Chapter 21 Nuclear Chemistry Nuclear chemistry is the











- Slides: 25

Chapter 21 Nuclear Chemistry

• Nuclear chemistry is the study of nuclear reactions, with an emphasis on their uses in chemistry and their effects on biological systems. 2

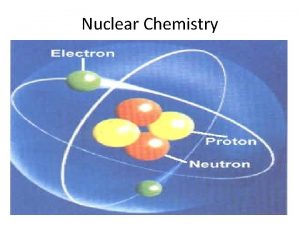
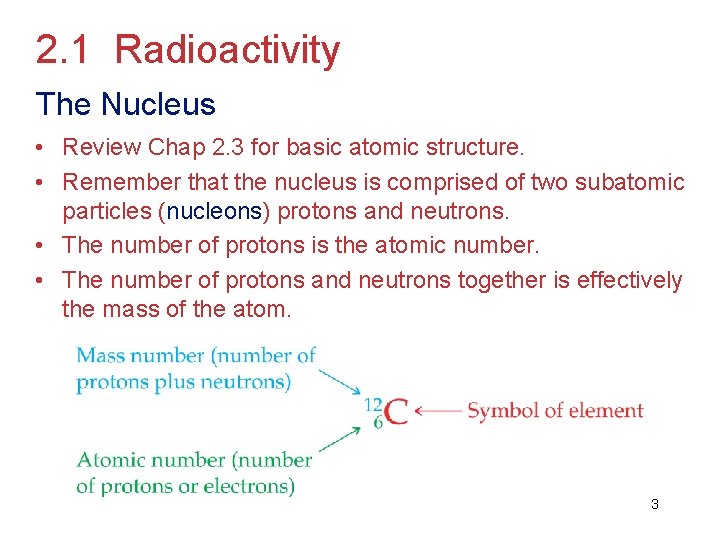
2. 1 Radioactivity The Nucleus • Review Chap 2. 3 for basic atomic structure. • Remember that the nucleus is comprised of two subatomic particles (nucleons) protons and neutrons. • The number of protons is the atomic number. • The number of protons and neutrons together is effectively the mass of the atom. 3

Isotopes • Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. • There are three naturally occurring isotopes of uranium: ØUranium-234 ØUranium-235 ØUranium-238 4

• A nuclide is a nucleus with a specific number of protons and neutrons. • It is not uncommon for some nuclides of an element to be unstable, or radioactive. • We refer to these as radionuclides. • There are several ways radionuclides can decay into a different nuclide. 5

Nuclear Equations • Radionuclides are unstable and spontaneously emit particles and electromagnetic radiation. • Emission of radiation is a way for an unstable nucleus to be transformed into a more stable one with less energy. • When a nucleus spontaneously decomposes in this way it is said to have decayed. 6

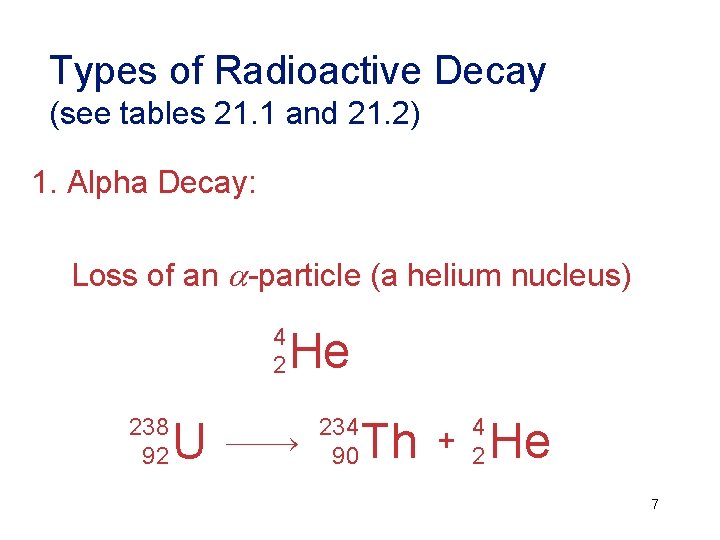
Types of Radioactive Decay (see tables 21. 1 and 21. 2) 1. Alpha Decay: Loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 Th + 4 2 He 7

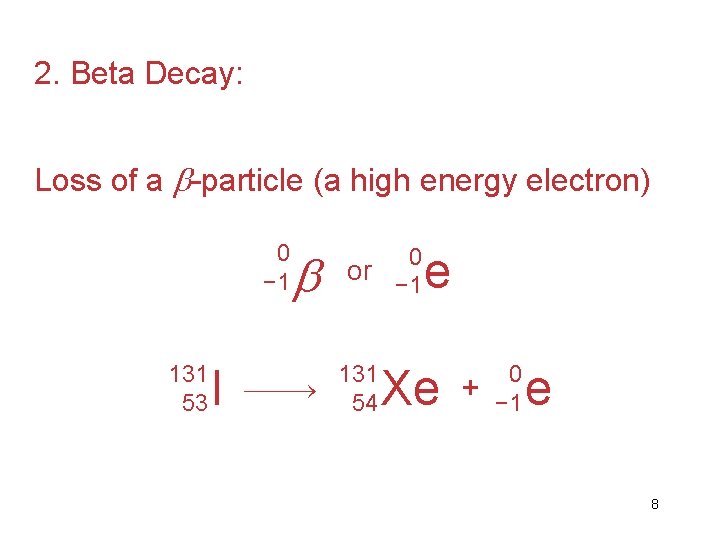
2. Beta Decay: Loss of a -particle (a high energy electron) 0 − 1 131 53 I 0 or − 1 131 54 e Xe + 0 − 1 e 8

3. Gamma Emission: • Loss of a -ray -- high-energy photon that almost always accompanies the loss of a nuclear particle. 0 0 • Generally, not shown in a nuclear equation. 9

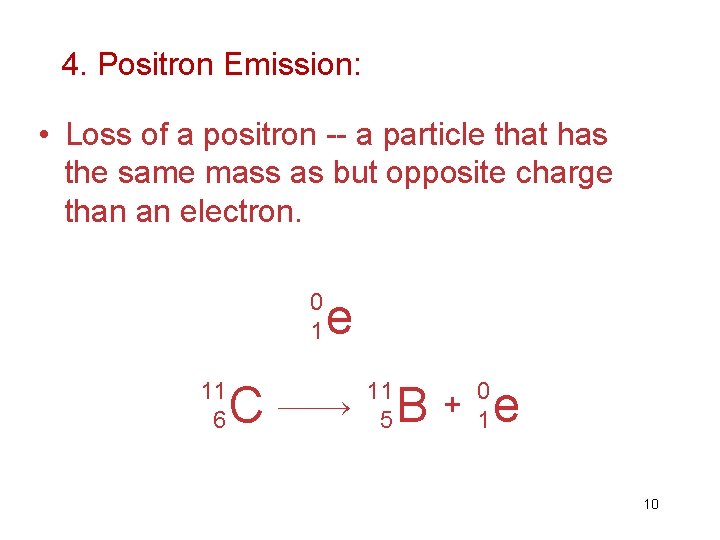
4. Positron Emission: • Loss of a positron -- a particle that has the same mass as but opposite charge than an electron. 0 1 11 6 C e 11 5 B + 0 1 e 10

5. Electron Capture • The addition of an electron to a proton in the nucleus. • As a result, a proton is transformed into a neutron. 1 1 p + 0 − 1 e 1 0 n 11

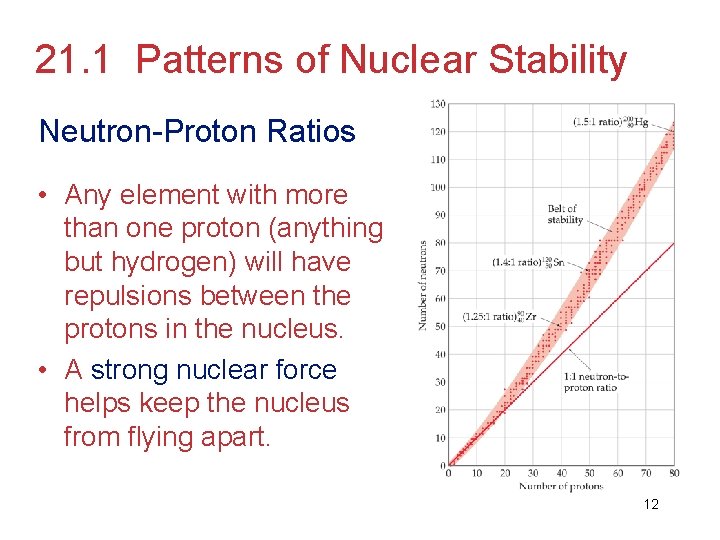
21. 1 Patterns of Nuclear Stability Neutron-Proton Ratios • Any element with more than one proton (anything but hydrogen) will have repulsions between the protons in the nucleus. • A strong nuclear force helps keep the nucleus from flying apart. 12

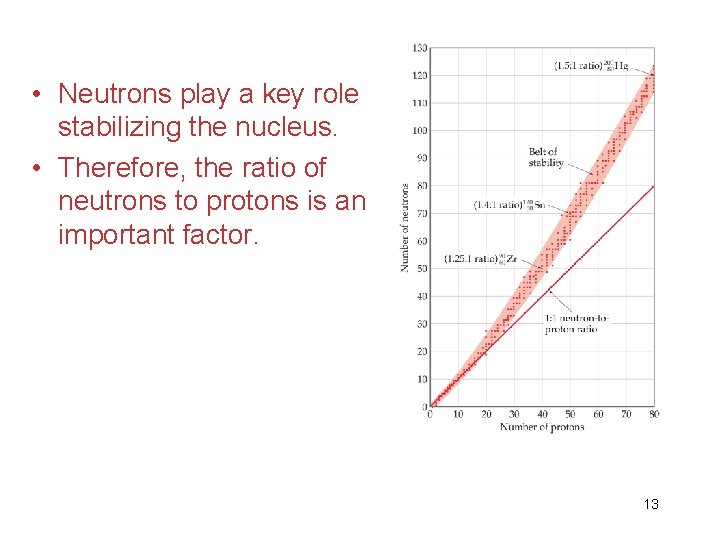
• Neutrons play a key role stabilizing the nucleus. • Therefore, the ratio of neutrons to protons is an important factor. 13

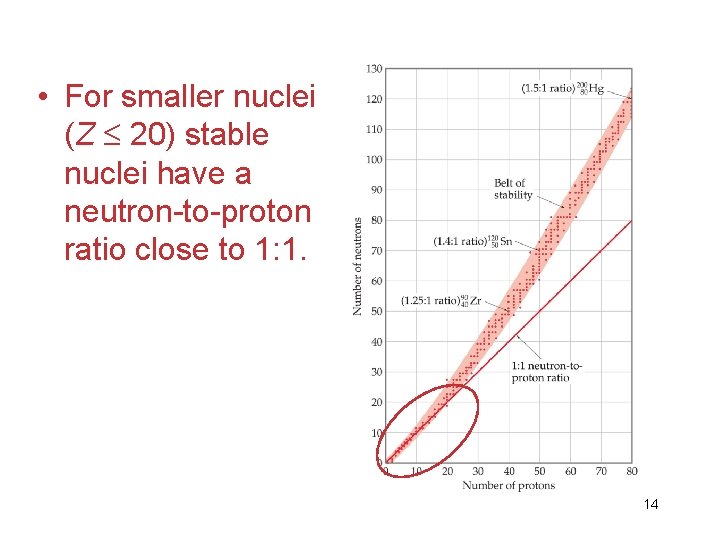
• For smaller nuclei (Z 20) stable nuclei have a neutron-to-proton ratio close to 1: 1. 14

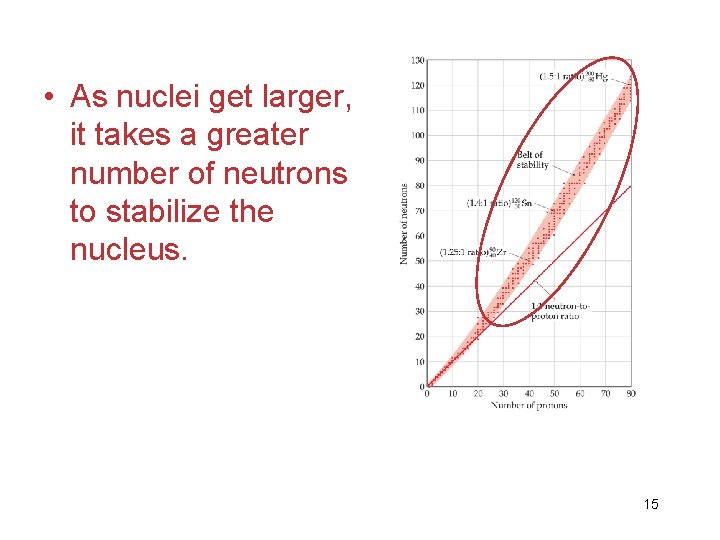
• As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. 15

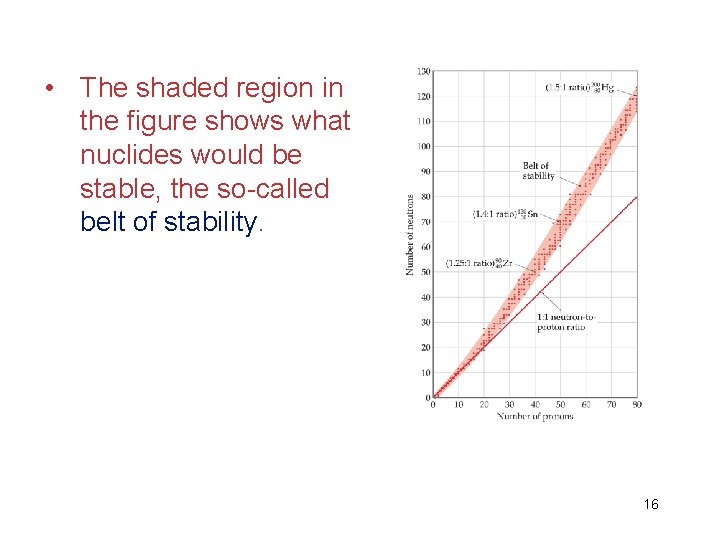
• The shaded region in the figure shows what nuclides would be stable, the so-called belt of stability. 16

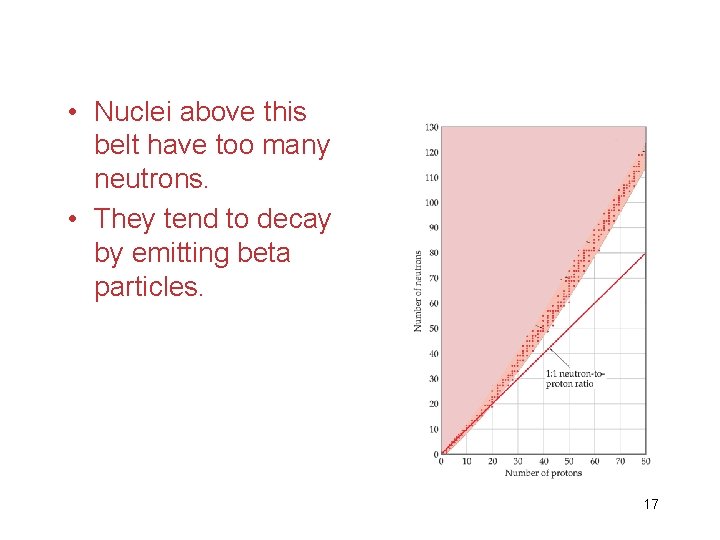
• Nuclei above this belt have too many neutrons. • They tend to decay by emitting beta particles. 17

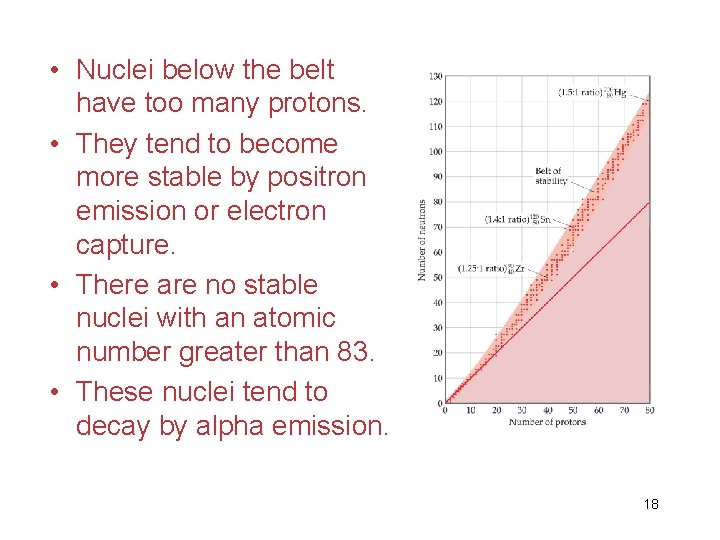
• Nuclei below the belt have too many protons. • They tend to become more stable by positron emission or electron capture. • There are no stable nuclei with an atomic number greater than 83. • These nuclei tend to decay by alpha emission. 18

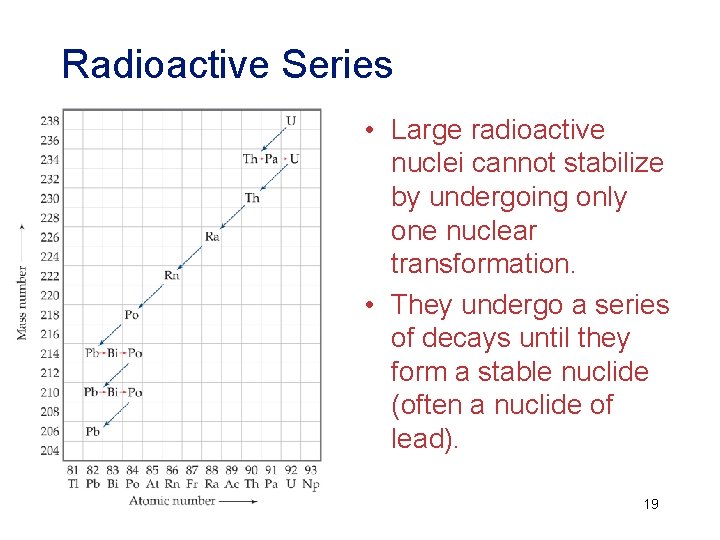
Radioactive Series • Large radioactive nuclei cannot stabilize by undergoing only one nuclear transformation. • They undergo a series of decays until they form a stable nuclide (often a nuclide of lead). 19

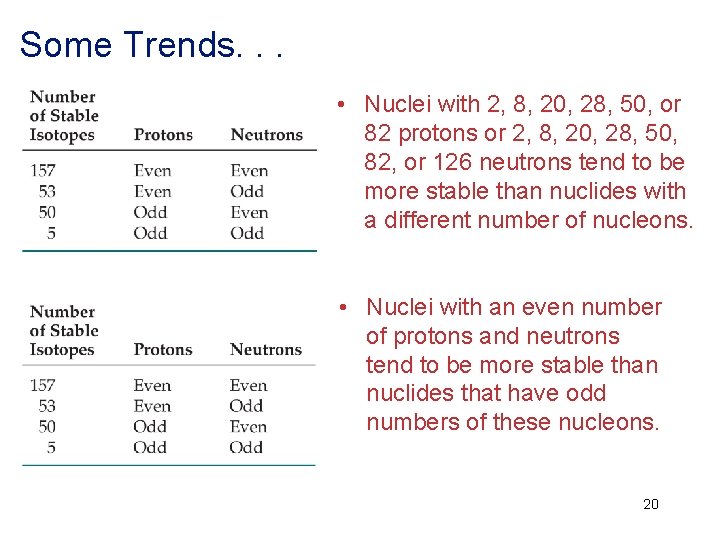
Some Trends. . . • Nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons tend to be more stable than nuclides with a different number of nucleons. • Nuclei with an even number of protons and neutrons tend to be more stable than nuclides that have odd numbers of these nucleons. 20

21. 3 Nuclear Transmutations • Nuclear transmutations can be induced by accelerating a particle (neutron or another nucleus) and colliding it with the nuclide. • Examples of particle accelerators: 21

• Synthetic isotopes used in medicine and research are made using neutrons as projectiles. • Example: cobalt-60 22

21. 4 Rates Radioactive Decay • Nuclear transmutation is a first-order process. • The kinetics of such a process, you will recall, obey this equation: Nt = kt ln N 0 23

• The half-life of such a process is: 0. 693 = t 1/2 k • Comparing the amount of a radioactive nuclide present at a given point in time with the amount normally present, one can find the age of an object. 24

Calculations Based on Half-life • Because the half-life of any particular nuclide is constant, the half-life serves as a nuclear clock to determine the ages of different objects. (See Table 21. 4) • The rate at which a sample decays is called its activity. • The SI unit for activity is the becquerel (Bq). • Another unit of activity is the curie (Ci), defined as 3. 7 x 1010 disintegrations per second. 25
 Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear fusion
Nuclear fusion Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear chemistry webquest
Nuclear chemistry webquest Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry Anatomy of a wave
Anatomy of a wave Applications of nuclear chemistry
Applications of nuclear chemistry Nuclear chemistry
Nuclear chemistry Rectangle real life examples
Rectangle real life examples Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào