 -2666. 4. Furuse J et al. Clin Oncol. 1999; 17: 2692 -2699. 5. Belderbos J et al. Eur J Cancer. 2007; 43: 114 -121. 6. Clamon G et al. J Clin Oncol. 1999; 17: 4 -11.

Radiation Can Be Immunogenic 1 • 46 case reports between 1969 and 2014 • Wide variety of tumor types, treatment sites, dose schedules • “Abscopal response” occurred at 0 to 12 months 1. Abuodeh Y et al. Curr Probl Cancer. 2016; 40: 25 -37.

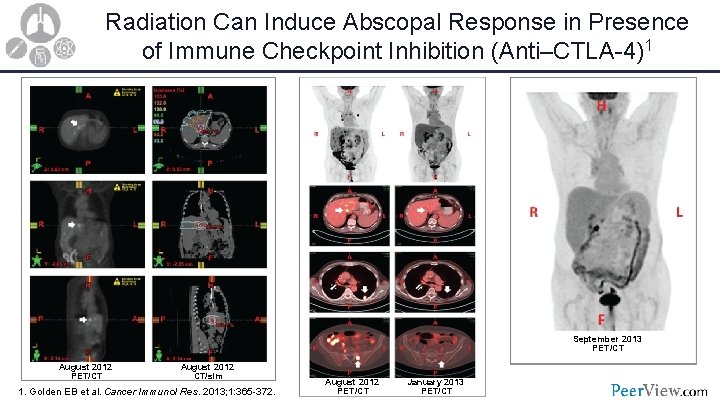
Radiation Can Induce Abscopal Response in Presence of Immune Checkpoint Inhibition (Anti–CTLA-4)1 September 2013 PET/CT August 2012 CT/sim 1. Golden EB et al. Cancer Immunol Res. 2013; 1: 365 -372. August 2012 PET/CT January 2013 PET/CT

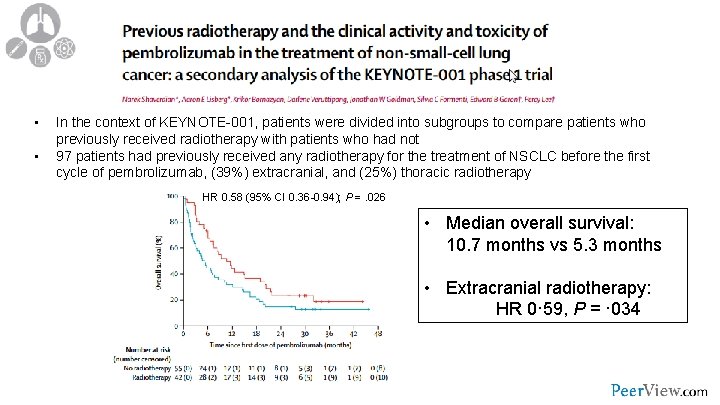
• • In the context of KEYNOTE-001, patients were divided into subgroups to compare patients who previously received radiotherapy with patients who had not 97 patients had previously received any radiotherapy for the treatment of NSCLC before the first cycle of pembrolizumab, (39%) extracranial, and (25%) thoracic radiotherapy HR 0. 58 (95% CI 0. 36 -0. 94); P =. 026 • Median overall survival: 10. 7 months vs 5. 3 months • Extracranial radiotherapy: HR 0· 59, P = · 034

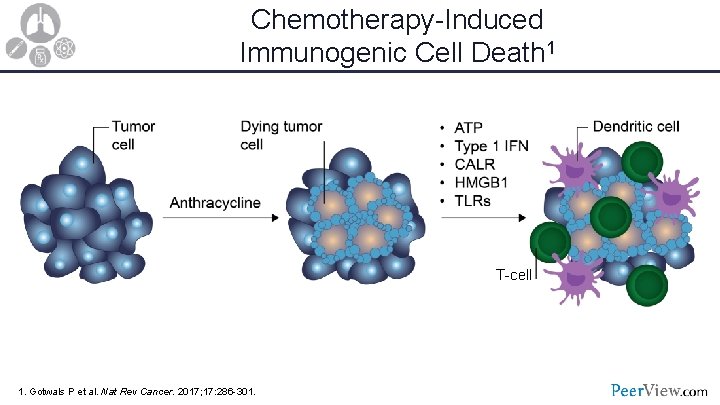
Chemotherapy-Induced Immunogenic Cell Death 1 T-cell 1. Gotwals P et al. Nat Rev Cancer. 2017; 17: 286 -301.

• What clinical evidence supports the use of immune checkpoint inhibition in locally advanced, unresectable, stage III NSCLC?

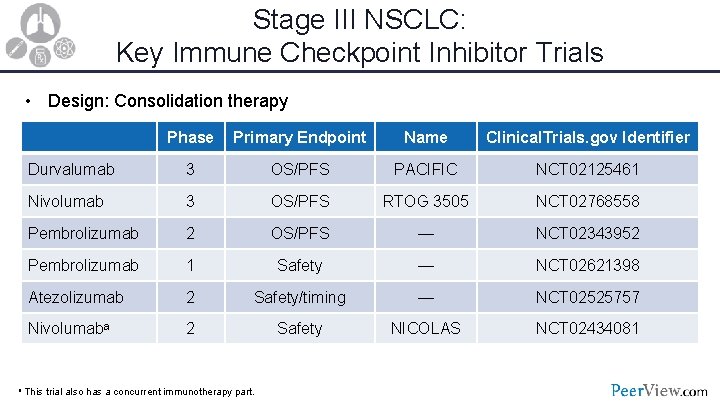
Stage III NSCLC: Key Immune Checkpoint Inhibitor Trials • Design: Consolidation therapy Phase Primary Endpoint Name Clinical. Trials. gov Identifier Durvalumab 3 OS/PFS PACIFIC NCT 02125461 Nivolumab 3 OS/PFS RTOG 3505 NCT 02768558 Pembrolizumab 2 OS/PFS — NCT 02343952 Pembrolizumab 1 Safety — NCT 02621398 Atezolizumab 2 Safety/timing — NCT 02525757 Nivolumaba 2 Safety NICOLAS NCT 02434081 a This trial also has a concurrent immunotherapy part.

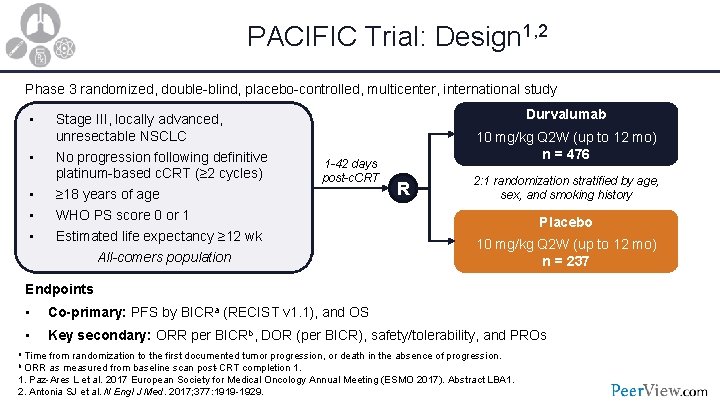
PACIFIC Trial: Design 1, 2 Phase 3 randomized, double-blind, placebo-controlled, multicenter, international study • • • Stage III, locally advanced, unresectable NSCLC No progression following definitive platinum-based c. CRT (≥ 2 cycles) ≥ 18 years of age WHO PS score 0 or 1 Estimated life expectancy ≥ 12 wk All-comers population Durvalumab 1 -42 days post-c. CRT 10 mg/kg Q 2 W (up to 12 mo) n = 476 R 2: 1 randomization stratified by age, sex, and smoking history Placebo 10 mg/kg Q 2 W (up to 12 mo) n = 237 Endpoints • Co-primary: PFS by BICRa (RECIST v 1. 1), and OS • Key secondary: ORR per BICRb, DOR (per BICR), safety/tolerability, and PROs a Time from randomization to the first documented tumor progression, or death in the absence of progression. b ORR as measured from baseline scan post-CRT completion 1. Paz-Ares L et al. 2017 European Society for Medical Oncology Annual Meeting (ESMO 2017). Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

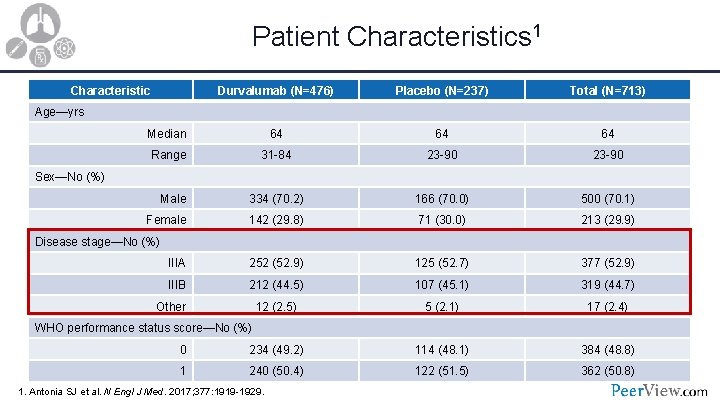
Patient Characteristics 1 Characteristic Durvalumab (N=476) Placebo (N=237) Total (N=713) Median 64 64 64 Range 31 -84 23 -90 Male 334 (70. 2) 166 (70. 0) 500 (70. 1) Female 142 (29. 8) 71 (30. 0) 213 (29. 9) IIIA 252 (52. 9) 125 (52. 7) 377 (52. 9) IIIB 212 (44. 5) 107 (45. 1) 319 (44. 7) 12 (2. 5) 5 (2. 1) 17 (2. 4) 0 234 (49. 2) 114 (48. 1) 384 (48. 8) 1 240 (50. 4) 122 (51. 5) 362 (50. 8) Age—yrs Sex—No (%) Disease stage—No (%) Other WHO performance status score—No (%) 1. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

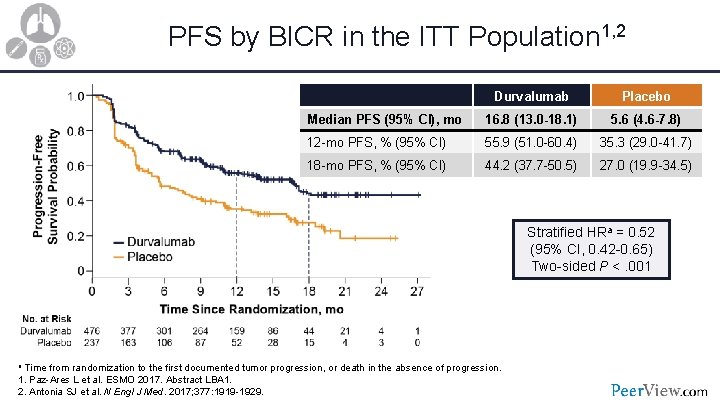
PFS by BICR in the ITT Population 1, 2 Durvalumab Placebo Median PFS (95% CI), mo 16. 8 (13. 0 -18. 1) 5. 6 (4. 6 -7. 8) 12 -mo PFS, % (95% CI) 55. 9 (51. 0 -60. 4) 35. 3 (29. 0 -41. 7) 18 -mo PFS, % (95% CI) 44. 2 (37. 7 -50. 5) 27. 0 (19. 9 -34. 5) Stratified HRa = 0. 52 (95% CI, 0. 42 -0. 65) Two-sided P <. 001 a Time from randomization to the first documented tumor progression, or death in the absence of progression. 1. Paz-Ares L et al. ESMO 2017. Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

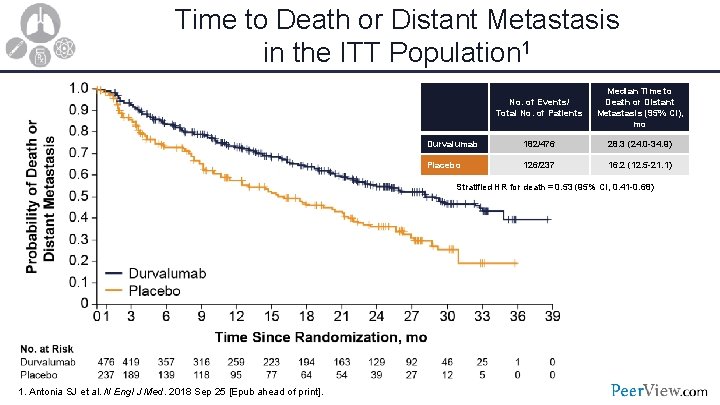
Time to Death or Distant Metastasis in the ITT Population 1 No. of Events/ Total No. of Patients Median Time to Death or Distant Metastasis (95% CI), mo Durvalumab 182/476 28. 3 (24. 0 -34. 9) Placebo 126/237 16. 2 (12. 5 -21. 1) Stratified HR for death = 0. 53 (95% CI, 0. 41 -0. 68) 1. Antonia SJ et al. N Engl J Med. 2018 Sep 25 [Epub ahead of print].

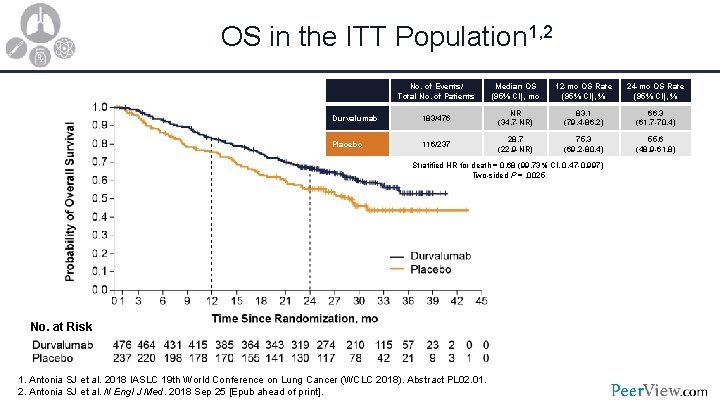
OS in the ITT Population 1, 2 No. of Events/ Total No. of Patients Median OS (95% CI), mo 12 -mo OS Rate (95% CI), % 24 -mo OS Rate (95% CI), % Durvalumab 183/476 NR (34. 7 -NR) 83. 1 (79. 4 -86. 2) 66. 3 (61. 7 -70. 4) Placebo 116/237 28. 7 (22. 9 -NR) 75. 3 (69. 2 -80. 4) 55. 6 (48. 9 -61. 8) Stratified HR for death = 0. 68 (99. 73% CI, 0. 47 -0. 997) Two-sided P =. 0025 No. at Risk 1. Antonia SJ et al. 2018 IASLC 19 th World Conference on Lung Cancer (WCLC 2018). Abstract PL 02. 01. 2. Antonia SJ et al. N Engl J Med. 2018 Sep 25 [Epub ahead of print].

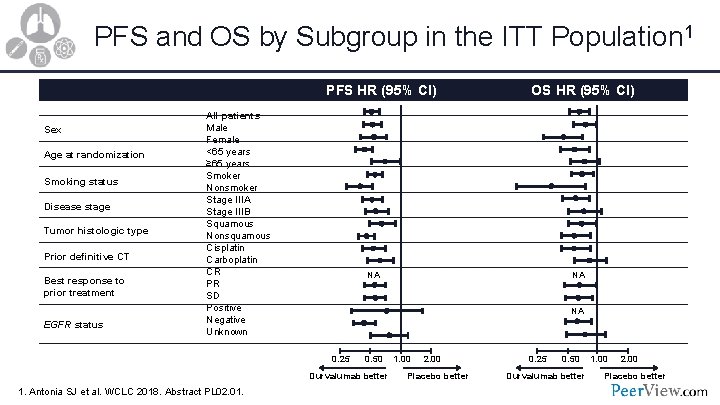
PFS and OS by Subgroup in the ITT Population 1 PFS HR (95% CI) Sex Age at randomization Smoking status Disease stage Tumor histologic type Prior definitive CT Best response to prior treatment EGFR status All patients Male Female <65 years ≥ 65 years Smoker Nonsmoker Stage IIIA Stage IIIB Squamous Nonsquamous Cisplatin Carboplatin CR PR SD Positive Negative Unknown NA NA NA 0. 25 0. 50 Durvalumab better 1. Antonia SJ et al. WCLC 2018. Abstract PL 02. 01. OS HR (95% CI) 1. 00 2. 00 Placebo better 0. 25 0. 50 Durvalumab better 1. 00 2. 00 Placebo better

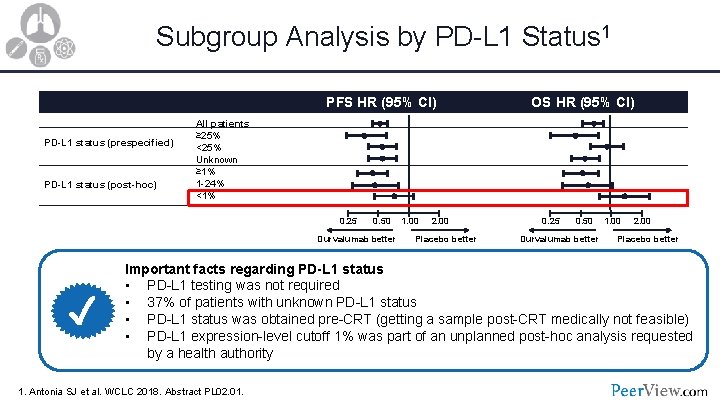
Subgroup Analysis by PD-L 1 Status 1 PFS HR (95% CI) PD-L 1 status (prespecified) PD-L 1 status (post-hoc) All patients ≥ 25% <25% Unknown ≥ 1% 1 -24% <1% 0. 25 0. 50 Durvalumab better ✓ OS HR (95% CI) 1. 00 2. 00 Placebo better 0. 25 0. 50 Durvalumab better 1. 00 2. 00 Placebo better Important facts regarding PD-L 1 status • PD-L 1 testing was not required • 37% of patients with unknown PD-L 1 status • PD-L 1 status was obtained pre-CRT (getting a sample post-CRT medically not feasible) • PD-L 1 expression-level cutoff 1% was part of an unplanned post-hoc analysis requested by a health authority 1. Antonia SJ et al. WCLC 2018. Abstract PL 02. 01.

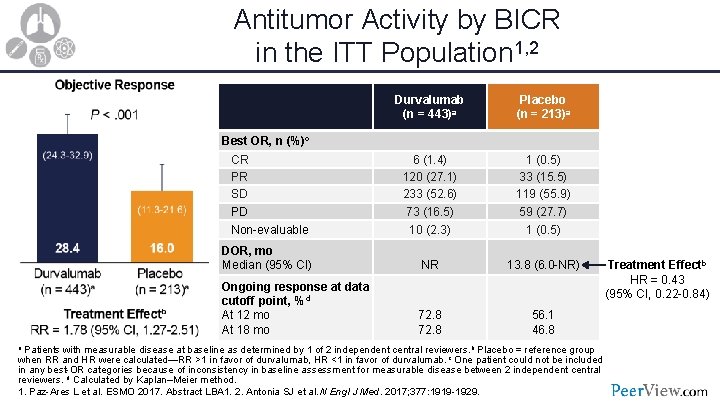
Antitumor Activity by BICR in the ITT Population 1, 2 Durvalumab (n = 443)a Placebo (n = 213)a 6 (1. 4) 120 (27. 1) 233 (52. 6) 73 (16. 5) 1 (0. 5) 33 (15. 5) 119 (55. 9) 59 (27. 7) Non-evaluable 10 (2. 3) 1 (0. 5) DOR, mo Median (95% CI) NR 13. 8 (6. 0 -NR) Ongoing response at data cutoff point, %d At 12 mo At 18 mo 72. 8 56. 1 46. 8 Best OR, n (%)c CR PR SD PD a Patients with measurable disease at baseline as determined by 1 of 2 independent central reviewers. b Placebo = reference group Treatment Effectb HR = 0. 43 (95% CI, 0. 22 -0. 84) when RR and HR were calculated—RR >1 in favor of durvalumab, HR <1 in favor of durvalumab. c One patient could not be included in any best-OR categories because of inconsistency in baseline assessment for measurable disease between 2 independent central reviewers. d Calculated by Kaplan–Meier method. 1. Paz-Ares L et al. ESMO 2017. Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

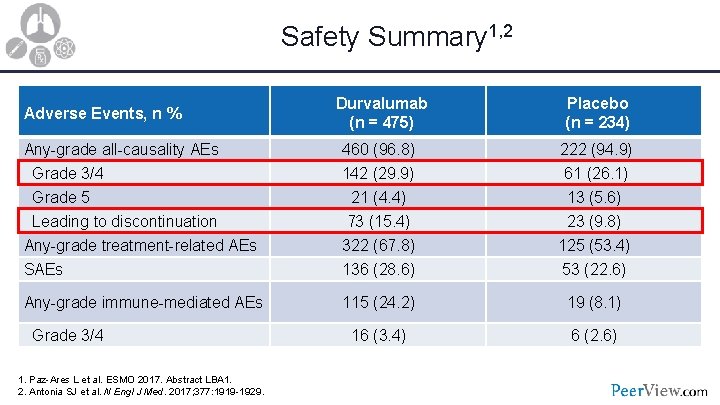
Safety Summary 1, 2 Durvalumab (n = 475) Placebo (n = 234) Any-grade all-causality AEs 460 (96. 8) 222 (94. 9) Grade 3/4 Grade 5 Leading to discontinuation Any-grade treatment-related AEs SAEs 142 (29. 9) 21 (4. 4) 73 (15. 4) 322 (67. 8) 136 (28. 6) 61 (26. 1) 13 (5. 6) 23 (9. 8) 125 (53. 4) 53 (22. 6) Any-grade immune-mediated AEs 115 (24. 2) 19 (8. 1) 16 (3. 4) 6 (2. 6) Adverse Events, n % Grade 3/4 1. Paz-Ares L et al. ESMO 2017. Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

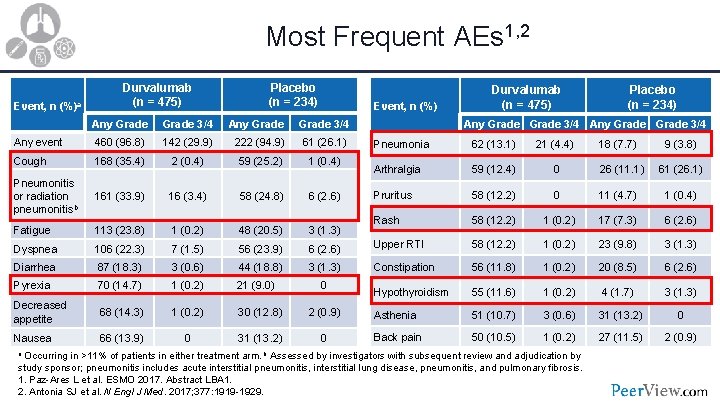
Most Frequent AEs 1, 2 Event, n (%)a Durvalumab (n = 475) Placebo (n = 234) Event, n (%) Any Grade 3/4 Any event 460 (96. 8) 142 (29. 9) 222 (94. 9) 61 (26. 1) Cough 168 (35. 4) 2 (0. 4) 59 (25. 2) 1 (0. 4) Pneumonitis or radiation pneumonitisb 161 (33. 9) 16 (3. 4) 58 (24. 8) 6 (2. 6) Fatigue 113 (23. 8) 1 (0. 2) 48 (20. 5) 3 (1. 3) Dyspnea 106 (22. 3) 7 (1. 5) 56 (23. 9) Diarrhea 87 (18. 3) 3 (0. 6) 44 (18. 8) Pyrexia 70 (14. 7) 1 (0. 2) 21 (9. 0) Decreased appetite 68 (14. 3) 1 (0. 2) 30 (12. 8) Nausea 66 (13. 9) 0 31 (13. 2) Durvalumab (n = 475) Placebo (n = 234) Any Grade 3/4 Pneumonia 62 (13. 1) 21 (4. 4) 18 (7. 7) 9 (3. 8) Arthralgia 59 (12. 4) 0 26 (11. 1) 61 (26. 1) Pruritus 58 (12. 2) 0 11 (4. 7) 1 (0. 4) Rash 58 (12. 2) 1 (0. 2) 17 (7. 3) 6 (2. 6) Upper RTI 58 (12. 2) 1 (0. 2) 23 (9. 8) 3 (1. 3) Constipation 56 (11. 8) 1 (0. 2) 20 (8. 5) 6 (2. 6) Hypothyroidism 55 (11. 6) 1 (0. 2) 4 (1. 7) 3 (1. 3) 2 (0. 9) Asthenia 51 (10. 7) 3 (0. 6) 31 (13. 2) 0 0 Back pain 50 (10. 5) 1 (0. 2) 27 (11. 5) 2 (0. 9) 0 a Occurring in >11% of patients in either treatment arm. b Assessed by investigators with subsequent review and adjudication by study sponsor; pneumonitis includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis, and pulmonary fibrosis. 1. Paz-Ares L et al. ESMO 2017. Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

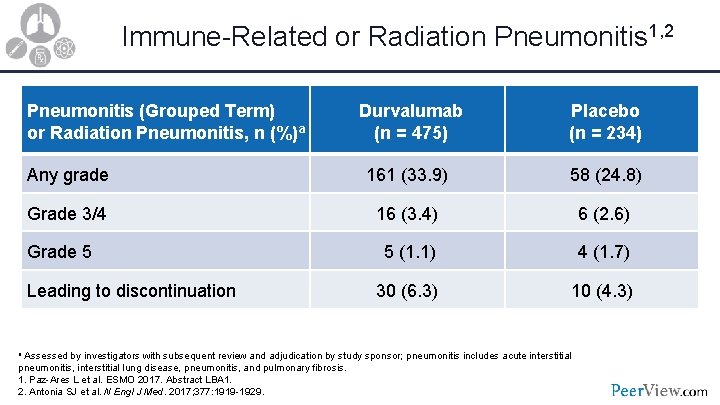
Immune-Related or Radiation Pneumonitis 1, 2 Pneumonitis (Grouped Term) or Radiation Pneumonitis, n (%)a Durvalumab (n = 475) Placebo (n = 234) Any grade 161 (33. 9) 58 (24. 8) Grade 3/4 16 (3. 4) 6 (2. 6) Grade 5 5 (1. 1) 4 (1. 7) Leading to discontinuation 30 (6. 3) 10 (4. 3) a Assessed by investigators with subsequent review and adjudication by study sponsor; pneumonitis includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis, and pulmonary fibrosis. 1. Paz-Ares L et al. ESMO 2017. Abstract LBA 1. 2. Antonia SJ et al. N Engl J Med. 2017; 377: 1919 -1929.

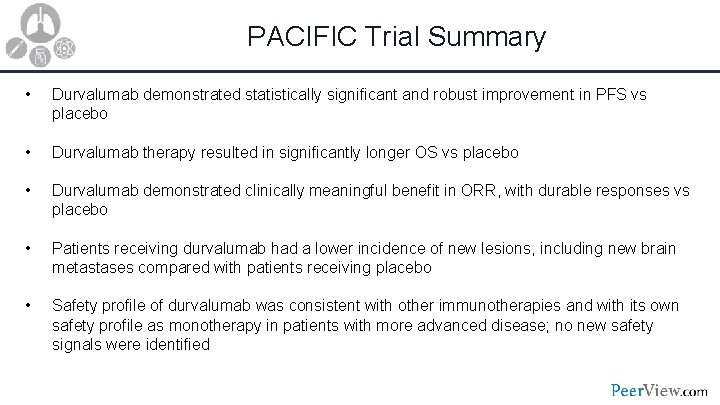
PACIFIC Trial Summary • Durvalumab demonstrated statistically significant and robust improvement in PFS vs placebo • Durvalumab therapy resulted in significantly longer OS vs placebo • Durvalumab demonstrated clinically meaningful benefit in ORR, with durable responses vs placebo • Patients receiving durvalumab had a lower incidence of new lesions, including new brain metastases compared with patients receiving placebo • Safety profile of durvalumab was consistent with other immunotherapies and with its own safety profile as monotherapy in patients with more advanced disease; no new safety signals were identified

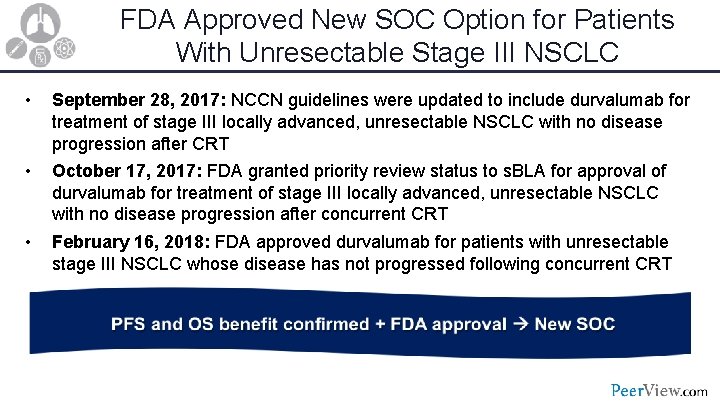
FDA Approved New SOC Option for Patients With Unresectable Stage III NSCLC • September 28, 2017: NCCN guidelines were updated to include durvalumab for treatment of stage III locally advanced, unresectable NSCLC with no disease progression after CRT • October 17, 2017: FDA granted priority review status to s. BLA for approval of durvalumab for treatment of stage III locally advanced, unresectable NSCLC with no disease progression after concurrent CRT • February 16, 2018: FDA approved durvalumab for patients with unresectable stage III NSCLC whose disease has not progressed following concurrent CRT

Module 2 Potential Role of Immunotherapy in Resectable NSCLC Benny Weksler, MBA, MD System Chief of Thoracic Surgery Allegheny Health Network Pittsburgh, Pennsylvania

• What is the rationale for using cancer immunotherapy in resectable NSCLC? • Are there any trials and data in neoadjuvant or adjuvant settings?

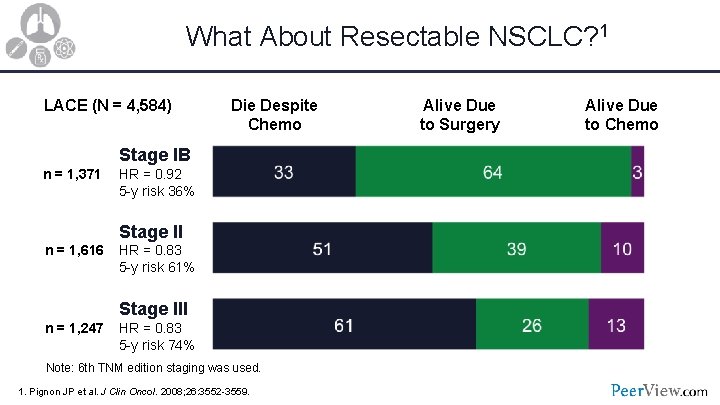
What About Resectable NSCLC? 1 LACE (N = 4, 584) Die Despite Chemo Stage IB n = 1, 371 HR = 0. 92 5 -y risk 36% Stage II n = 1, 616 HR = 0. 83 5 -y risk 61% Stage III n = 1, 247 HR = 0. 83 5 -y risk 74% Note: 6 th TNM edition staging was used. 1. Pignon JP et al. J Clin Oncol. 2008; 26: 3552 -3559. Alive Due to Surgery Alive Due to Chemo

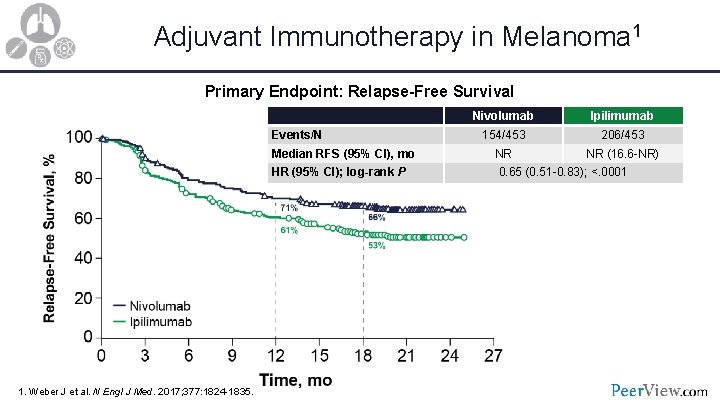
Adjuvant Immunotherapy in Melanoma 1 Primary Endpoint: Relapse-Free Survival Events/N Median RFS (95% CI), mo HR (95% CI); log-rank P 1. Weber J et al. N Engl J Med. 2017; 377: 1824 -1835. Nivolumab Ipilimumab 154/453 206/453 NR NR (16. 6 -NR) 0. 65 (0. 51 -0. 83); <. 0001

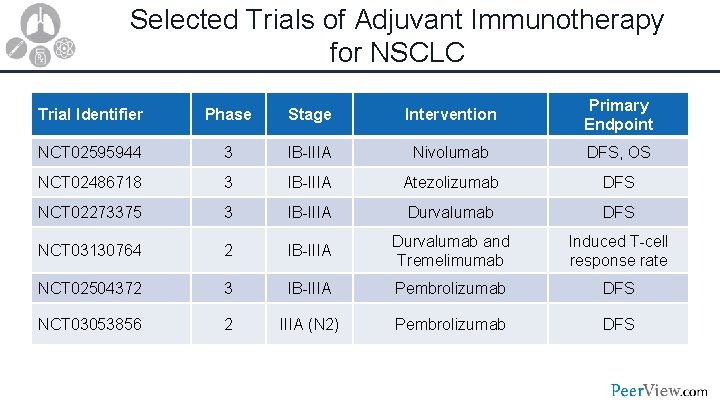
Selected Trials of Adjuvant Immunotherapy for NSCLC Trial Identifier Phase Stage Intervention Primary Endpoint NCT 02595944 3 IB-IIIA Nivolumab DFS, OS NCT 02486718 3 IB-IIIA Atezolizumab DFS NCT 02273375 3 IB-IIIA Durvalumab DFS NCT 03130764 2 IB-IIIA Durvalumab and Tremelimumab Induced T-cell response rate NCT 02504372 3 IB-IIIA Pembrolizumab DFS NCT 03053856 2 IIIA (N 2) Pembrolizumab DFS

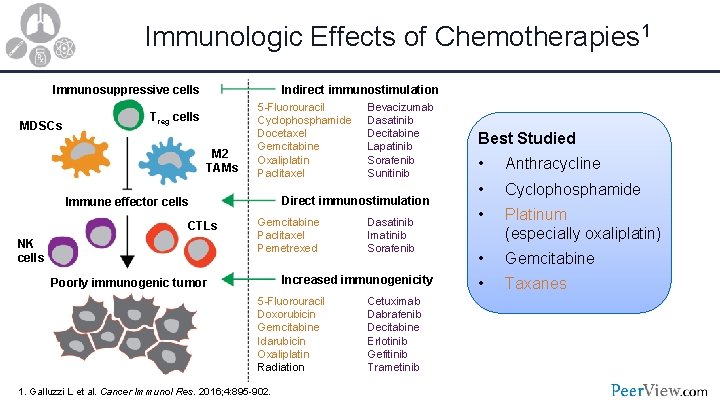
Immunologic Effects of Chemotherapies 1 Immunosuppressive cells MDSCs Indirect immunostimulation Treg cells M 2 TAMs 5 -Fluorouracil Cyclophosphamide Docetaxel Gemcitabine Oxaliplatin Paclitaxel Direct immunostimulation Immune effector cells CTLs NK cells Bevacizumab Dasatinib Decitabine Lapatinib Sorafenib Sunitinib Gemcitabine Paclitaxel Pemetrexed Dasatinib Imatinib Sorafenib Increased immunogenicity Poorly immunogenic tumor 5 -Fluorouracil Doxorubicin Gemcitabine Idarubicin Oxaliplatin Radiation 1. Galluzzi L et al. Cancer Immunol Res. 2016; 4: 895 -902. Cetuximab Dabrafenib Decitabine Erlotinib Gefitinib Trametinib Best Studied • Anthracycline • Cyclophosphamide • Platinum (especially oxaliplatin) • Gemcitabine • Taxanes

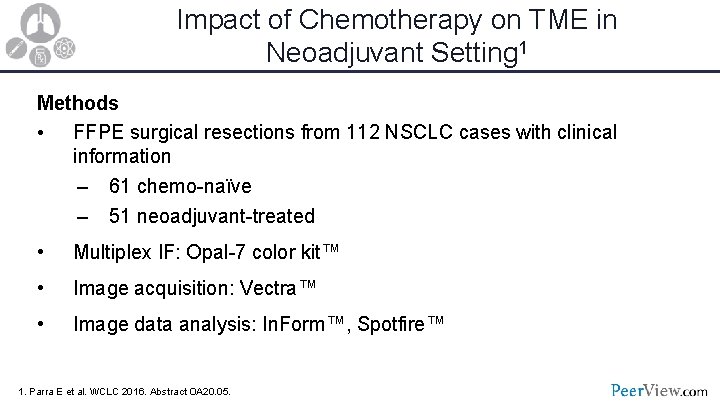
Impact of Chemotherapy on TME in Neoadjuvant Setting 1 Methods • FFPE surgical resections from 112 NSCLC cases with clinical information – 61 chemo-naïve – 51 neoadjuvant-treated • Multiplex IF: Opal-7 color kit™ • Image acquisition: Vectra™ • Image data analysis: In. Form™, Spotfire™ 1. Parra E et al. WCLC 2016. Abstract OA 20. 05.

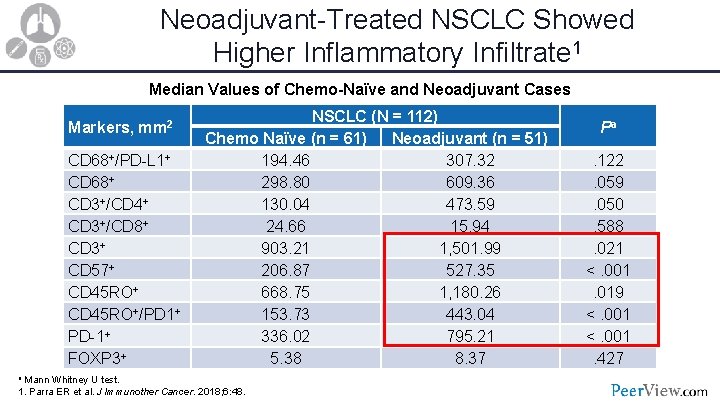
Neoadjuvant-Treated NSCLC Showed Higher Inflammatory Infiltrate 1 Median Values of Chemo-Naïve and Neoadjuvant Cases Markers, mm 2 CD 68+/PD-L 1+ CD 68+ CD 3+/CD 4+ CD 3+/CD 8+ CD 3+ CD 57+ CD 45 RO+/PD 1+ PD-1+ FOXP 3+ NSCLC (N = 112) Chemo Naïve (n = 61) Neoadjuvant (n = 51) 194. 46 307. 32 298. 80 609. 36 130. 04 473. 59 24. 66 15. 94 903. 21 1, 501. 99 206. 87 527. 35 668. 75 1, 180. 26 153. 73 443. 04 336. 02 795. 21 5. 38 8. 37 a Mann Whitney U test. 1. Parra ER et al. J Immunother Cancer. 2018; 6: 48. Pa. 122. 059. 050. 588. 021 <. 001. 019 <. 001. 427

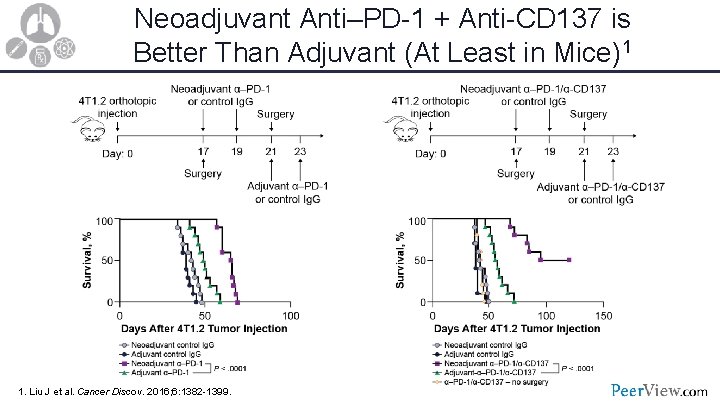
Neoadjuvant Anti–PD-1 + Anti-CD 137 is Better Than Adjuvant (At Least in Mice)1 1. Liu J et al. Cancer Discov. 2016; 6: 1382 -1399.

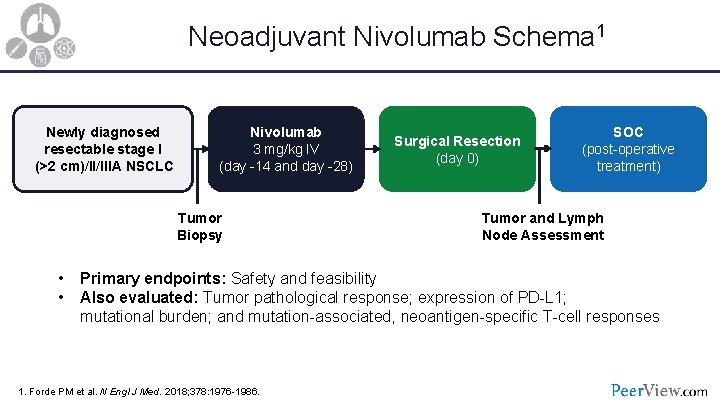
Neoadjuvant Nivolumab Schema 1 Newly diagnosed resectable stage I (>2 cm)/II/IIIA NSCLC Nivolumab 3 mg/kg IV (day -14 and day -28) Tumor Biopsy • • Surgical Resection (day 0) SOC (post-operative treatment) Tumor and Lymph Node Assessment Primary endpoints: Safety and feasibility Also evaluated: Tumor pathological response; expression of PD-L 1; mutational burden; and mutation-associated, neoantigen-specific T-cell responses 1. Forde PM et al. N Engl J Med. 2018; 378: 1976 -1986.

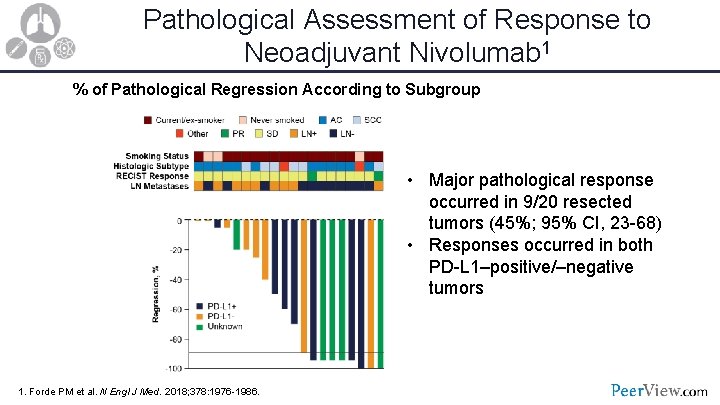
Pathological Assessment of Response to Neoadjuvant Nivolumab 1 % of Pathological Regression According to Subgroup • Major pathological response occurred in 9/20 resected tumors (45%; 95% CI, 23 -68) • Responses occurred in both PD-L 1–positive/–negative tumors 1. Forde PM et al. N Engl J Med. 2018; 378: 1976 -1986.

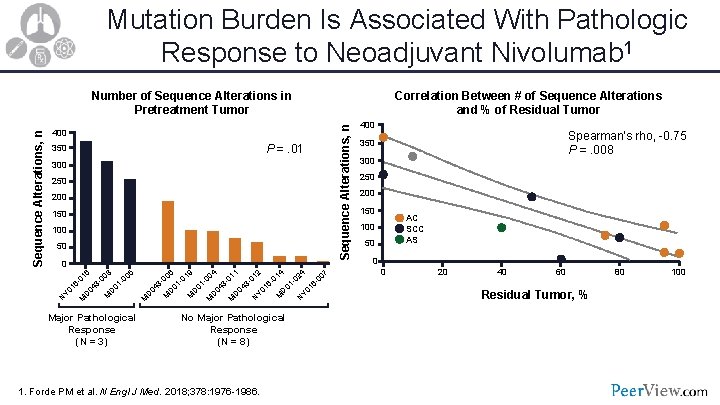
Mutation Burden Is Associated With Pathologic Response to Neoadjuvant Nivolumab 1 400 P =. 01 350 300 250 200 150 100 50 0 5 8 6 00 00 01 1360 4 1 0 D Y 0 D M N M Major Pathological Response (N = 3) 4 9 4 6 1 2 7 4 02 01 00 00 01 01 00 01 113 - 0133660 0 4 4 4 1 1 0 0 0 D D D Y 0 D D D M M M N N M M M No Major Pathological Response (N = 8) 1. Forde PM et al. N Engl J Med. 2018; 378: 1976 -1986. Correlation Between # of Sequence Alterations and % of Residual Tumor Sequence Alterations, n Number of Sequence Alterations in Pretreatment Tumor 400 Spearman’s rho, -0. 75 P =. 008 350 300 250 200 150 AC SCC AS 100 50 0 0 20 40 60 Residual Tumor, % 80 100

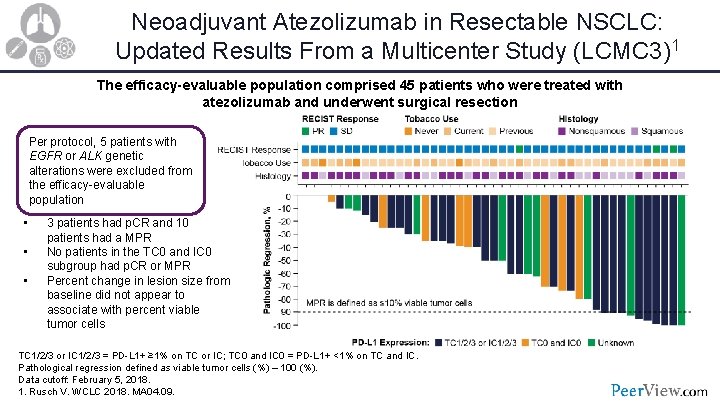
Neoadjuvant Atezolizumab in Resectable NSCLC: Updated Results From a Multicenter Study (LCMC 3)1 The efficacy-evaluable population comprised 45 patients who were treated with atezolizumab and underwent surgical resection Per protocol, 5 patients with EGFR or ALK genetic alterations were excluded from the efficacy-evaluable population • • • 3 patients had p. CR and 10 patients had a MPR No patients in the TC 0 and IC 0 subgroup had p. CR or MPR Percent change in lesion size from baseline did not appear to associate with percent viable tumor cells TC 1/2/3 or IC 1/2/3 = PD-L 1+ ≥ 1% on TC or IC; TC 0 and IC 0 = PD-L 1+ <1% on TC and IC. Pathological regression defined as viable tumor cells (%) – 100 (%). Data cutoff: February 5, 2018. 1. Rusch V. WCLC 2018. MA 04. 09.

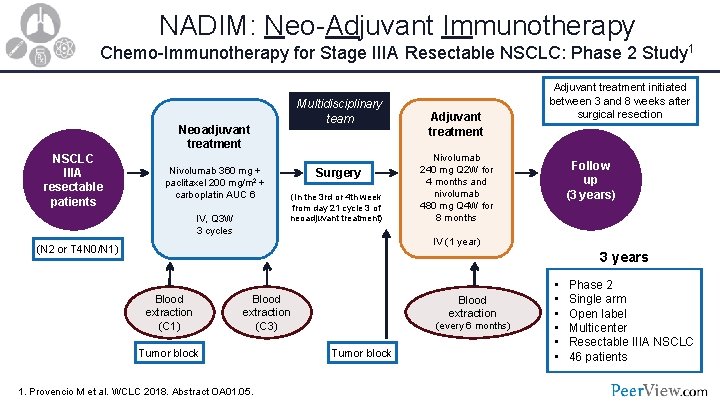
NADIM: Neo-Adjuvant Immunotherapy Chemo-Immunotherapy for Stage IIIA Resectable NSCLC: Phase 2 Study 1 Multidisciplinary team Neoadjuvant treatment NSCLC IIIA resectable patients Nivolumab 360 mg + paclitaxel 200 mg/m 2 + carboplatin AUC 6 IV, Q 3 W 3 cycles Surgery (In the 3 rd or 4 th week from day 21 cycle 3 of neoadjuvant treatment) Adjuvant treatment initiated between 3 and 8 weeks after surgical resection Nivolumab 240 mg Q 2 W for 4 months and nivolumab 480 mg Q 4 W for 8 months Follow up (3 years) IV (1 year) (N 2 or T 4 N 0/N 1) 3 years Blood extraction (C 1) Blood extraction (C 3) Tumor block 1. Provencio M et al. WCLC 2018. Abstract OA 01. 05. Blood extraction (every 6 months) Tumor block • • • Phase 2 Single arm Open label Multicenter Resectable IIIA NSCLC 46 patients

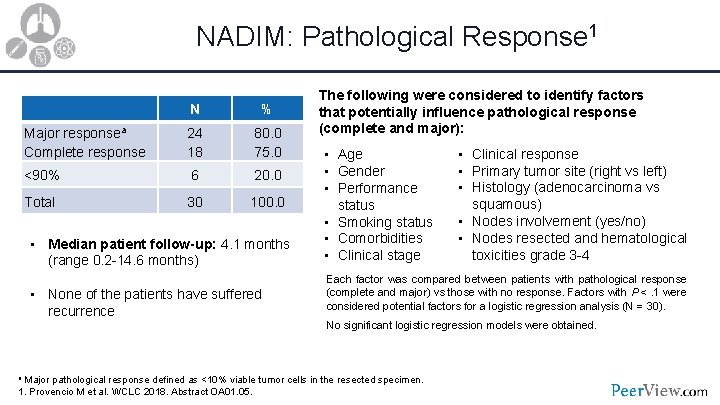
NADIM: Pathological Response 1 N % Major responsea Complete response 24 18 80. 0 75. 0 <90% 6 20. 0 Total 30 100. 0 • Median patient follow-up: 4. 1 months (range 0. 2 -14. 6 months) • None of the patients have suffered recurrence The following were considered to identify factors that potentially influence pathological response (complete and major): • Age • Gender • Performance status • Smoking status • Comorbidities • Clinical stage • Clinical response • Primary tumor site (right vs left) • Histology (adenocarcinoma vs squamous) • Nodes involvement (yes/no) • Nodes resected and hematological toxicities grade 3 -4 Each factor was compared between patients with pathological response (complete and major) vs those with no response. Factors with P <. 1 were considered potential factors for a logistic regression analysis (N = 30). No significant logistic regression models were obtained. a Major pathological response defined as <10% viable tumor cells in the resected specimen. 1. Provencio M et al. WCLC 2018. Abstract OA 01. 05.

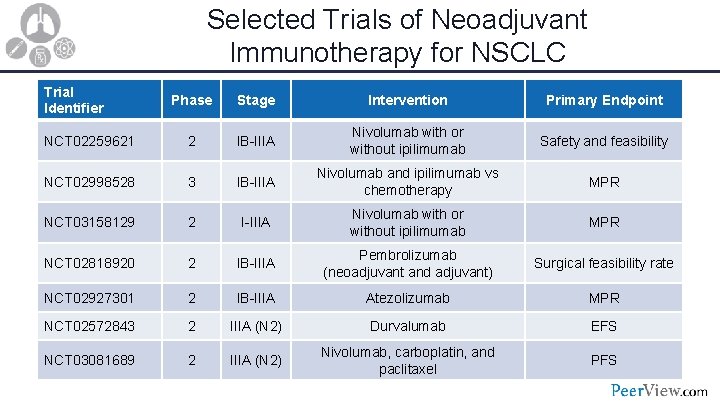
Selected Trials of Neoadjuvant Immunotherapy for NSCLC Trial Identifier Phase Stage Intervention Primary Endpoint NCT 02259621 2 IB-IIIA Nivolumab with or without ipilimumab Safety and feasibility NCT 02998528 3 IB-IIIA Nivolumab and ipilimumab vs chemotherapy MPR NCT 03158129 2 I-IIIA Nivolumab with or without ipilimumab MPR NCT 02818920 2 IB-IIIA Pembrolizumab (neoadjuvant and adjuvant) Surgical feasibility rate NCT 02927301 2 IB-IIIA Atezolizumab MPR NCT 02572843 2 IIIA (N 2) Durvalumab EFS NCT 03081689 2 IIIA (N 2) Nivolumab, carboplatin, and paclitaxel PFS

Practicum Moving Immunotherapy Into Earlier Stages of Lung Cancer Practicalities, Challenges, Questions

Let’s Consider a Case 64 -year-old man with NSCLC • History of 50 pack-years of smoking, but quit 10 years ago • Found to have a 4. 5 -cm lung lesion in the right upper lobe

Case (Cont’d) Pathological findings • Adenocarcinoma • No mutations in EGFR, ALK, ROS 1, or BRAF • PD-L 1: 10% using the 22 C 3 antibody • TMB status not available (not routinely tested)

Case (Cont’d) • Mediastinoscopy was performed • Despite negative PET scan in the mediastinum, all 3 ipsilateral lymph node stations evaluated (level 2, 4, and 7) had evidence of metastatic adenocarcinoma Q Is this patient a candidate for resection? Q Should neoadjuvant immunotherapy protocol be considered?

Case (Cont’d) • Patient receives treatment: Cisplatin and etoposide along with 65 Gy of radiation Q What treatment would you recommend for this patient next?

Please remember to complete and submit your Post-Test and Evaluation for CME/MOC/CE credit. Missed anything? Visit us at: www. peerview. com/Lung 18 • Download slides and Practice Aids • Watch for the on. Demand version of this symposium • Continue the conversation on Twitter @Peer. View Thank you and good day.