Chapter 1 Nuclear Energy I Radioactivity A Definitions






- Slides: 18

Chapter 1 Nuclear Energy I. Radioactivity

A. Definitions ª Radioactivity w emission of high-energy radiation from the nucleus of an atom ª Nuclide w nucleus of an isotope ª Transmutation w process of changing one element into another via nuclear decay

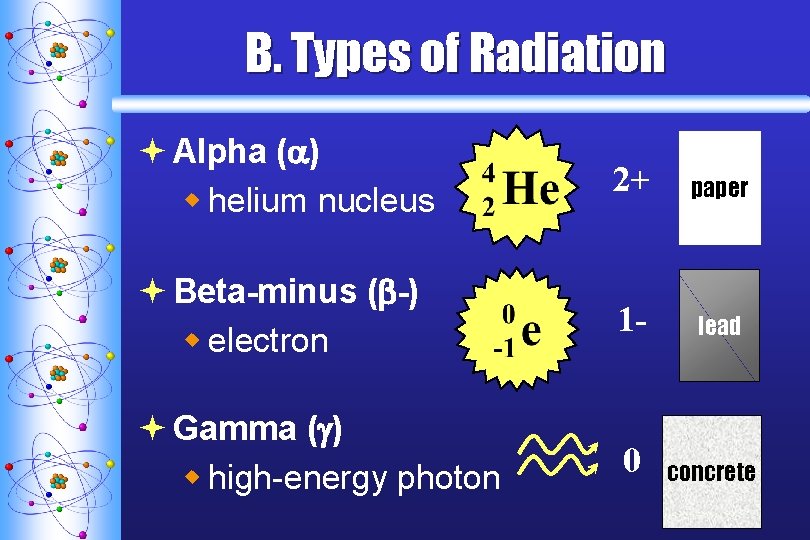
B. Types of Radiation ª Alpha ( ) w helium nucleus 2+ paper ª Beta-minus ( -) w electron 1 - lead ª Gamma ( ) w high-energy photon 0 concrete

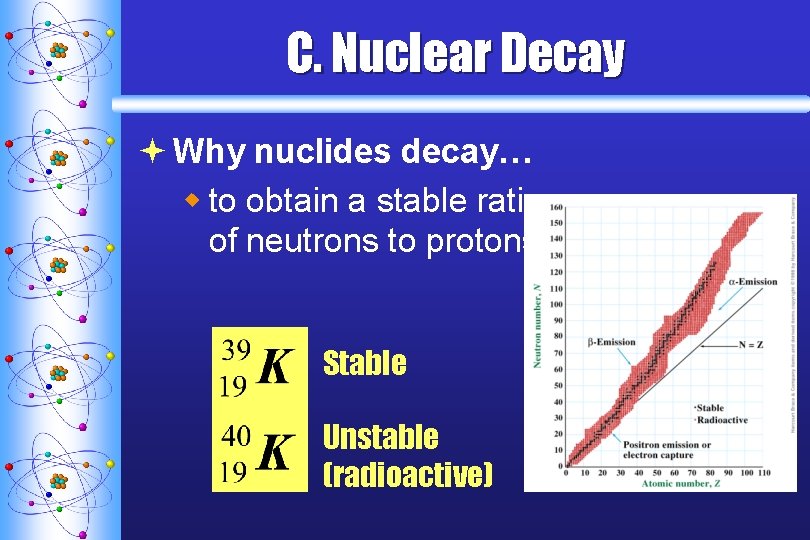
C. Nuclear Decay ª Why nuclides decay… w to obtain a stable ratio of neutrons to protons Stable Unstable (radioactive)

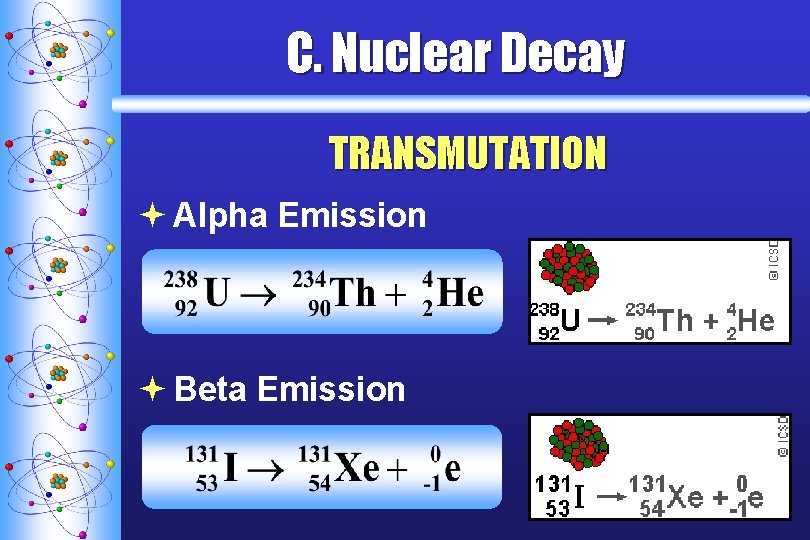
C. Nuclear Decay TRANSMUTATION ª Alpha Emission ª Beta Emission

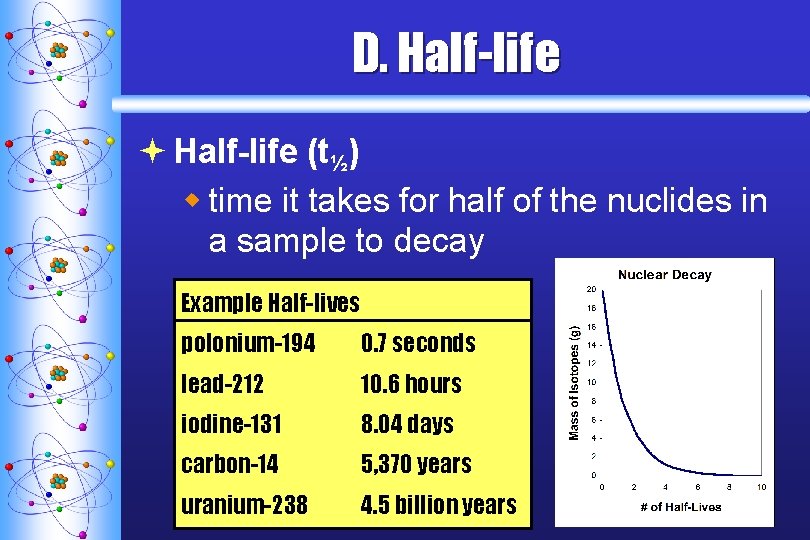
D. Half-life ª Half-life (t½) w time it takes for half of the nuclides in a sample to decay Example Half-lives polonium-194 0. 7 seconds lead-212 10. 6 hours iodine-131 8. 04 days carbon-14 5, 370 years uranium-238 4. 5 billion years

D. Half-life ª How much of a 20 -g sample of sodium-24 would remain after decaying for 30 hours? Sodium-24 has a half-life of 15 hours. GIVEN: WORK: total time = 30 hours number of half-lives = 2 t 1/2 = 15 hours 20 g ÷ 2 = 10 g (1 half-life) original mass = 20 g 10 g ÷ 2 = 5 g (2 half-lives) 5 g of 24 Na would remain.

CHAPTER 10 Nuclear Energy II. Nuclear Reactions (p. 689 -691)

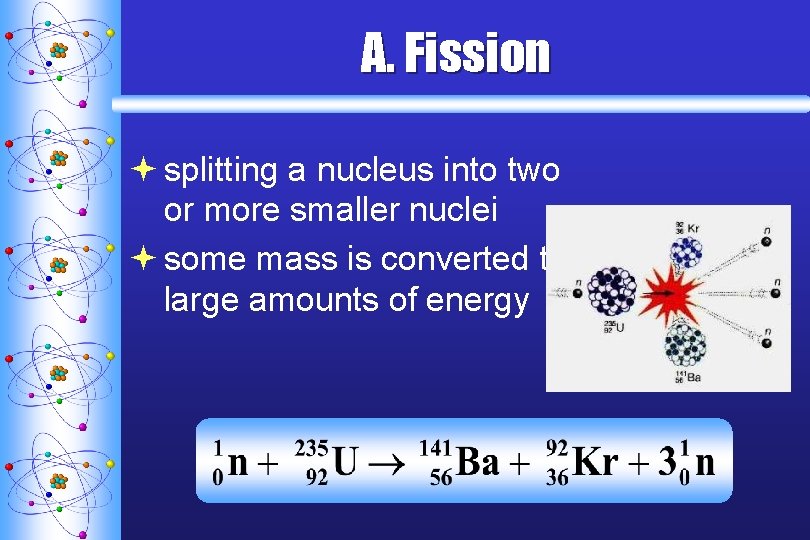
A. F ission ª splitting a nucleus into two or more smaller nuclei ª some mass is converted to large amounts of energy

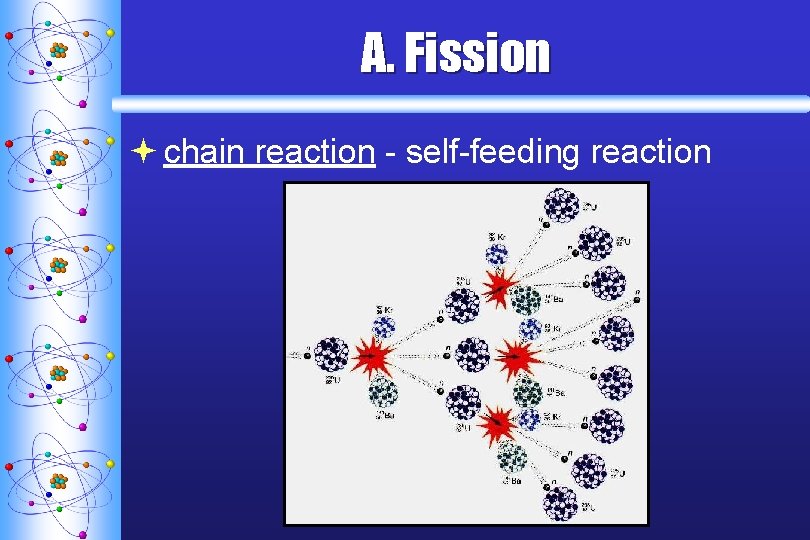
A. F ission ª chain reaction - self-feeding reaction

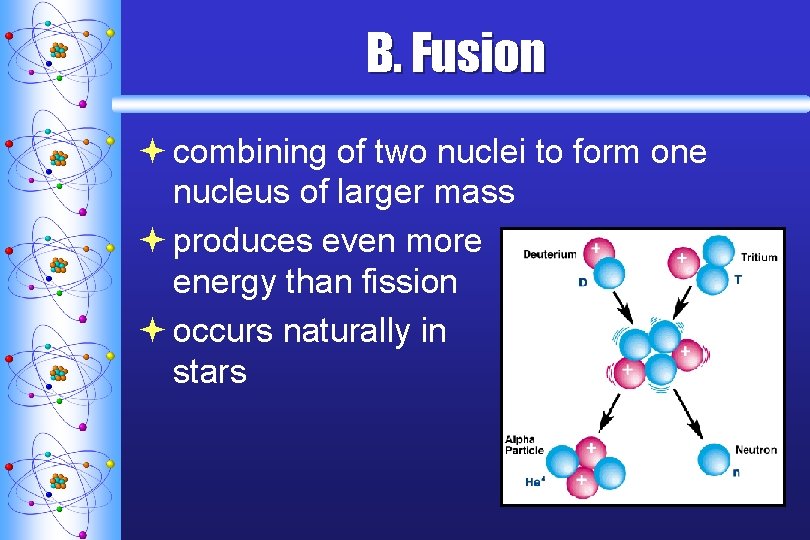
B. Fusion ª combining of two nuclei to form one nucleus of larger mass ª produces even more energy than fission ª occurs naturally in stars

Nuclear Energy III. Applications

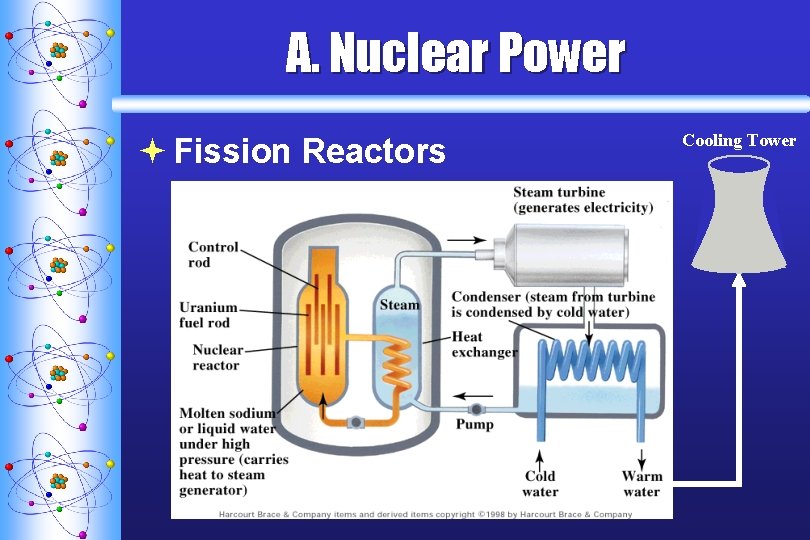
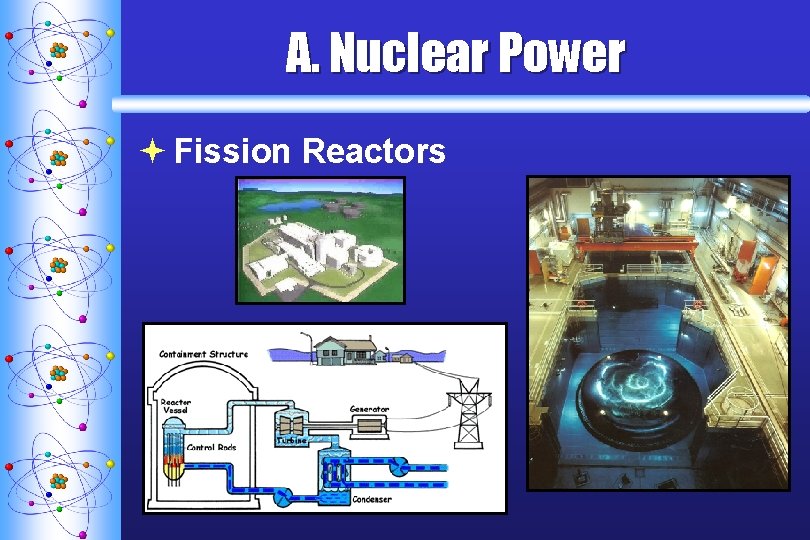
A. Nuclear Power ª Fission Reactors Cooling Tower

A. Nuclear Power ª Fission Reactors

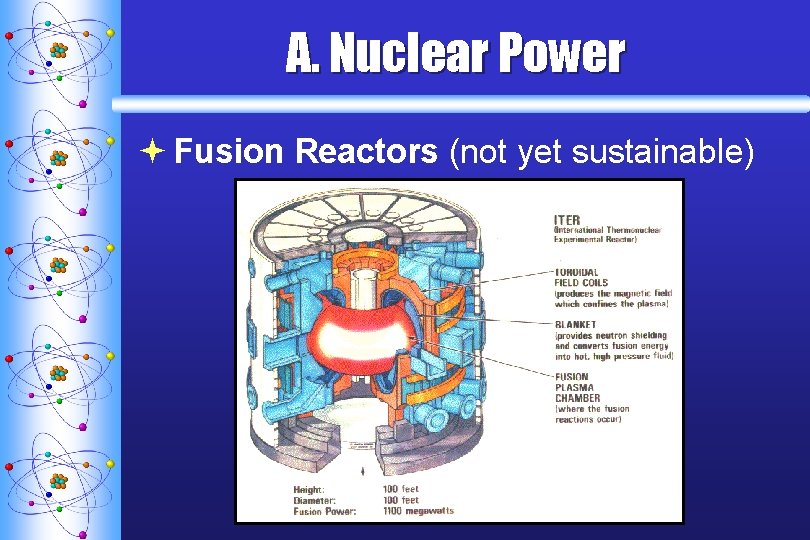
A. Nuclear Power ª Fusion Reactors (not yet sustainable)

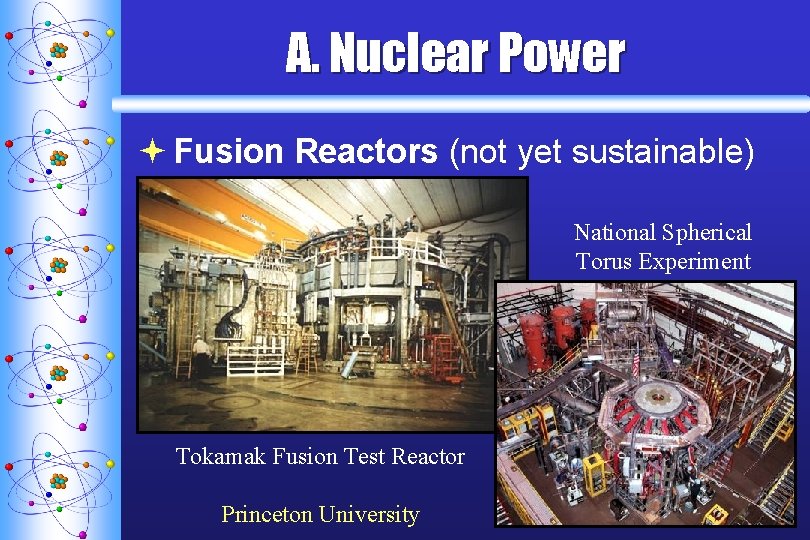
A. Nuclear Power ª Fusion Reactors (not yet sustainable) National Spherical Torus Experiment Tokamak Fusion Test Reactor Princeton University

A. Nuclear Power F I S S I O N ª 235 U is limited ª danger of meltdown ª toxic waste ª thermal pollution vs. F U S I O N ª Hydrogen is abundant ª no danger of meltdown ª no toxic waste ª not yet sustainable

B. Others ª Choose one of the following to investigate: w Irradiated Food w Radioactive Dating w Nuclear Medicine Make a mini-poster in your book to display what you have learned.