Chapter 22 Nuclear Chemistry Nuclear Chemistry Nuclear NOT






























- Slides: 64

Chapter 22 Nuclear Chemistry

Nuclear Chemistry Nuclear (NOT Nucular) chemistry involves changes in the nuclear composition (protons and neutrons) of atoms that are radioactive. n Nuclear reactions can be used as energy sources, for medical diagnosis, and for medical treatment. n

Chemical Reactions Breaking/forming bonds via electrons n Start and end with same number and type of atoms (conservation of mass) n DH ≈ -1 - 1000 k. J n Rates affected by temperature, pressure, concentration, catalyst n

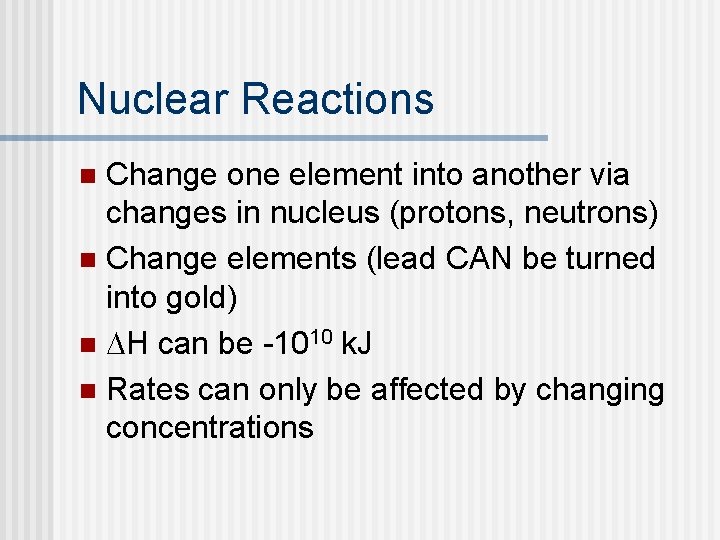
Nuclear Reactions Change one element into another via changes in nucleus (protons, neutrons) n Change elements (lead CAN be turned into gold) n DH can be -1010 k. J n Rates can only be affected by changing concentrations n

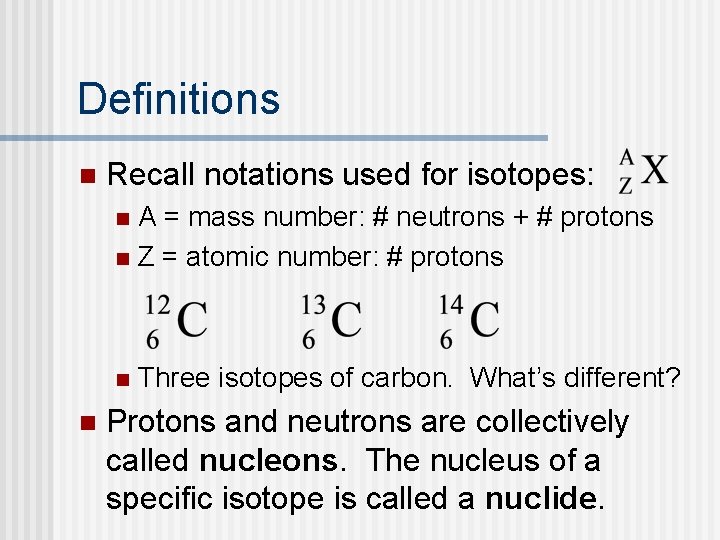
Definitions n Recall notations used for isotopes: A = mass number: # neutrons + # protons n Z = atomic number: # protons n n n Three isotopes of carbon. What’s different? Protons and neutrons are collectively called nucleons. The nucleus of a specific isotope is called a nuclide.

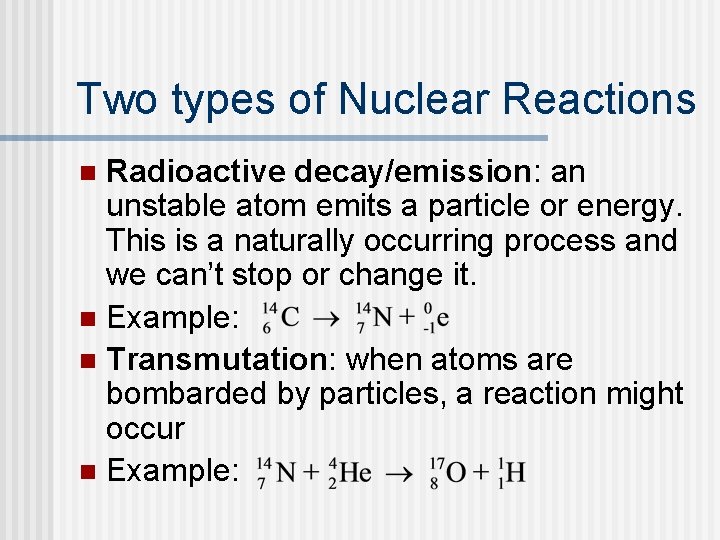
Two types of Nuclear Reactions Radioactive decay/emission: an unstable atom emits a particle or energy. This is a naturally occurring process and we can’t stop or change it. n Example: n Transmutation: when atoms are bombarded by particles, a reaction might occur n Example: n

22. 1 Nuclear Reactions n Radiation arises from nuclear reactions: parent nuclide daughter nuclide + radiation To balance, two conditions must be met: • Conserve mass number (A) • Conserve nuclear charge (Z) n If we know two of the nuclear particles, we can use these rules to identify the third particle. n

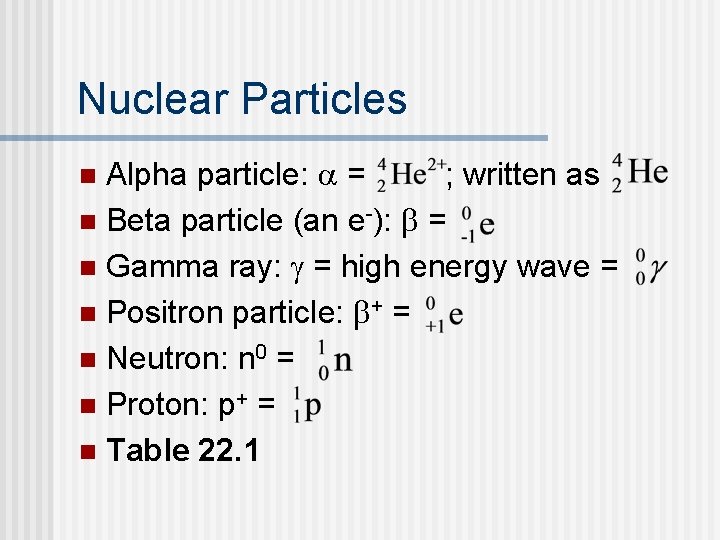
Nuclear Particles Alpha particle: a = ; written as n Beta particle (an e-): b = n Gamma ray: g = high energy wave = n Positron particle: b+ = n Neutron: n 0 = n Proton: p+ = n Table 22. 1 n

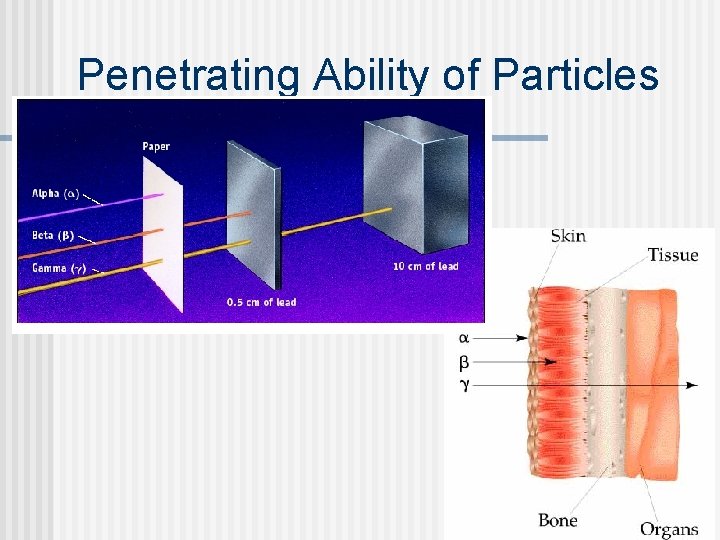
Penetrating Ability of Particles

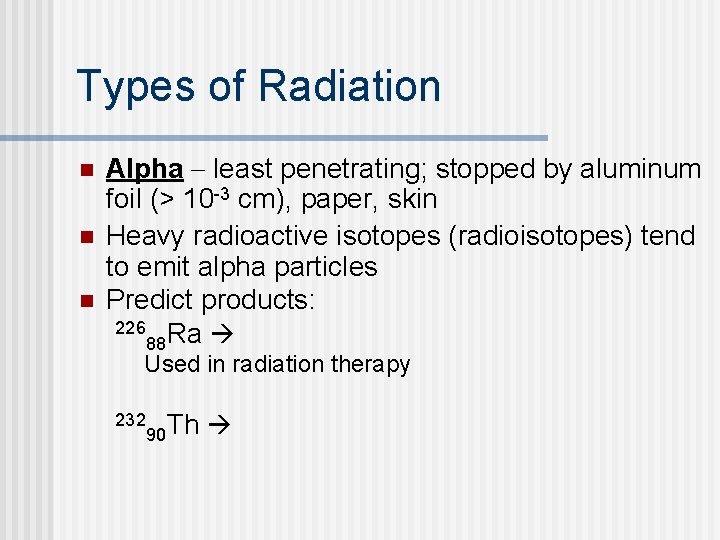
Types of Radiation n Alpha least penetrating; stopped by aluminum foil (> 10 -3 cm), paper, skin Heavy radioactive isotopes (radioisotopes) tend to emit alpha particles Predict products: 226 Ra 88 Used in radiation therapy 232 90 Th

Beta Emission/Decay, Positron Emission/Decay n Beta - b = 0 -1 e; more penetrating - stopped by 0. 05 - 0. 1 cm of aluminum n n n Beta emission: neutron is converted to a proton and an electron Positron emission: proton is converted to a neutron and a positron Predict products: n n Beta emission: 239 U Beta emission: 131 I Positron emission: 207 Po Positron emission: 40 K

Gamma Radiation (Emission) Gamma - g = energy with no mass or charge; most penetrating radiation n Stopped by 5 - 11 cm of aluminum or thick layer of concrete or lead n Lead is commonly used to enclose radioactive materials because radiation does not penetrate readily n In the 1950 s, it was common to build thick concrete bomb shelters n

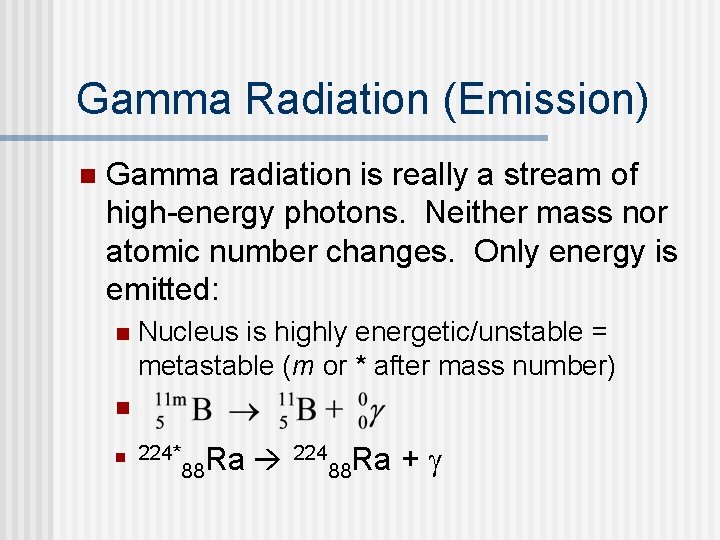
Gamma Radiation (Emission) n Gamma radiation is really a stream of high-energy photons. Neither mass nor atomic number changes. Only energy is emitted: Nucleus is highly energetic/unstable = metastable (m or * after mass number) n. n n 224* 224 Ra + g Ra 88 88

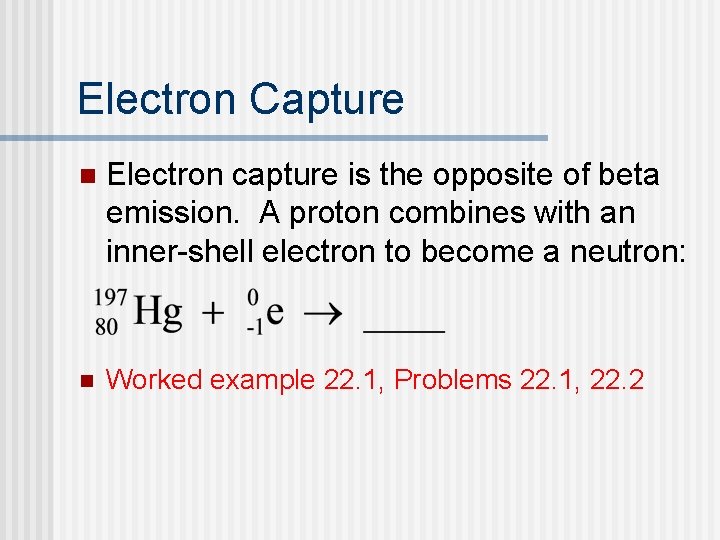
Electron Capture n Electron capture is the opposite of beta emission. A proton combines with an inner-shell electron to become a neutron: n Worked example 22. 1, Problems 22. 1, 22. 2

Group Work n Write balanced equations for: 1. Alpha emission from curium-242 2. Beta emission from magnesium-28 3. Positron emission from xenon-118 4. Electron capture by polonium-204 n What particle is produced by decay of thorium-214 to radium-210?

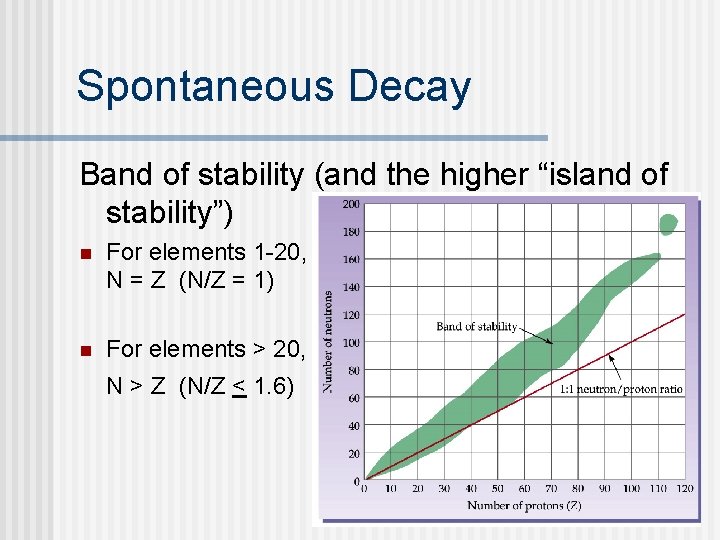
22. 4 Nuclear Stability n n Unstable isotopes are the radioactive ones they decay too rapidly to be measured. “Stable” isotopes have measurable isotopes. Every element up to Bi (except Tc) has one or more stable isotopes. Elements above Bi are all unstable. The “Band of Stability” plots the stable atoms (protons vs neutrons). n Neutron/proton ratio helps predict stability.

Spontaneous Decay Band of stability (and the higher “island of stability”) n For elements 1 -20, N = Z (N/Z = 1) n For elements > 20, N > Z (N/Z < 1. 6)

Radioactivity n n Nuclides above the band (upper left) often undergo beta decay; nuclides below the band undergo positron emission, electron capture, or alpha emission. The heavier elements (93 and higher) are synthetic and radioactive. Elements with molar mass in parentheses on the periodic table are radioactive. Why don’t these have a more precise molar mass?

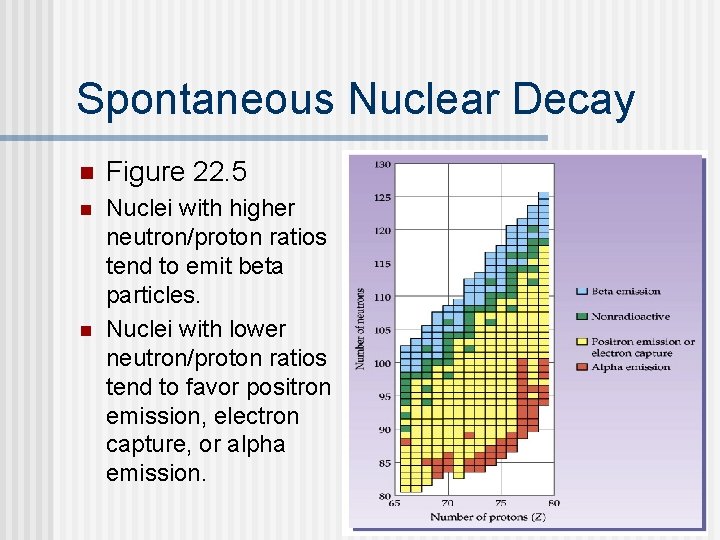
Spontaneous Nuclear Decay n Figure 22. 5 n Nuclei with higher neutron/proton ratios tend to emit beta particles. Nuclei with lower neutron/proton ratios tend to favor positron emission, electron capture, or alpha emission. n

Nuclear Stability Figure 22. 4 n Applet: http: //www. colorado. e du/physics/2000/appl ets/iso. html n

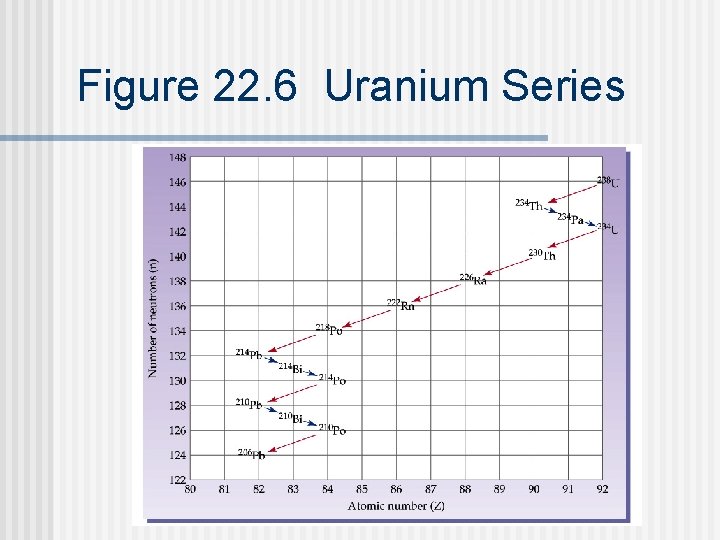
Figure 22. 6 Uranium Series

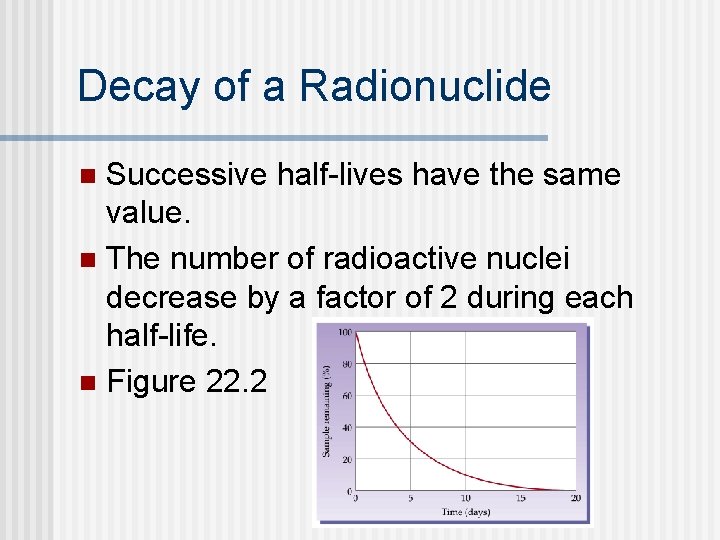
Kinetics of Radioactive Decay n n The rate of decay, as well as the type and energy of the radiation, determines the damage caused by radiation. Nuclear decay follows first-order kinetics, which gives a constant t 1/2 over the course of the decay. Nuclear decay rates are also independent of temperature. Usually we cite t 1/2 instead of a rate constant.

Decay of a Radionuclide Successive half-lives have the same value. n The number of radioactive nuclei decrease by a factor of 2 during each half-life. n Figure 22. 2 n

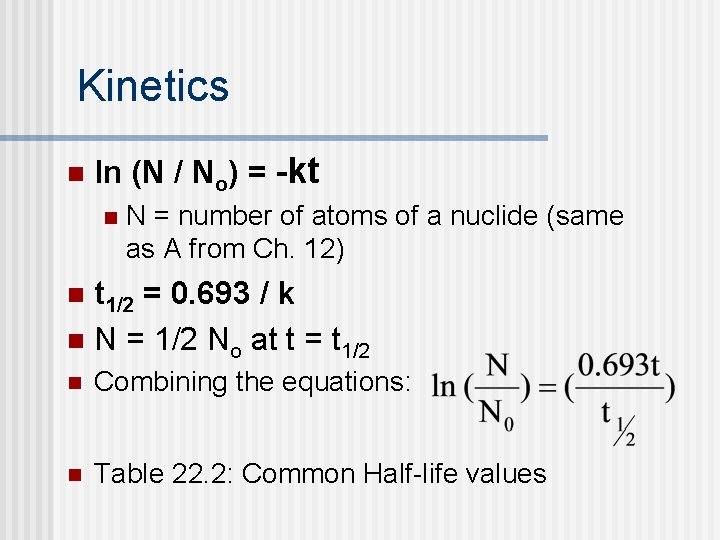
Kinetics n ln (N / No) = -kt n N = number of atoms of a nuclide (same as A from Ch. 12) n t 1/2 = 0. 693 / k n N = 1/2 No at t = t 1/2 n Combining the equations: n Table 22. 2: Common Half-life values

Kinetics n n Can use kinetics, just like in Chapter 12, to do various calculations. ln(N/No) = -0. 693 t / t 1/2 n n n How much nuclide is left after ___ time? How much time has elapsed if decay is ___% complete? Use kinetics to determine storage time of nuclear waste. (Usually 10 half-lives: 2 -10 = 0. 000977, so 99. 9023% has decayed) 51 Cr (t 1/2 = 27. 8 days) is stored for > 10 months

Group Work n The decay constant for sodium-24, a radioisotope used medically in blood studies, is 4. 63 x 10– 2 h– 1. What is the half-life of 24 Na? n Gold-128 undergoes beta decay to give mercury -128 with a half-life of 2. 7 days. What percent of gold-128 is left after 14 days? n Worked example 22. 2 -5, Problems 22. 3 -8

C -14 Dating http: //www. youtube. com/watch? v=81 d. WTereg. EA&feature=related 22. 10 Archeological Dating n n The decay of radioactive isotopes allows for the relative age of once-living things to be calculated. Radiocarbon dating uses 14 C n Living things have a dynamic equilibrium of 14 C in their system (ex: inhaling/exhaling) When a plant or animal dies, it no longer incorporates new 14 C, and the 14 C begins to decay, causing the 14 C content to become less than that in the atmosphere. 14 C 14 N + 0 e 6 7 -1

Geological Dating n n Radiometric Dating http: //www. youtube. com/watch? v=1920 gi 3 swe 4 Geological dating is similar to archeological dating, but uses longer-lived nuclides Measure ratio of 40 K to 40 Ar in rocks 0 e 40 Ar n 40 K + 19 -1 18 n 40 40 Ca + 0 e K 19 20 -1 n 40 K n Rocks are crushed, 40 Ar escapes, and the ratio of 40 K to 40 Ar is measured. This allowed the age of the earth to be calculated to be 4. 5 billion years. n has a half-life of 1. 28 x 109 years

Global Energy Sources n n http: //www. world-nuclear. org/info/inf 01. html http: //www. nucleartourist. com/basics/why. htm n List of advantages/disadvantages of each energy source.

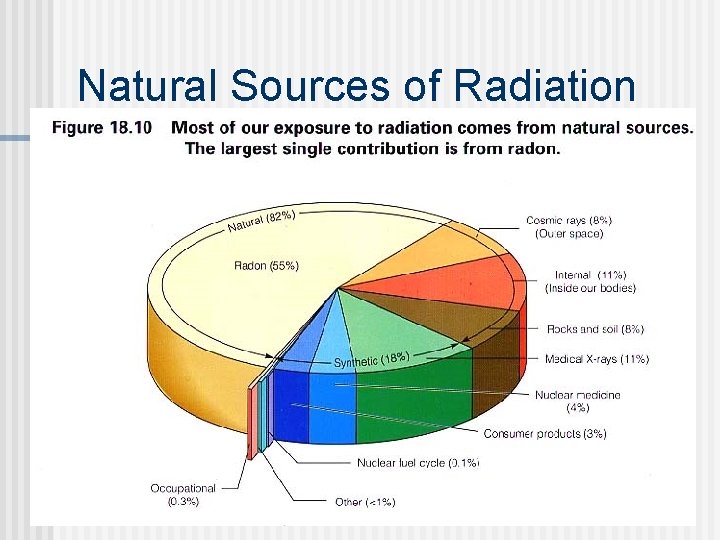
Natural Sources of Radiation n Can damage tissue cells Radiation comes continuously from many sources besides nuclear power plants and applications of isotopes Natural radiation sources: granite soil water food air brick concrete cosmic rays (airplane flights) radon in houses

Nuclear Reactions n n n Nuclear fission: bombarding nuclei of heavier isotopes by neutrons splits them into lighter, more stable nuclei. Nuclear fusion: nuclei of light atoms are joined together or fused. This requires very hot conditions (i. e. , the sun). Bombardment/Transmutation: two elements (usually one heavy, one light) are bombarded by each other to create heavier elements

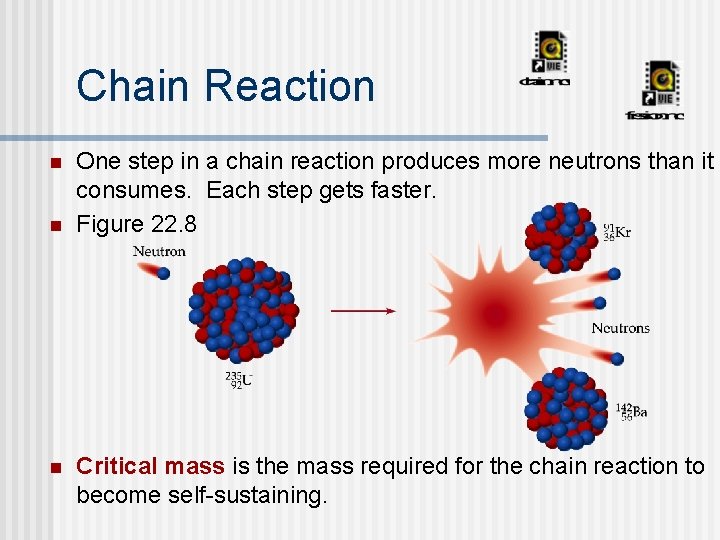
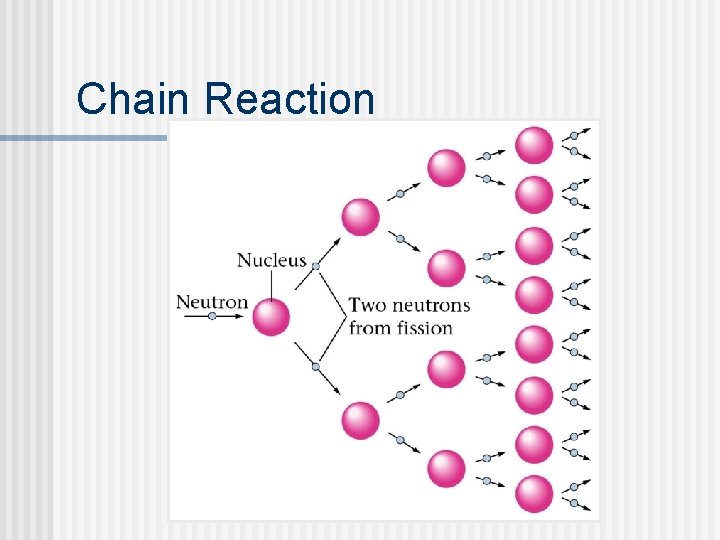
Chain Reaction n One step in a chain reaction produces more neutrons than it consumes. Each step gets faster. Figure 22. 8 Critical mass is the mass required for the chain reaction to become self-sustaining.

Chain Reaction

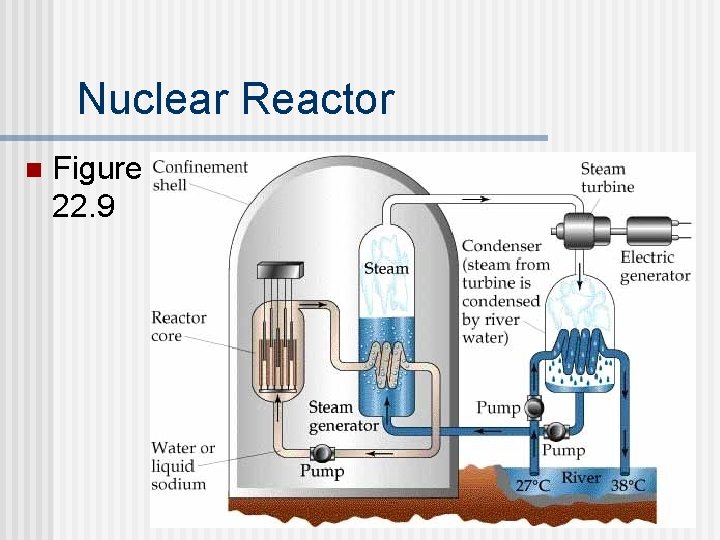
Nuclear Reactor The same fission process that leads to a nuclear explosion can be used to generate electric power. n Nuclear Reactors “control” the fission of 235 U and use the energy produced to heat water that drives steam turbines. (Figure 22. 9) n

Nuclear Reactor n Figure 22. 9

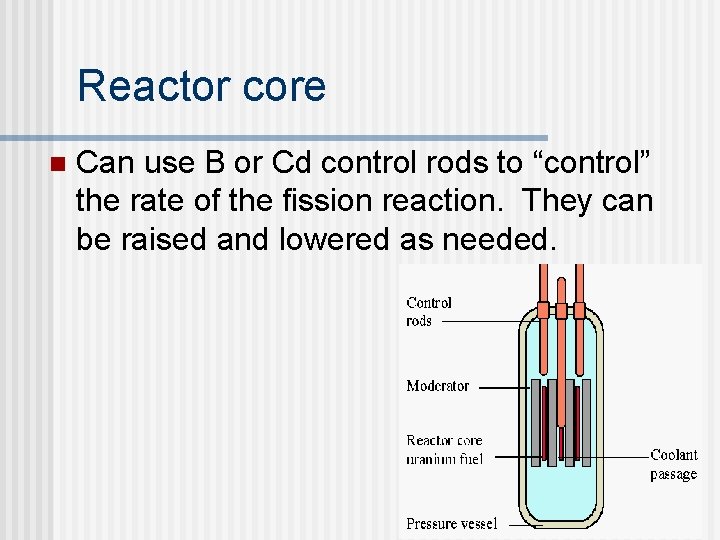
Reactor core n Can use B or Cd control rods to “control” the rate of the fission reaction. They can be raised and lowered as needed.

Nuclear Power n n Energy released from 200 g U = 2 x 1013 J Energy released from 1 ton coal = 5 x 107 J It would take 1 million tons of coal to create the same amount of energy. Nuclear power plants don’t explode (like TNT unless an uncontrolled nuclear chain reaction occurs). When nuclear power plants have meltdowns, the threat is from leakage of radioactive material (water, airborne, soil contamination, etc. )

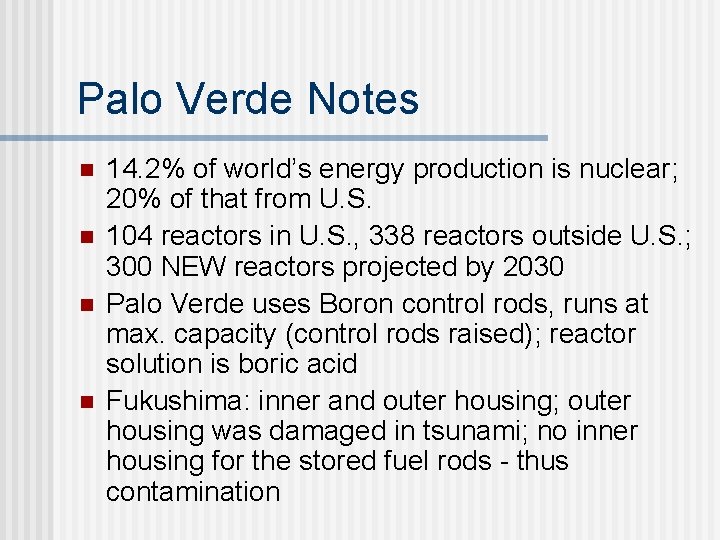
Palo Verde Notes n n 14. 2% of world’s energy production is nuclear; 20% of that from U. S. 104 reactors in U. S. , 338 reactors outside U. S. ; 300 NEW reactors projected by 2030 Palo Verde uses Boron control rods, runs at max. capacity (control rods raised); reactor solution is boric acid Fukushima: inner and outer housing; outer housing was damaged in tsunami; no inner housing for the stored fuel rods - thus contamination

Nuclear Reactor Storage of fission products is a major challenge. n Chernobyl (1986) used no containment, so the radiation leak was worse than from Three Mile Island (1979). n

n Schematic of underground storage system

Chernobyl Ukraine, 1986 n Only 2 people died in the initial explosion; most died from or were affected by radiation exposure n

Three Mile Island - PA; 1979 n The accident started at 4 am when the water pumps that supplied the steam generators of the plant stopped abruptly. The subsequent lack of steam was detected by the plant's safety system which then automatically shut off the steamturbine powering the generator. The three primary causes of the Three Mile Island nuclear accident included mistakes made by employees, faulty plant design, and the breakdown of key elements in the properation of the plant. n http: //www. eoearth. org/article/Three_Mile_Island, _Pennsylvania

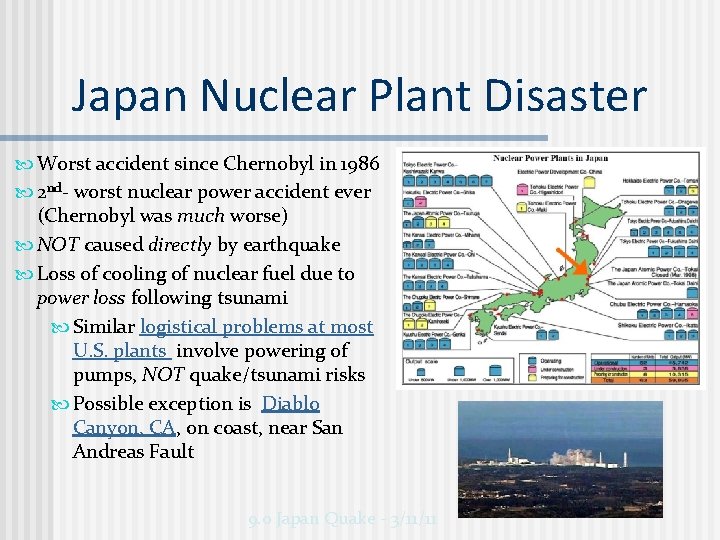
Japan Nuclear Plant Disaster Worst accident since Chernobyl in 1986 2 nd- worst nuclear power accident ever (Chernobyl was much worse) NOT caused directly by earthquake Loss of cooling of nuclear fuel due to power loss following tsunami Similar logistical problems at most U. S. plants involve powering of pumps, NOT quake/tsunami risks Possible exception is Diablo Canyon, CA, on coast, near San Andreas Fault 9. 0 Japan Quake - 3/11/11

Japan Nuclear Plant Disaster Does not appear any dangerous radiation will affect U. S. (Hawaii or mainland) Buying iodine or Geiger counters in AZ is a waste of money and time Online resources for Japan nuclear power plant: http: //serc. carleton. edu/NAGTWorkshops/energy/nuclear. html Online resources for 9. 0 Japan quake and tsunami: http: //serc. carleton. edu/NAGTWorkshops/visualization/collections/jap an 2011. html 9. 0 Japan Quake - 3/11/11

Nuclear Fusion n n n Nuclear Fusion is the formation of heavier nuclei by the joining of lighter ones. Fusion products are generally not radioactive. Smaller atoms are fused together into one larger atom: 11 H + 21 H 32 He (i. e. , in the sun) Called thermonuclear because they require extremely high temperatures. Positive side: H is cheap, plentiful; He is waste which isn’t toxic Negative side: Still have trouble controlling the reactions.

Nuclear Fusion n Iron is the end of fusion and the heaviest element found in stars but it can become unstable through neutron capture. Once it turns into Fe-59 it is unstable enough to beginning decaying and will eventually become Co-59 which will become the unstable Co-60 and decay into Ni, etc. Supernovas are able to form even heavier elements due to the sheer amount of neutrons bombarding elements before they have time to decay. http: //lifeng. lamost. org/courses/astrotoday/CHAISSON/A T 321/HTML/AT 32104. HTM

Atomic vs Hydrogen Bombs n n n Atomic weapons utilize the splitting of atoms (235 U) and are detonated through fission and its resulting chain reaction. Hiroshima = 13 ktons Hydrogen bombs are detonated through fusion. They are 1000 times more explosive than atomic bombs. Energy is released because of overall loss of mass. Typical = 10 megatons! Great site - summary of nuclear weapons: http: //library. thinkquest. org/3471/abomb. html

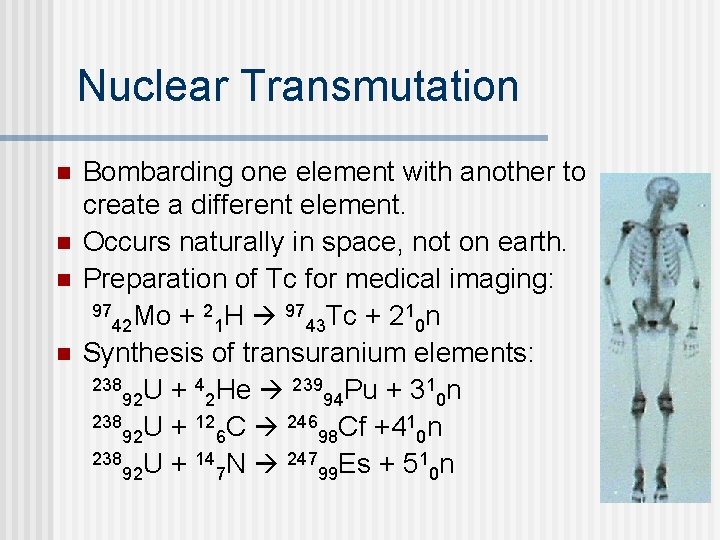
Nuclear Transmutation n n Bombarding one element with another to create a different element. Occurs naturally in space, not on earth. Preparation of Tc for medical imaging: 97 Mo + 2 H 97 Tc + 21 n 42 1 43 0 Synthesis of transuranium elements: 238 U + 4 He 239 Pu + 31 n 92 2 94 0 238 U + 12 C 246 Cf +41 n 92 6 98 0 238 U + 14 N 247 Es + 51 n 92 7 99 0

Bombardment Reactions n n In September 1982, two new elements were formed by bombardment with heavy nuclides: 58 Fe + 206 Pb 265 1 n Hs + 26 82 108 0 58 Fe + 209 Bi 266 1 n Mt + 26 83 109 0 Prepared only a few atoms of each The interest in preparing new elements is generated in part by the prediction that there will be another island of stability among elements with Z~114 or 126, N~184. Worked example 22. 9, Problem 22. 15 -16

Particle Accelerators n Linear Accelerator A beam of charged particles is accelerated by passing it through a series of tubes having alternating electrical charge. n The tubes get successively longer because the particles are moving faster. n

Linear Accelerator Australian Synchotron

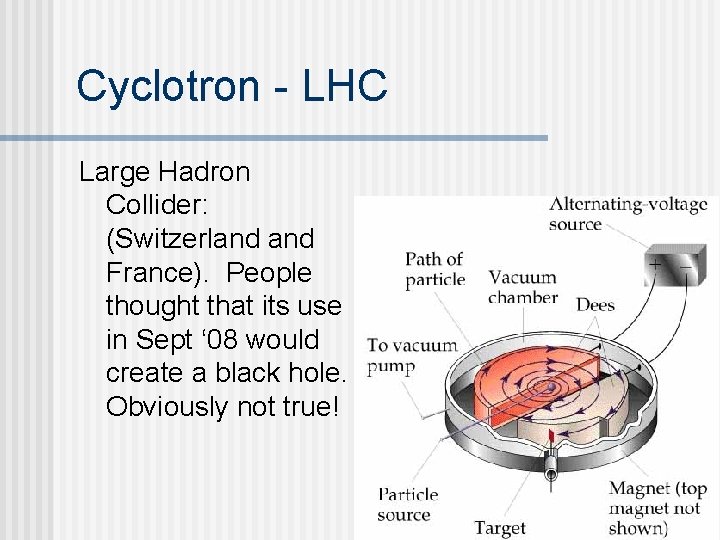
Circular Accelerators n Circular accelerator cyclotron n A magnetic field is used to keep the particles in a circular pathway. n Alternating current on the “D”s is used to accelerate the particles. n Some cyclotrons have a radius of 1 km. n The Large Hadron Collider (LHC) is 27 km/16. 8 miles in diameter. n

Cyclotron - LHC Large Hadron Collider: (Switzerland France). People thought that its use in Sept ‘ 08 would create a black hole. Obviously not true!

Detection of Radioactive Decay n Geiger n counter activity = number of nuclei disintegrating per unit time; units = Curie (Ci)

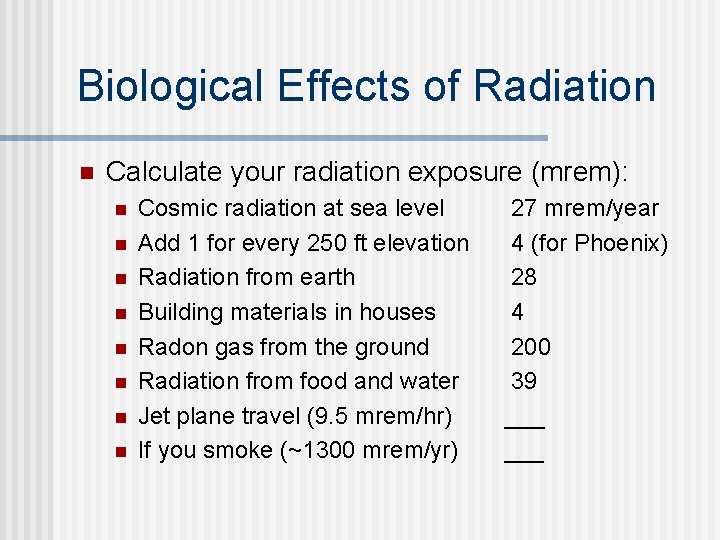
Biological Effects of Radiation n Calculate your radiation exposure (mrem): n n n n Cosmic radiation at sea level Add 1 for every 250 ft elevation Radiation from earth Building materials in houses Radon gas from the ground Radiation from food and water Jet plane travel (9. 5 mrem/hr) If you smoke (~1300 mrem/yr) 27 mrem/year 4 (for Phoenix) 28 4 200 39 ___

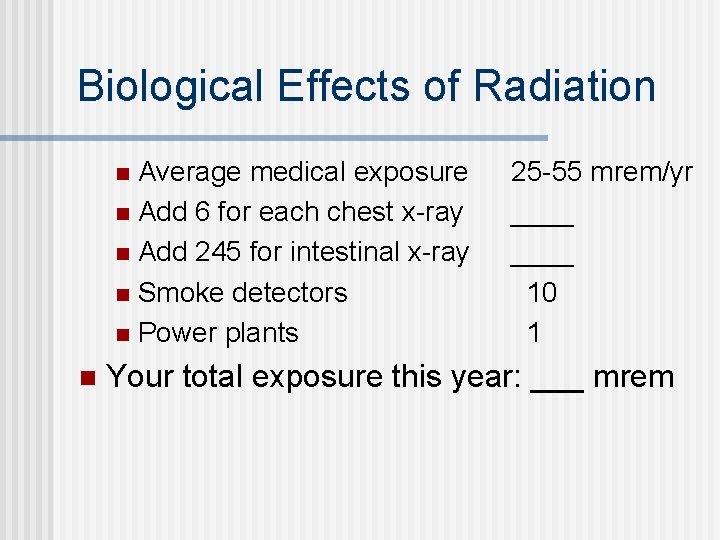
Biological Effects of Radiation Average medical exposure n Add 6 for each chest x-ray n Add 245 for intestinal x-ray n Smoke detectors n Power plants n n 25 -55 mrem/yr ____ 10 1 Your total exposure this year: ___ mrem

Biological Effects of Radiation n Normal exposure = 200 mrem (0. 2 rem) per year, which produces no observable effects n 0 -25 rem: no effect n 25 -100 rem: temporary blood cell changes n 100 -300 rem: radiation sickness n 400 rem: 50% chance of death n > ~600 rem: 100% chance of death n 50, 000 rem needed to kill bacteria n 1, 000 rem needed to kill viruses

Applications of Nuclear Chem. Archeological/geological dating (already discussed) n Nuclear medicine and radiotracers n Food irradiation n Small power generators for space vehicles, submarines, and pacemakers n Smoke alarms n Warfare n

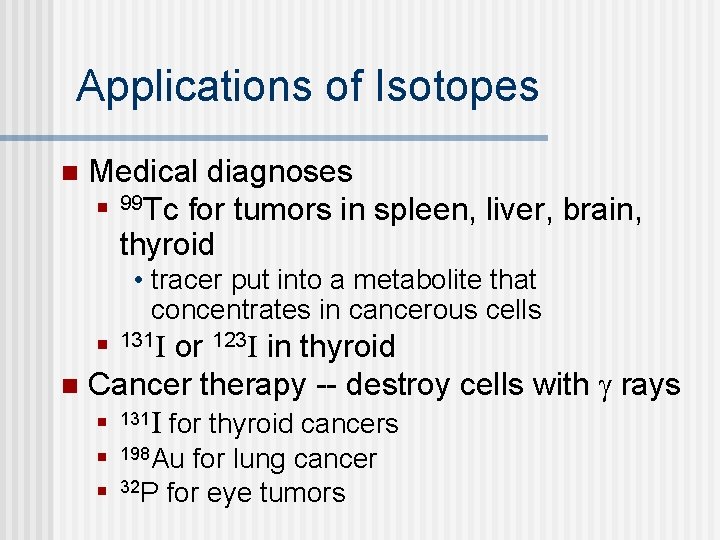
Applications of Isotopes n Medical diagnoses n 99 Tc for tumors in spleen, liver, brain, thyroid • tracer put into a metabolite that concentrates in cancerous cells or 123 I in thyroid n Cancer therapy -- destroy cells with g rays n 131 I for thyroid cancers n 131 I n 198 Au n for lung cancer 32 P for eye tumors

Imaging Procedures X-Rays n MRI: magnetic resonance imaging (no radioisotopes used) - radio waves stimulate H nuclei that give off a signal that can be interpreted for anomalies n PET scans: Positron Emission Tomography n

X-Ray

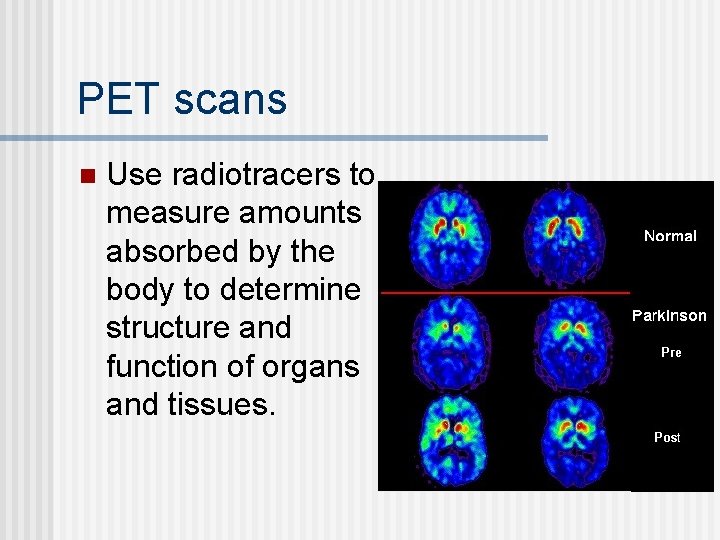
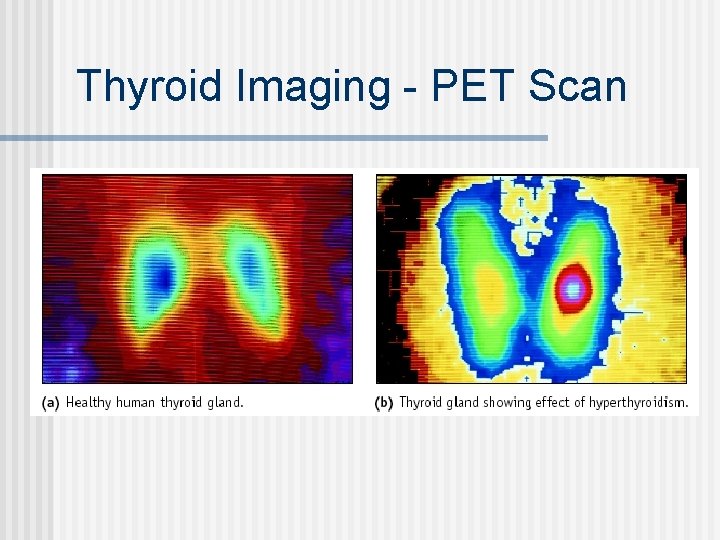
PET scans n Use radiotracers to measure amounts absorbed by the body to determine structure and function of organs and tissues.

Thyroid Imaging - PET Scan

Food Irradiation Food can be irradiated with gamma rays from 60 Co or 137 Cs. n Irradiated milk has a shelf life of 3 mo. without refrigeration. n USDA has approved irradiation of meats and eggs. n
 Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear fusion
Nuclear fusion Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Vocabulary workshop level d unit 1
Vocabulary workshop level d unit 1 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry Anatomy of a wave
Anatomy of a wave 25 m/s
25 m/s Nuclear chemistry
Nuclear chemistry Types of decay
Types of decay Copyright
Copyright Nuclear chemistry
Nuclear chemistry Irradiated food
Irradiated food Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry If you are not confused you're not paying attention
If you are not confused you're not paying attention Informal vs casual
Informal vs casual Attention is not explanation
Attention is not explanation Being too broad
Being too broad Negation of if
Negation of if Too big too small just right
Too big too small just right Love is not all: it is not meat nor drink
Love is not all: it is not meat nor drink Eyes that see and ears that hear
Eyes that see and ears that hear P ran
P ran If you don't measure it you can't manage it
If you don't measure it you can't manage it We will not be moved you're standing with us
We will not be moved you're standing with us Not a rustling leaf not a bird
Not a rustling leaf not a bird You can not not communicate
You can not not communicate Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Introduction to organic chemistry
Introduction to organic chemistry Chapter 9 section 3 stoichiometry
Chapter 9 section 3 stoichiometry Love formula in chemistry
Love formula in chemistry Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 review solutions answer key
Chapter 12 review solutions answer key Avogadro's law relationship
Avogadro's law relationship Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry: matter and change chapter 10 the mole answer key
Chemistry: matter and change chapter 10 the mole answer key Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas What functional group is ch3
What functional group is ch3 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers All acids owe their chemical reactivity to
All acids owe their chemical reactivity to Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Acid chloride + grignard reagent
Acid chloride + grignard reagent Modern chemistry chapter 4
Modern chemistry chapter 4 Chapter 1 chapter assessment the central science
Chapter 1 chapter assessment the central science Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 11 stoichiometry assessment answer key
Chapter 11 stoichiometry assessment answer key Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry