The Role of Immunotherapy for the Treatment of








- Slides: 35

The Role of Immunotherapy for the Treatment of Hematologic Malignancies Patrick Stiff MD Coleman Professor of Oncology Loyola University Medical Center 708 -327 -3216

The use of Immune therapy to fight cancer is not new • Allogeneic Bone Marrow Transplantation: adoptive immunotherapy • Antibodies: rituximab, brentuximab • Vaccines: anti-HPV, Hepatitis B vaccines • Dendritic Cell Vaccines • Engineered T cells • Chimeric Antigen Receptor T Cells: CAR-T

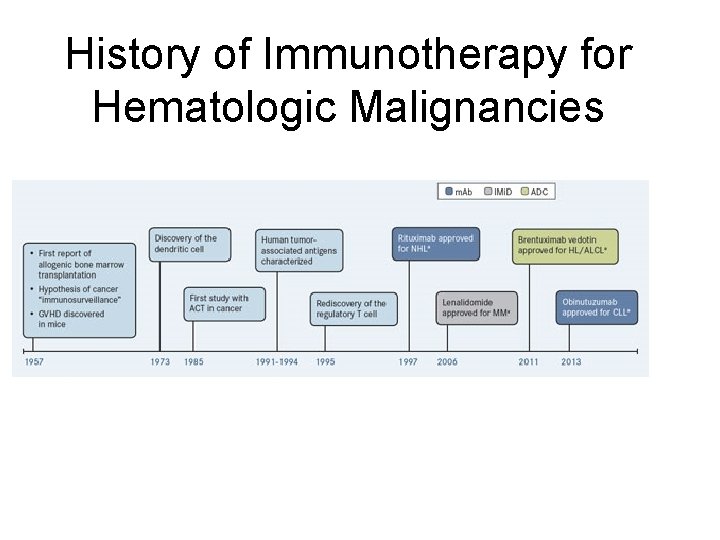
History of Immunotherapy for Hematologic Malignancies

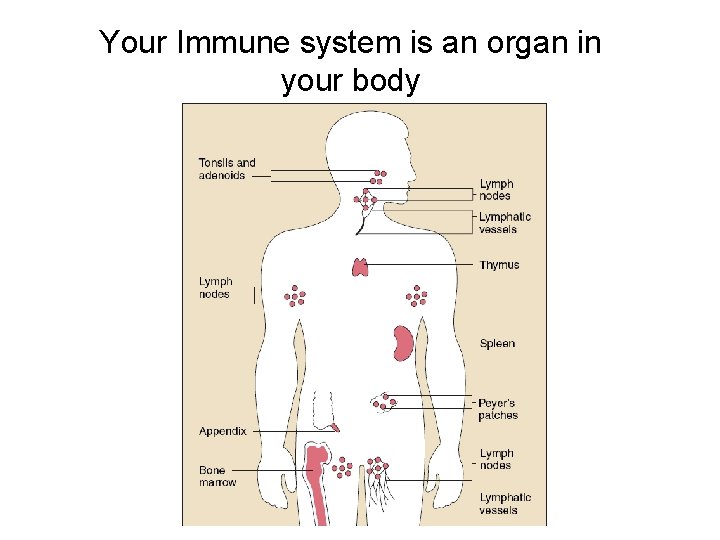
Your Immune system is an organ in your body


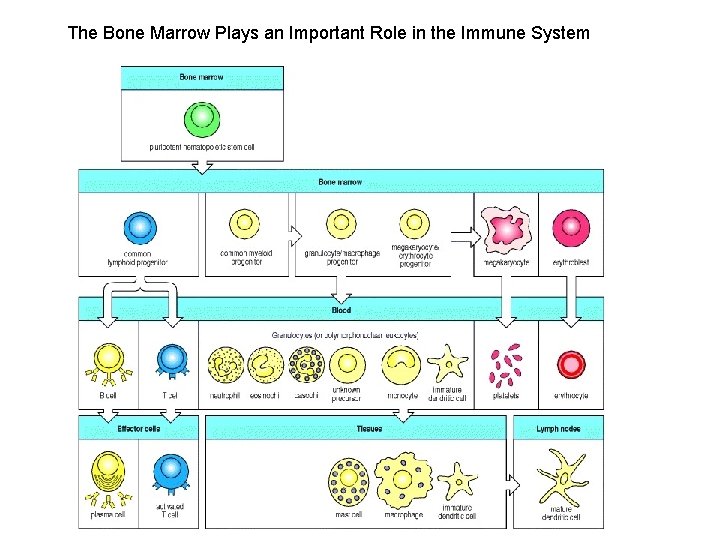
The Bone Marrow Plays an Important Role in the Immune System

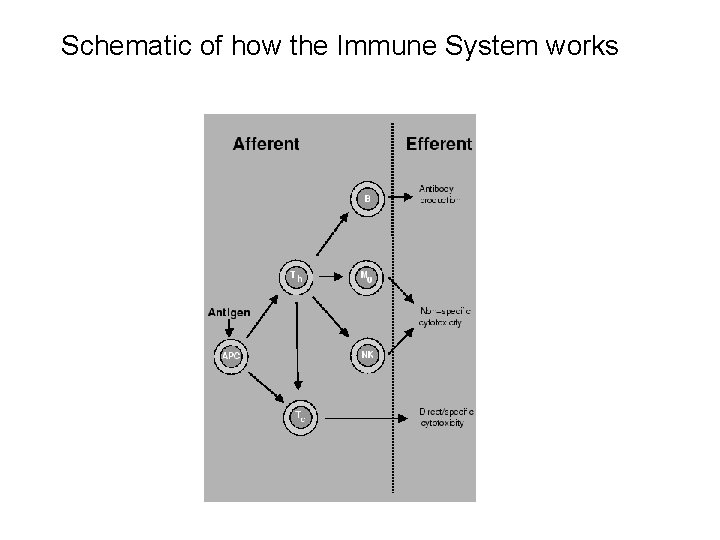
Schematic of how the Immune System works

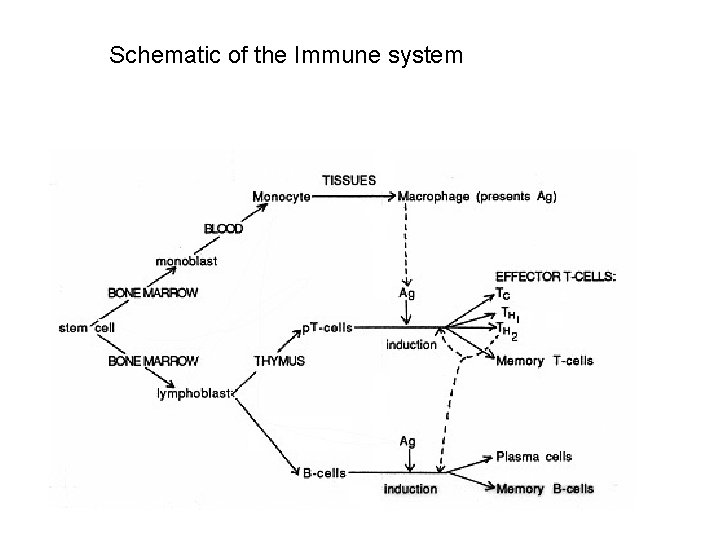
Schematic of the Immune system

For cancer, T cells do most of the work in preventing and eliminating disease

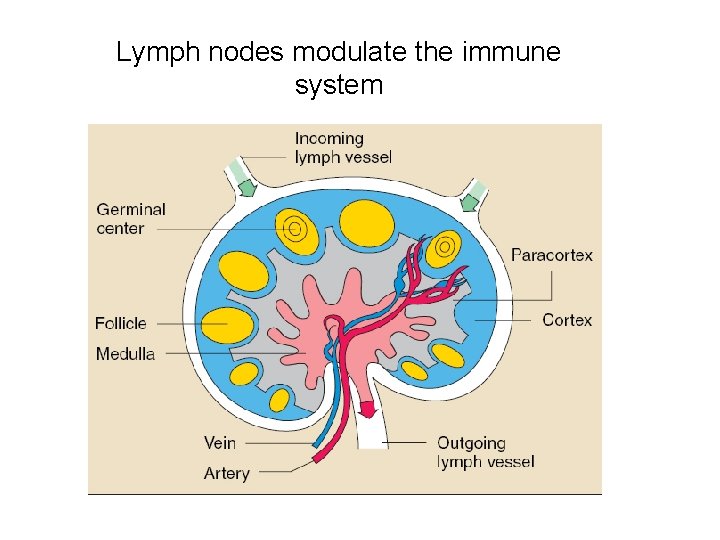
Lymph nodes modulate the immune system

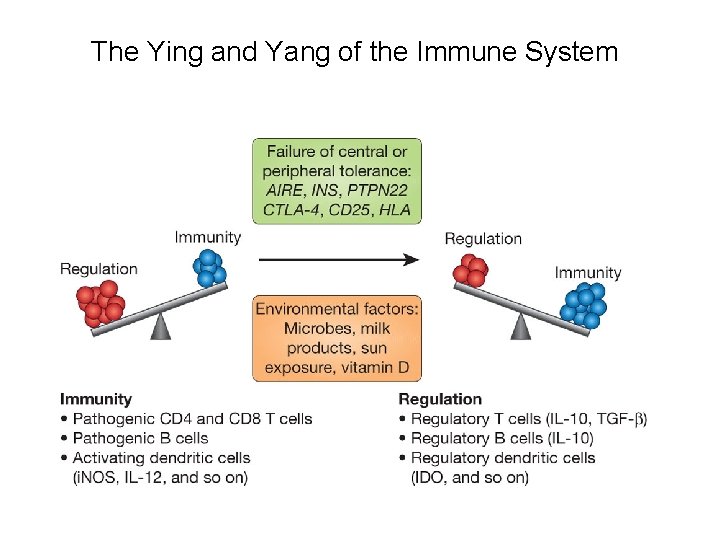
The Ying and Yang of the Immune System

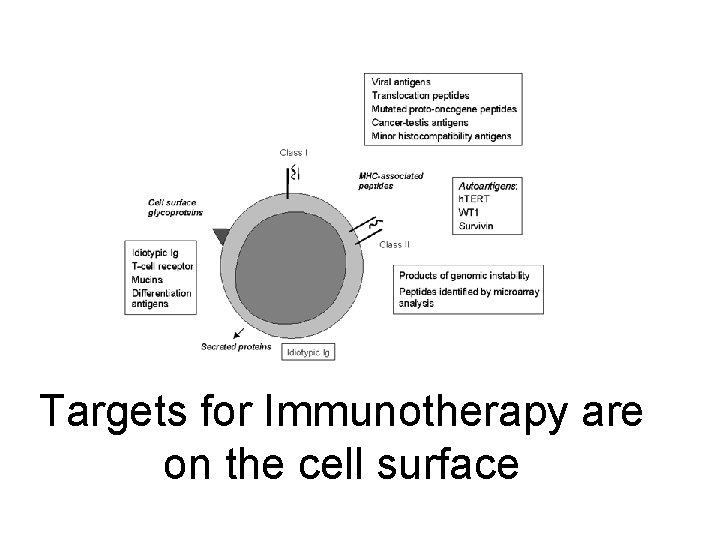
To invoke the immune system you need a target This is called an antigen and is usually on the surface of the cell Antigens can be targeted by antibodies or by cells or in fact by both simultaneously

Targets for Immunotherapy are on the cell surface

In cancer the immune system does not recognize the specific antigens on the tumor cells No response means no cancer cell killing

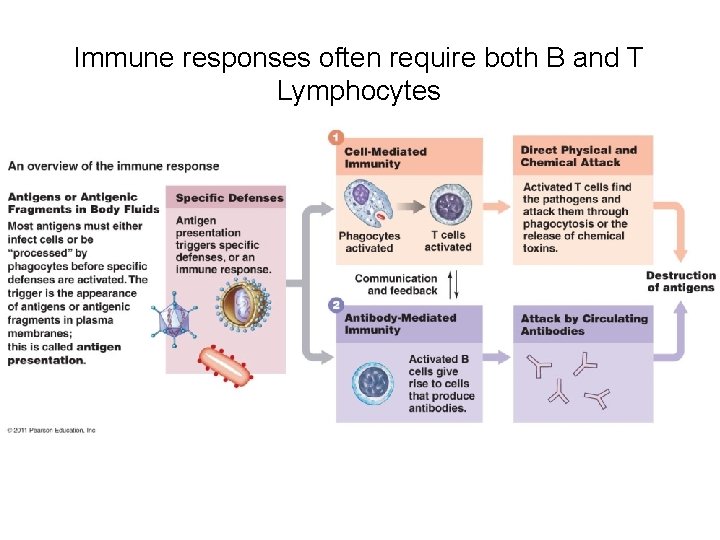
Immune responses often require both B and T Lymphocytes

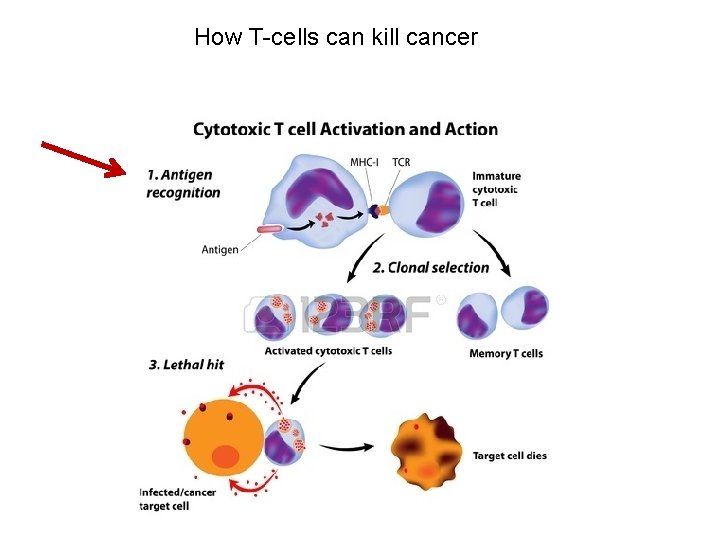
How T-cells can kill cancer

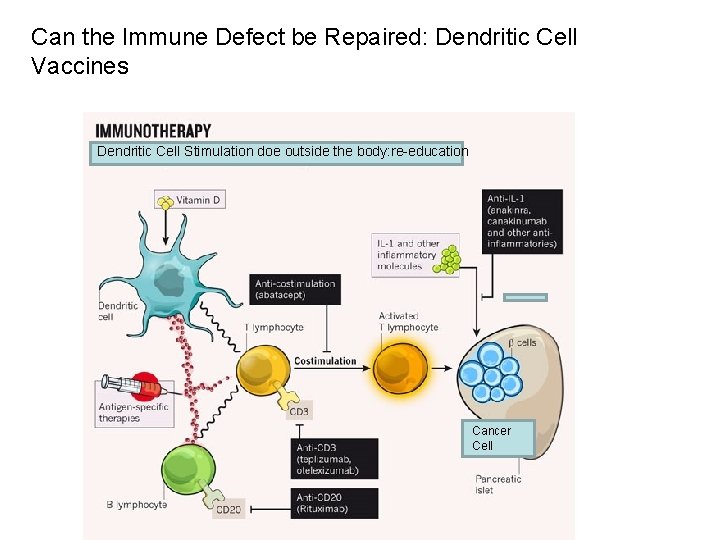
Can the Immune Defect be Repaired: Dendritic Cell Vaccines Dendritic Cell Stimulation doe outside the body: re-education Cancer Cell

CAR-T Cell Therapies • Several Platforms/strategies being developed( U Penn, MSKCC, NCI) • NCI is working collaboratively with Kite Pharmaceuticals to develop therapy for lymphoma and in the future leukemia using an anti-CD 19 CAR-T cell

DISCLAIMER / FORWARD LOOKING STATEMENTS am neither an employee nor representative of Kite Pharma, Inc. (“Kite”). Any statements made by me during this presentation are mine alone, have not been authorized or otherwise endorsed by Kite and are not to be attributed to Kite. As a courtesy as the trial sponsor of KTE-C 19 -101, Kite has provided publicly available information for my presentation. I

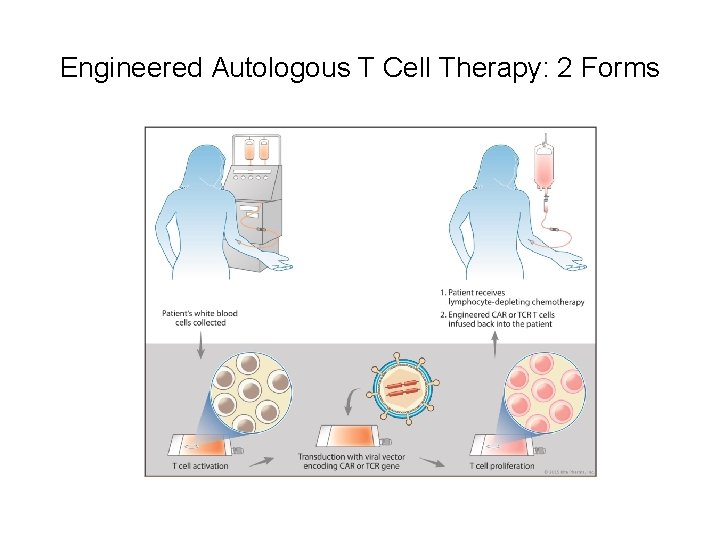
Engineered Autologous T Cell Therapy: 2 Forms

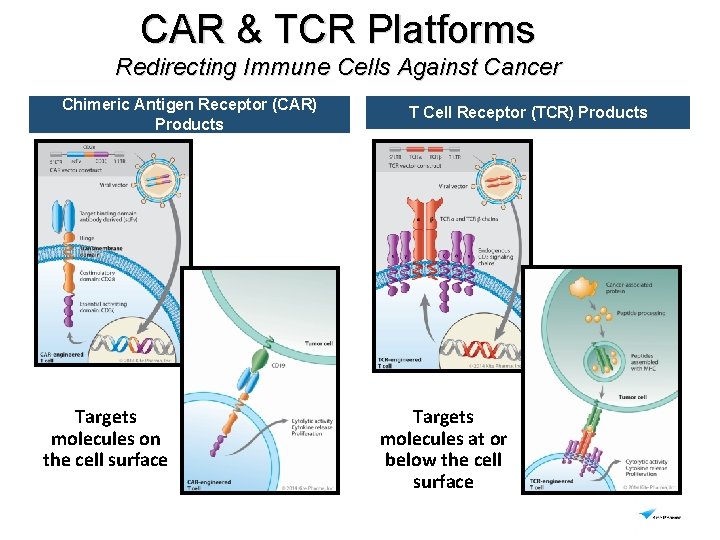
CAR & TCR Platforms Redirecting Immune Cells Against Cancer Chimeric Antigen Receptor (CAR) Products Targets molecules on the cell surface T Cell Receptor (TCR) Products Targets molecules at or below the cell surface

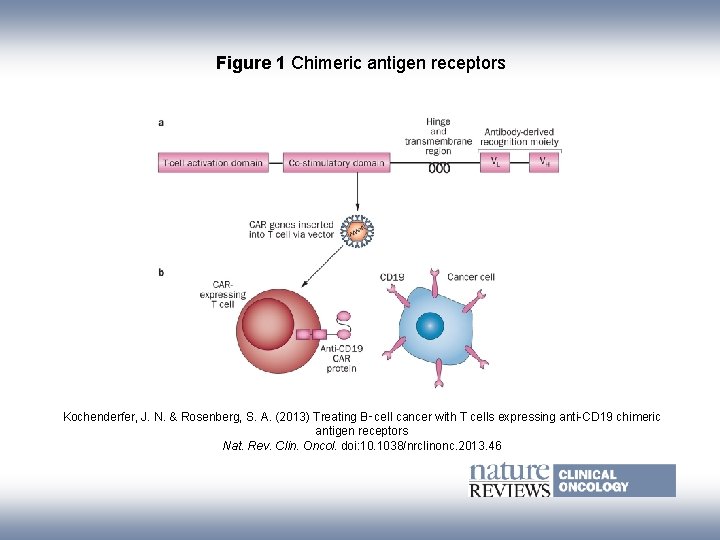
Figure 1 Chimeric antigen receptors Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

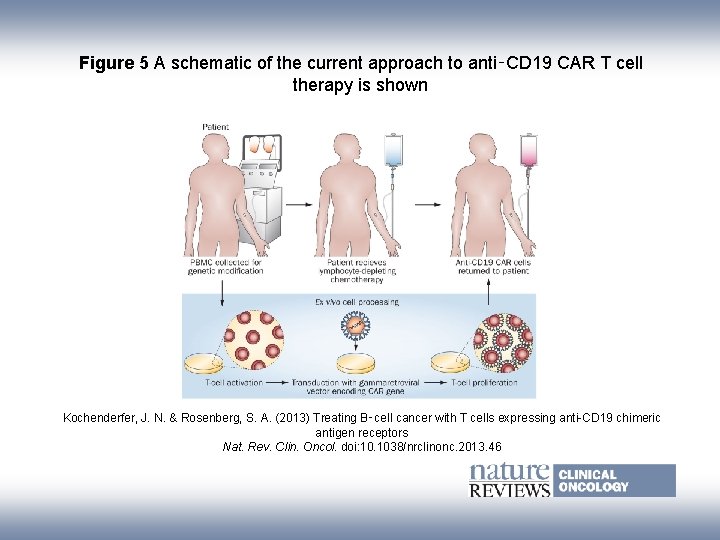
Figure 5 A schematic of the current approach to anti‑CD 19 CAR T cell therapy is shown Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

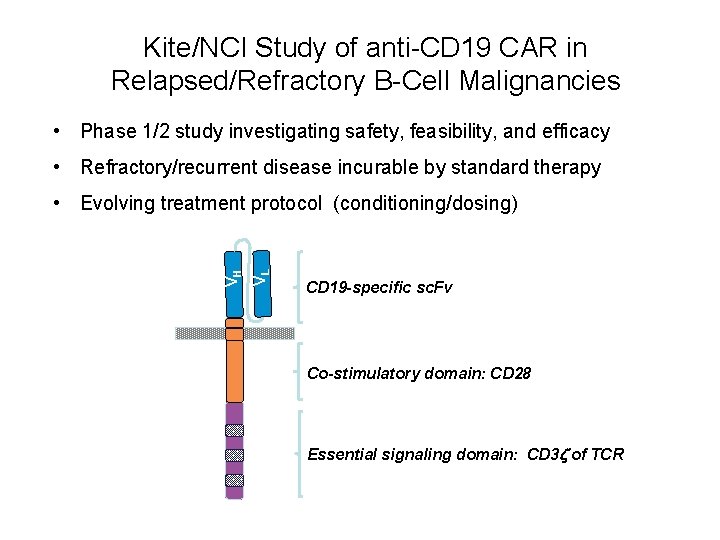
Kite/NCI Study of anti-CD 19 CAR in Relapsed/Refractory B-Cell Malignancies • Phase 1/2 study investigating safety, feasibility, and efficacy • Refractory/recurrent disease incurable by standard therapy VL VH • Evolving treatment protocol (conditioning/dosing) CD 19 -specific sc. Fv Co-stimulatory domain: CD 28 Essential signaling domain: CD 3 z of TCR

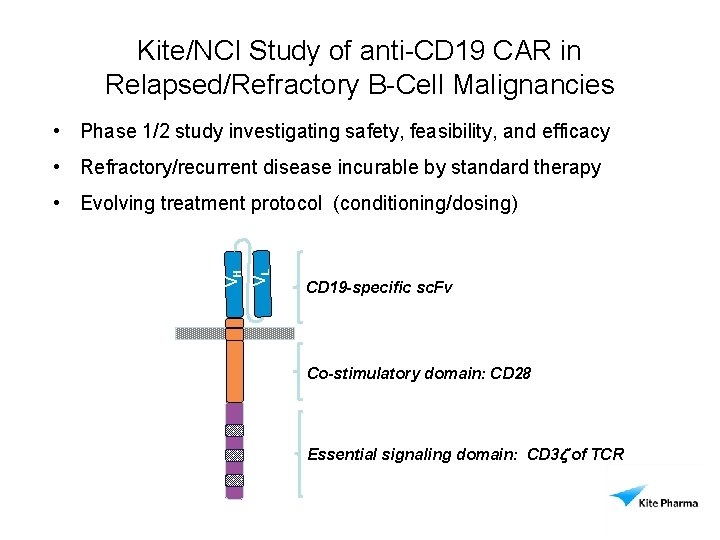
Kite/NCI Study of anti-CD 19 CAR in Relapsed/Refractory B-Cell Malignancies • Phase 1/2 study investigating safety, feasibility, and efficacy • Refractory/recurrent disease incurable by standard therapy VL VH • Evolving treatment protocol (conditioning/dosing) CD 19 -specific sc. Fv Co-stimulatory domain: CD 28 Essential signaling domain: CD 3 z of TCR

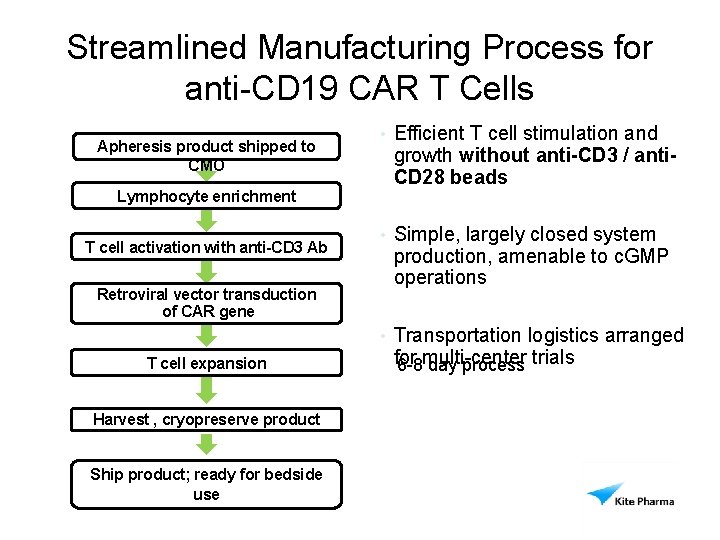
Streamlined Manufacturing Process for anti-CD 19 CAR T Cells Apheresis product shipped to CMO Lymphocyte enrichment T cell activation with anti-CD 3 Ab Retroviral vector transduction of CAR gene • Efficient T cell stimulation and growth without anti-CD 3 / anti. CD 28 beads • Simple, largely closed system production, amenable to c. GMP operations • Transportation logistics arranged T cell expansion Harvest , cryopreserve product Ship product; ready for bedside use for multi-center trials 6 -8 day process

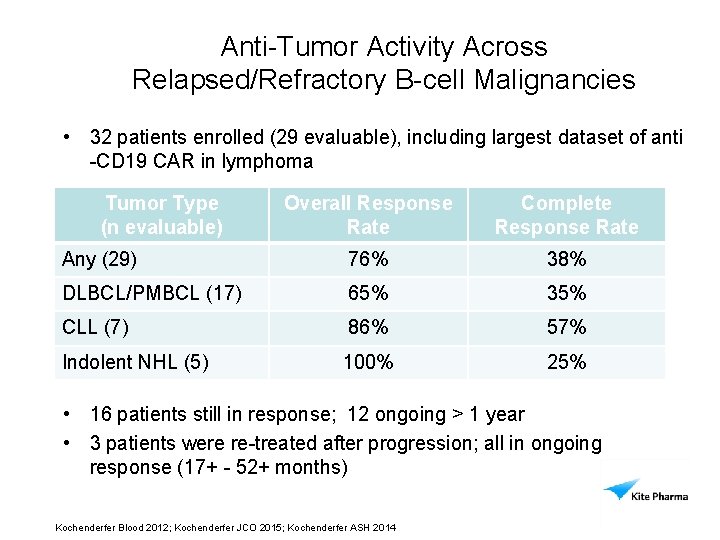
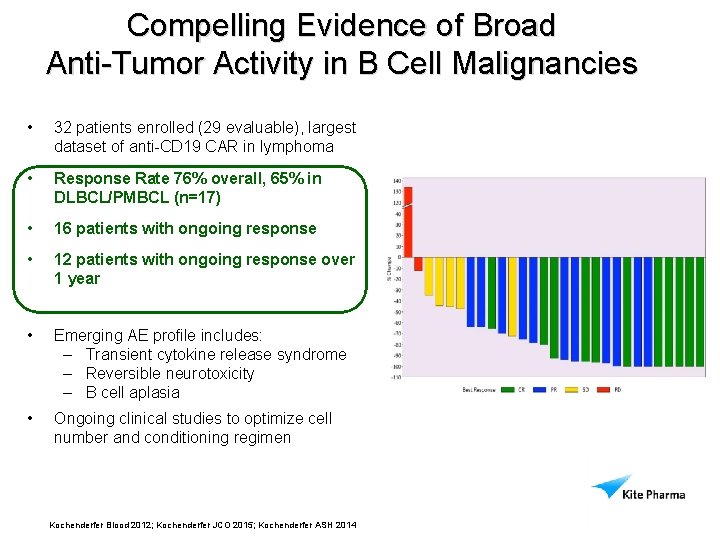
Anti-Tumor Activity Across Relapsed/Refractory B-cell Malignancies • 32 patients enrolled (29 evaluable), including largest dataset of anti -CD 19 CAR in lymphoma Tumor Type (n evaluable) Overall Response Rate Complete Response Rate Any (29) 76% 38% DLBCL/PMBCL (17) 65% 35% CLL (7) 86% 57% 100% 25% Indolent NHL (5) • 16 patients still in response; 12 ongoing > 1 year • 3 patients were re-treated after progression; all in ongoing response (17+ - 52+ months) Source: S: CD 19Clinical Developmentdata30 NOV 2014derived Kochenderfer Blood 2012; Kochenderfer JCO 2015; Kochenderfer ASH 2014

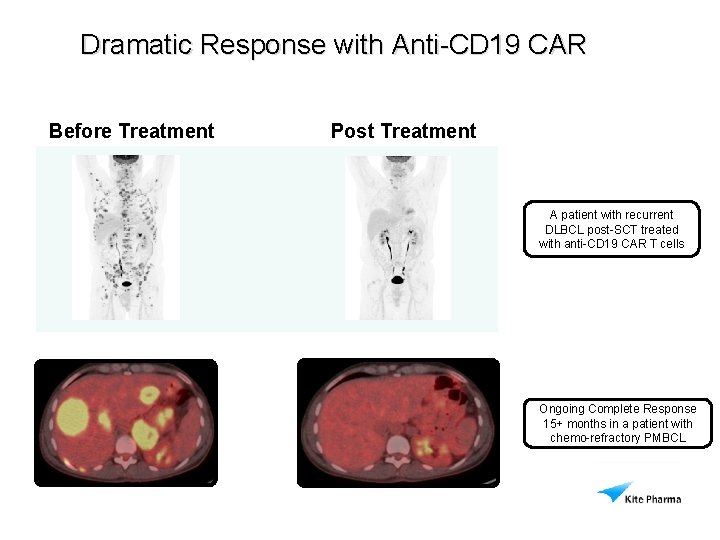
Dramatic Response with Anti-CD 19 CAR Before Treatment Post Treatment A patient with recurrent DLBCL post-SCT treated with anti-CD 19 CAR T cells Ongoing Complete Response 15+ months in a patient with chemo-refractory PMBCL Scans from Dr. Rosenberg NCI

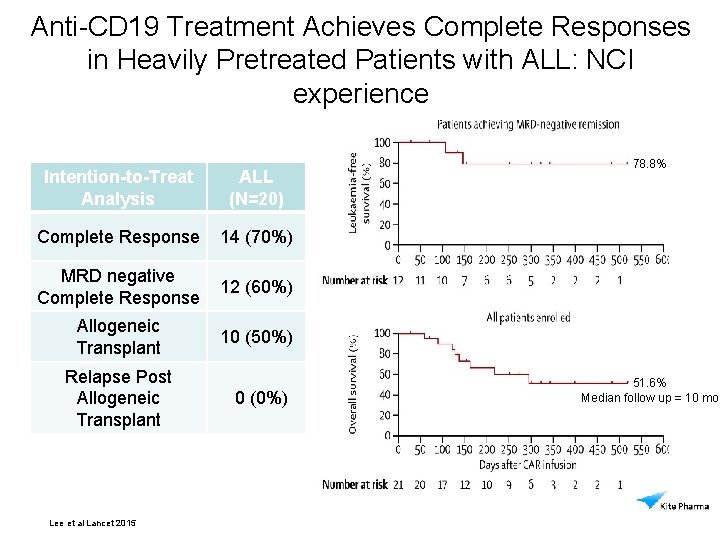
Anti-CD 19 Treatment Achieves Complete Responses in Heavily Pretreated Patients with ALL: NCI experience Intention-to-Treat Analysis ALL (N=20) 78. 8% Complete Response 14 (70%) MRD negative Complete Response 12 (60%) Allogeneic Transplant 10 (50%) Relapse Post Allogeneic Transplant Lee et al Lancet 2015 0 (0%) 51. 6% Median follow up = 10 mo

Compelling Evidence of Broad Anti-Tumor Activity in B Cell Malignancies • 32 patients enrolled (29 evaluable), largest dataset of anti-CD 19 CAR in lymphoma • Response Rate 76% overall, 65% in DLBCL/PMBCL (n=17) • 16 patients with ongoing response • 12 patients with ongoing response over 1 year • Emerging AE profile includes: – Transient cytokine release syndrome – Reversible neurotoxicity – B cell aplasia • Ongoing clinical studies to optimize cell number and conditioning regimen Kochenderfer Blood 2012; Kochenderfer JCO 2015; Kochenderfer ASH 2014

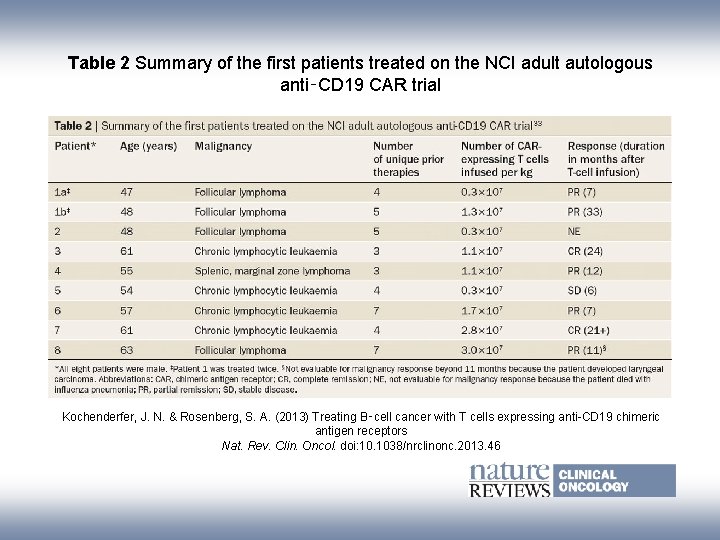
Table 2 Summary of the first patients treated on the NCI adult autologous anti‑CD 19 CAR trial Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

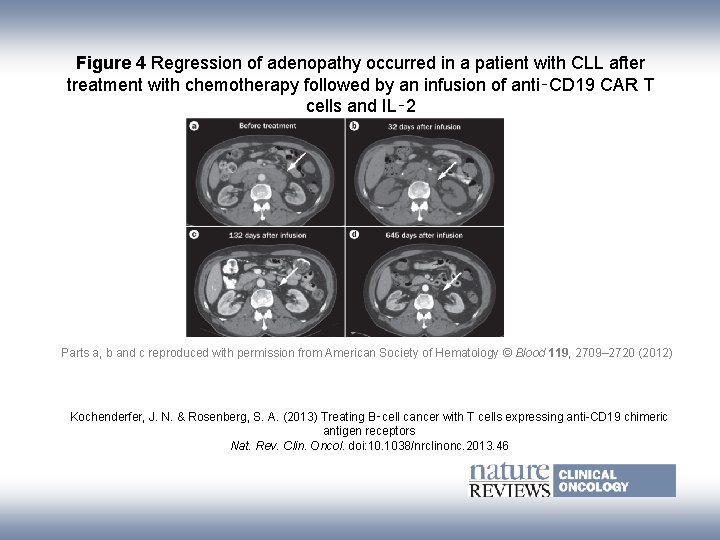
Figure 4 Regression of adenopathy occurred in a patient with CLL after treatment with chemotherapy followed by an infusion of anti‑CD 19 CAR T cells and IL‑ 2 Parts a, b and c reproduced with permission from American Society of Hematology © Blood 119, 2709– 2720 (2012) Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

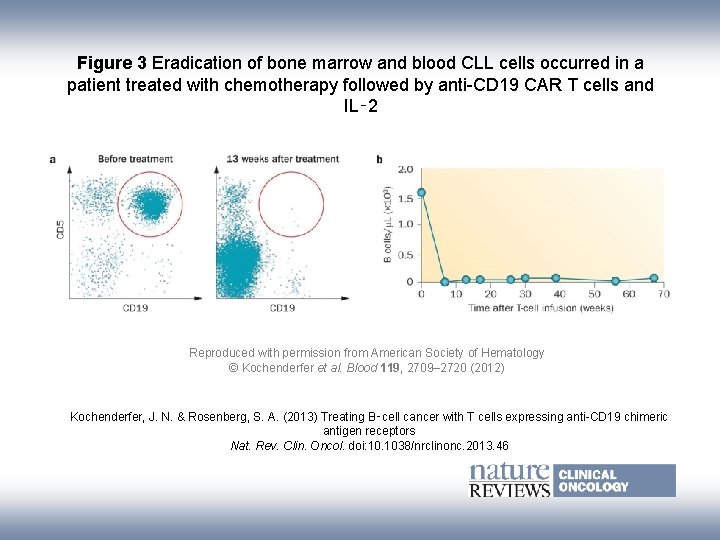
Figure 3 Eradication of bone marrow and blood CLL cells occurred in a patient treated with chemotherapy followed by anti-CD 19 CAR T cells and IL‑ 2 Reproduced with permission from American Society of Hematology © Kochenderfer et al. Blood 119, 2709– 2720 (2012) Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

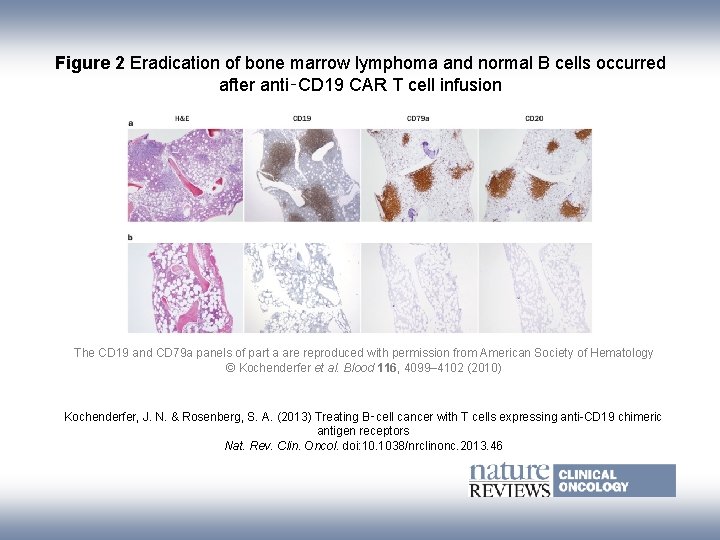
Figure 2 Eradication of bone marrow lymphoma and normal B cells occurred after anti‑CD 19 CAR T cell infusion The CD 19 and CD 79 a panels of part a are reproduced with permission from American Society of Hematology © Kochenderfer et al. Blood 116, 4099– 4102 (2010) Kochenderfer, J. N. & Rosenberg, S. A. (2013) Treating B‑cell cancer with T cells expressing anti-CD 19 chimeric antigen receptors Nat. Rev. Clin. Oncol. doi: 10. 1038/nrclinonc. 2013. 46

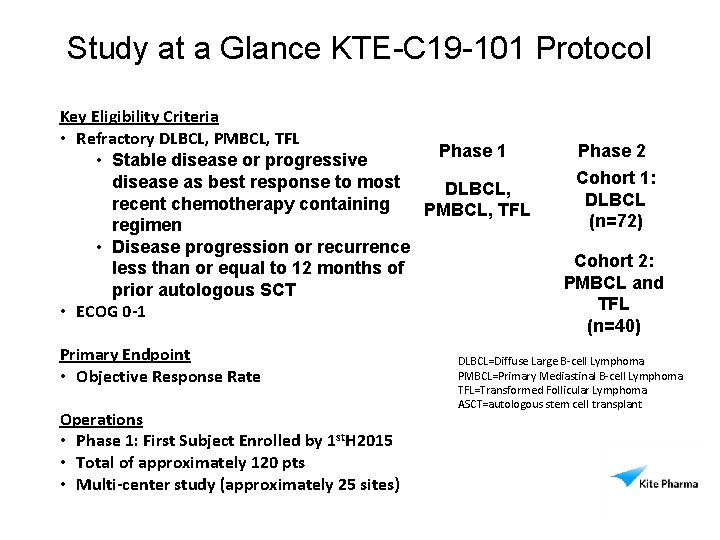
Study at a Glance KTE-C 19 -101 Protocol Key Eligibility Criteria • Refractory DLBCL, PMBCL, TFL Phase 1 • Stable disease or progressive disease as best response to most DLBCL, recent chemotherapy containing PMBCL, TFL regimen • Disease progression or recurrence less than or equal to 12 months of prior autologous SCT • ECOG 0 -1 Primary Endpoint • Objective Response Rate Operations • Phase 1: First Subject Enrolled by 1 st. H 2015 • Total of approximately 120 pts • Multi-center study (approximately 25 sites) Phase 2 Cohort 1: DLBCL (n=72) Cohort 2: PMBCL and TFL (n=40) DLBCL=Diffuse Large B-cell Lymphoma PMBCL=Primary Mediastinal B-cell Lymphoma TFL=Transformed Follicular Lymphoma ASCT=autologous stem cell transplant
 Immunotherapy for pots
Immunotherapy for pots Immunotherapy
Immunotherapy Ukons immunotherapy guidelines
Ukons immunotherapy guidelines Azure web role worker role example
Azure web role worker role example Symbolischer interaktionismus krappmann
Symbolischer interaktionismus krappmann Role conflict occurs when fulfilling the role expectations
Role conflict occurs when fulfilling the role expectations Presentera för publik crossboss
Presentera för publik crossboss Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Myndigheten för delaktighet
Myndigheten för delaktighet Debattinlägg mall
Debattinlägg mall Kung dog 1611
Kung dog 1611 Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Vad är referatmarkeringar
Vad är referatmarkeringar Karttecken ruin
Karttecken ruin Fimbrietratt
Fimbrietratt Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? Verifikationsplan
Verifikationsplan Rbk mätning
Rbk mätning Formel för lufttryck
Formel för lufttryck Densitet vatten
Densitet vatten Elektronik för barn
Elektronik för barn Borra hål för knoppar
Borra hål för knoppar Tack för att ni har lyssnat
Tack för att ni har lyssnat Anatomi organ reproduksi
Anatomi organ reproduksi Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Bris för vuxna
Bris för vuxna Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Typiska novell drag
Typiska novell drag Trög för kemist
Trög för kemist Magnetsjukhus
Magnetsjukhus Ellika andolf
Ellika andolf Matte större än tecken
Matte större än tecken Toppslätskivling dos
Toppslätskivling dos Redogör för vad psykologi är
Redogör för vad psykologi är Mat för idrottare
Mat för idrottare Gumman cirkel sång
Gumman cirkel sång