Chapter 18 Nuclear Chemistry Overview Natural radioactivity Nuclear





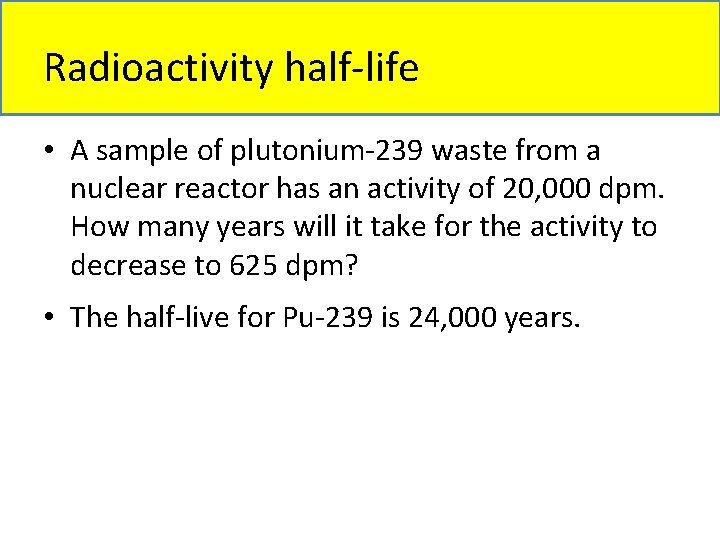

- Slides: 25

Chapter 18: Nuclear Chemistry

Overview • • Natural radioactivity Nuclear equations Radioactive decay series Radioactivity half-life Application of radionuclides Induced radioactivity Nuclear fission Nuclear fusion

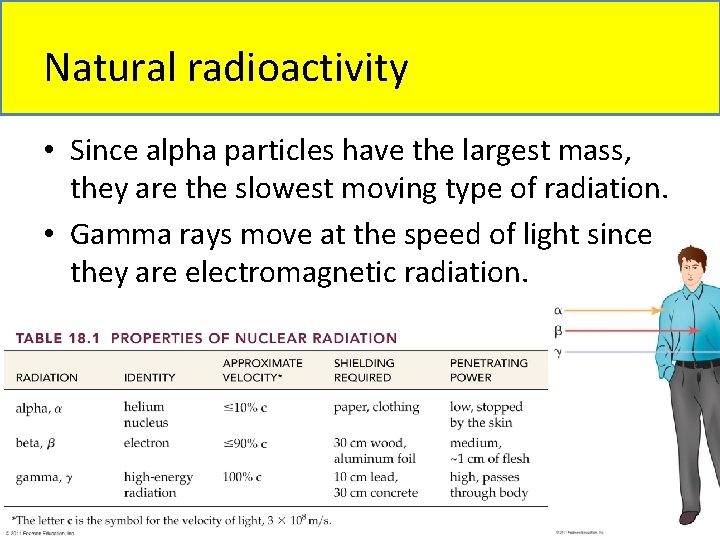
What is Radioactivity? • Radioactivity: the spontaneous emission of a stream of particles, or electromagnetic rays, in nuclear decay of unstable atoms. • There are three types of radioactivity: 1. Alpha particles (a) are identical to helium nuclei, containing two protons and two neutrons. 2. Beta particles (b) are identical to electrons. 3. Gamma rays (g) are high-energy photons.

Natural radioactivity • Since alpha particles have the largest mass, they are the slowest moving type of radiation. • Gamma rays move at the speed of light since they are electromagnetic radiation.

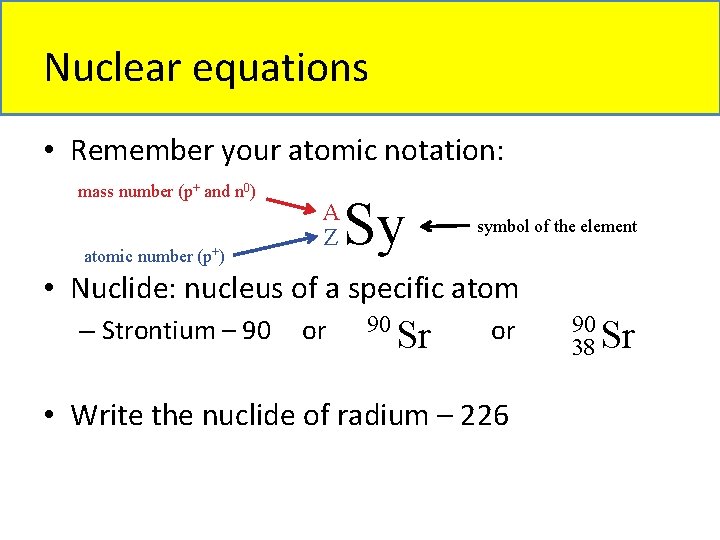
Nuclear equations • Remember your atomic notation: mass number (p+ and n 0) atomic number (p+) A Z Sy symbol of the element • Nuclide: nucleus of a specific atom 90 – Strontium – 90 or or Sr • Write the nuclide of radium – 226 90 38 Sr

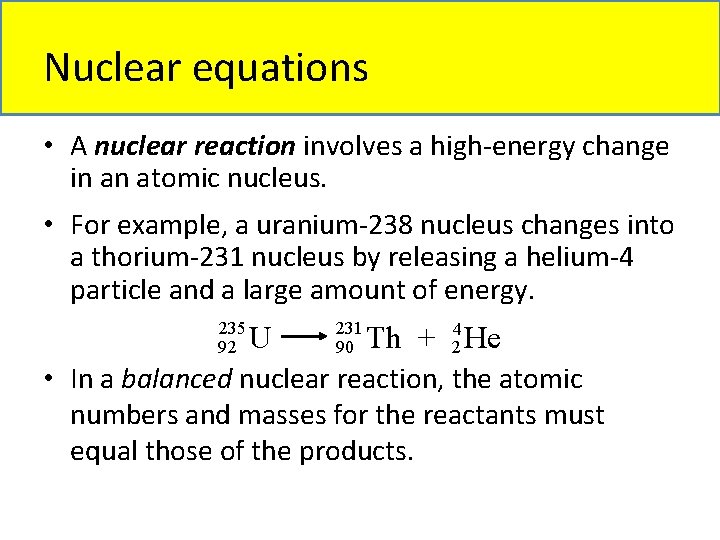
Nuclear equations • A nuclear reaction involves a high-energy change in an atomic nucleus. • For example, a uranium-238 nucleus changes into a thorium-231 nucleus by releasing a helium-4 particle and a large amount of energy. 235 92 U 231 90 Th + 24 He • In a balanced nuclear reaction, the atomic numbers and masses for the reactants must equal those of the products.

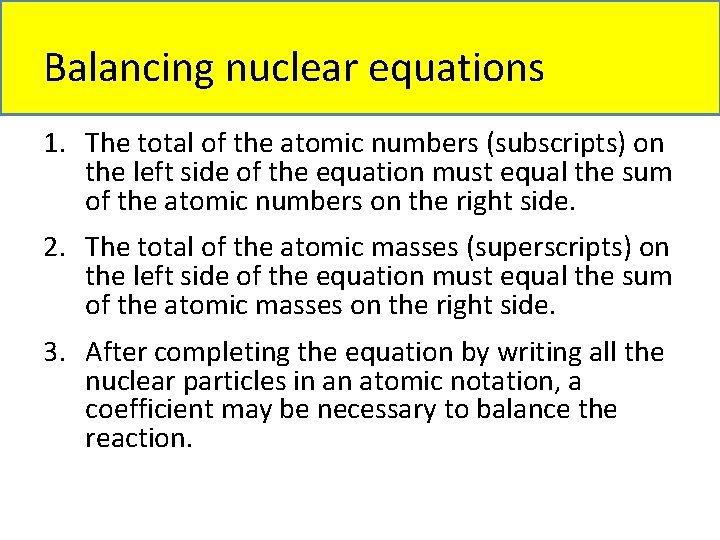
Balancing nuclear equations 1. The total of the atomic numbers (subscripts) on the left side of the equation must equal the sum of the atomic numbers on the right side. 2. The total of the atomic masses (superscripts) on the left side of the equation must equal the sum of the atomic masses on the right side. 3. After completing the equation by writing all the nuclear particles in an atomic notation, a coefficient may be necessary to balance the reaction.

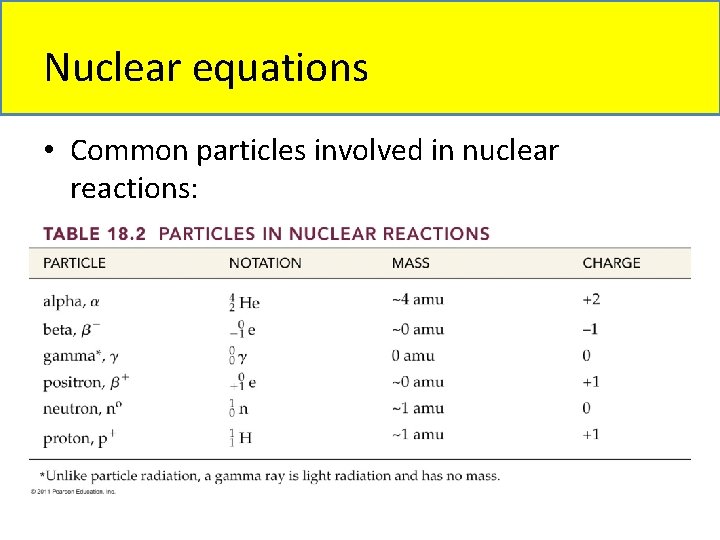
Nuclear equations • Common particles involved in nuclear reactions:

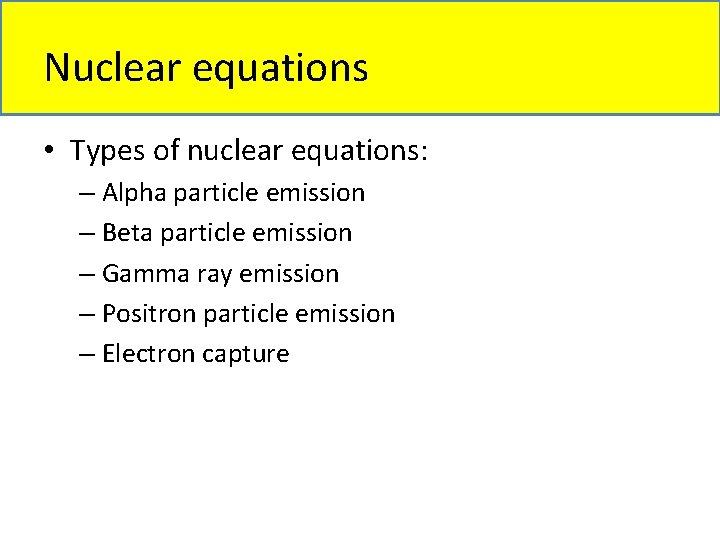
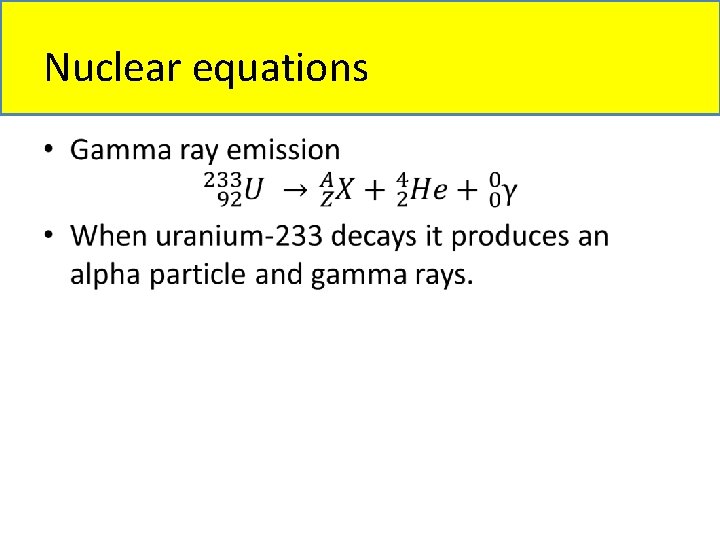
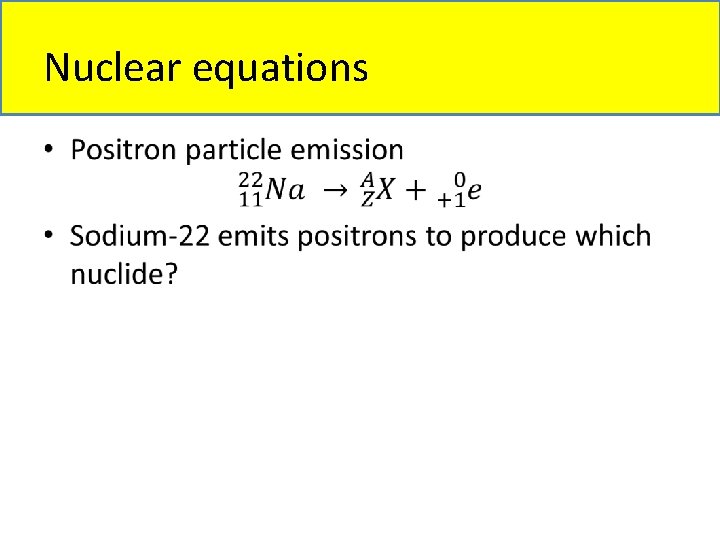
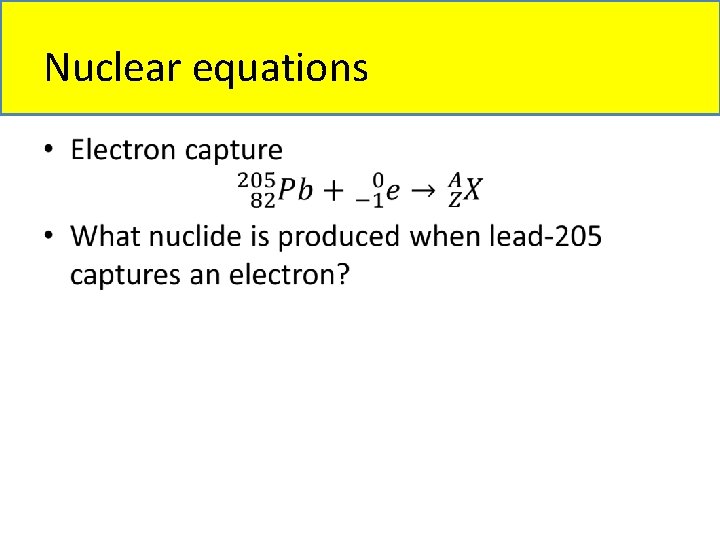
Nuclear equations • Types of nuclear equations: – Alpha particle emission – Beta particle emission – Gamma ray emission – Positron particle emission – Electron capture

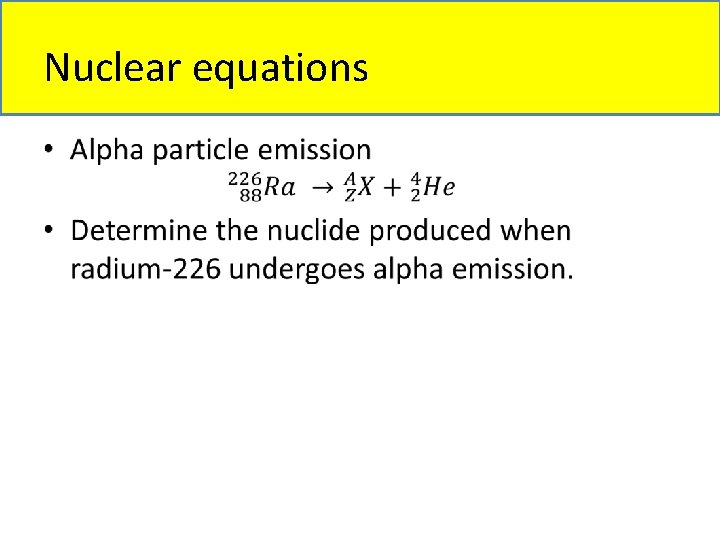
Nuclear equations •

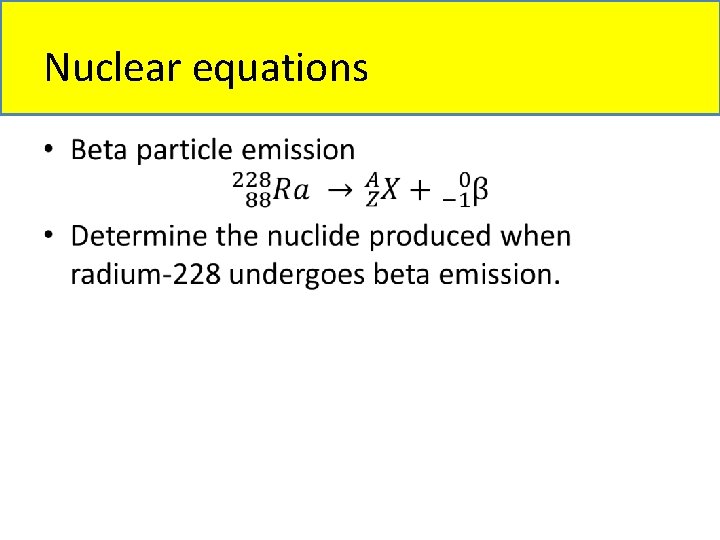
Nuclear equations •

Nuclear equations •

Nuclear equations •

Nuclear equations •

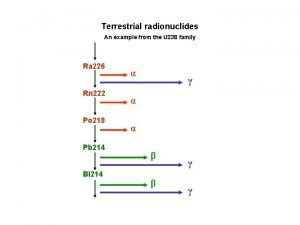
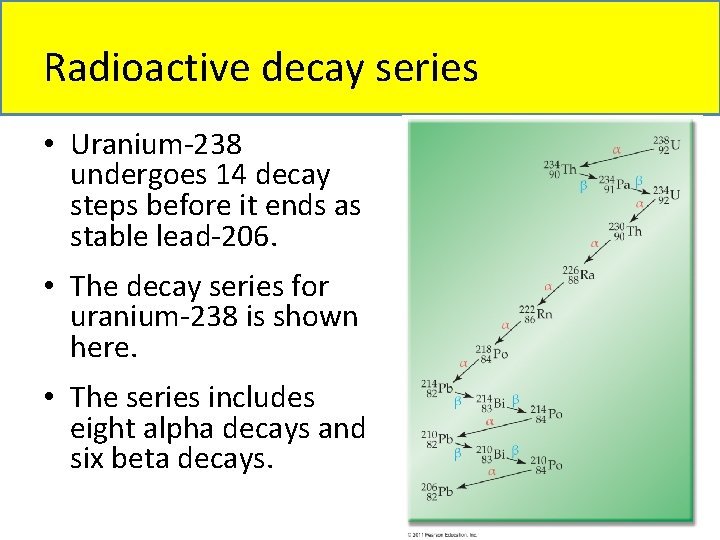
Radioactive decay series • Some heavy nuclides must go through a series of decay steps to reach a nuclide that is stable. • This stepwise disintegration of a radioactive nuclide until a stable nucleus is reached is called a radioactive decay series. • For example, uranium-235 requires 11 decay steps until it reaches the stable nuclide lead 207.

Radioactive decay series • Uranium-238 undergoes 14 decay steps before it ends as stable lead-206. • The decay series for uranium-238 is shown here. • The series includes eight alpha decays and six beta decays.

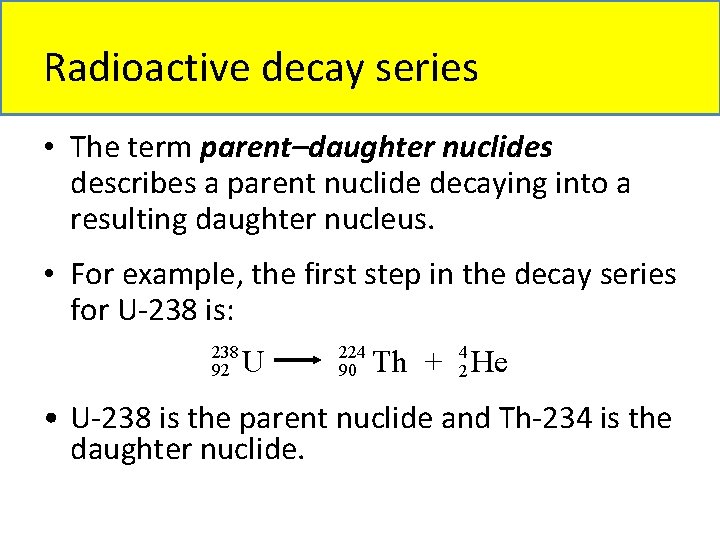
Radioactive decay series • The term parent–daughter nuclides describes a parent nuclide decaying into a resulting daughter nucleus. • For example, the first step in the decay series for U-238 is: 238 92 U 224 90 Th + 24 He • U-238 is the parent nuclide and Th-234 is the daughter nuclide.

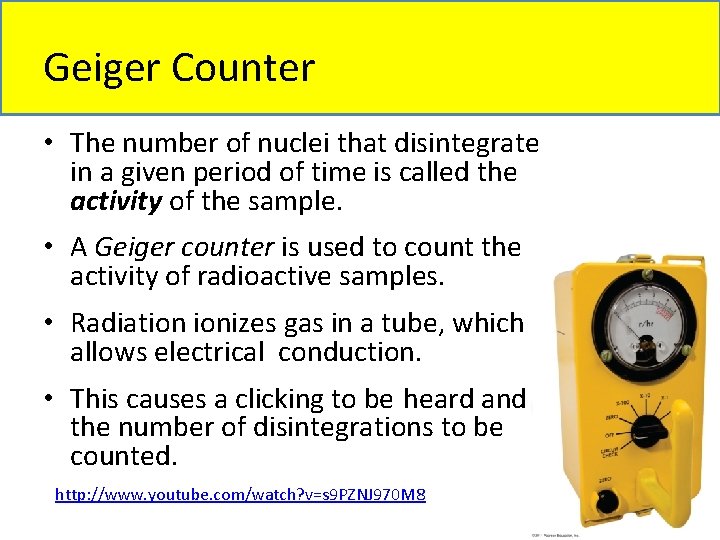
Geiger Counter • The number of nuclei that disintegrate in a given period of time is called the activity of the sample. • A Geiger counter is used to count the activity of radioactive samples. • Radiation ionizes gas in a tube, which allows electrical conduction. • This causes a clicking to be heard and the number of disintegrations to be counted. http: //www. youtube. com/watch? v=s 9 PZNJ 970 M 8

Radioactivity half-life • The level of radioactivity for all radioactive samples decreases over time. • Radioactive decay shows a systematic progression. • If we start with a sample that has an activity of 1000 disintegrations per minute (dpm), the level will drop to 500 dpm after a given amount of time. After the same amount of time, the activity will drop to 250 dpm. • The amount of time for the activity to decrease by half is called the half-life, t½.
Radioactivity half-life • A sample of plutonium-239 waste from a nuclear reactor has an activity of 20, 000 dpm. How many years will it take for the activity to decrease to 625 dpm? • The half-live for Pu-239 is 24, 000 years.

Radioactivity half-life • Iodine-131 is used to measure the activity of the thyroid gland. If 88 mg of I-131 are ingested, how much remains after 24 days (t½ = 8 days). • First, find out how many half-lives have passed. • Next, calculate how much I-131 is left.

Application of radionuclides • • • Radiocarbon dating Agricultural applications of radionuclides Medical applications Nuclear fission Nuclear fussion

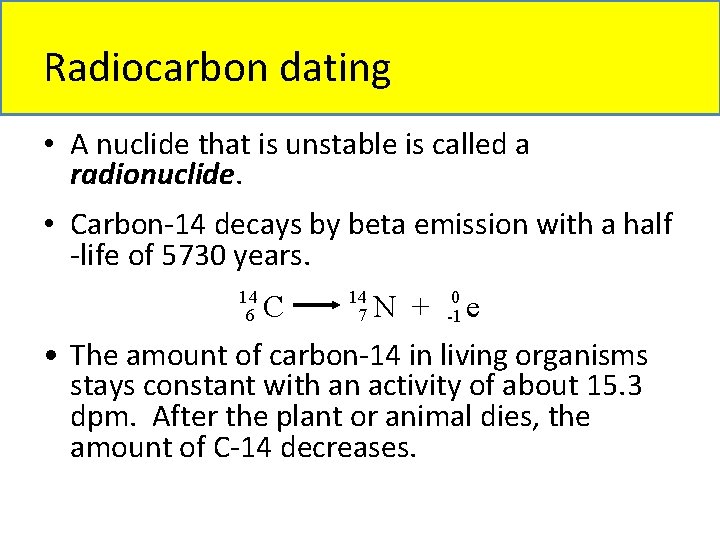
Radiocarbon dating • A nuclide that is unstable is called a radionuclide. • Carbon-14 decays by beta emission with a half -life of 5730 years. 14 6 C 14 7 N + 0 -1 e • The amount of carbon-14 in living organisms stays constant with an activity of about 15. 3 dpm. After the plant or animal dies, the amount of C-14 decreases.

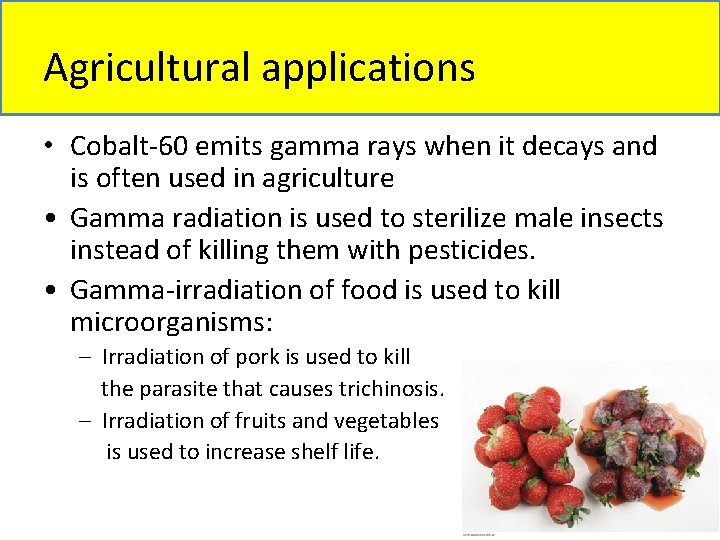
Agricultural applications • Cobalt-60 emits gamma rays when it decays and is often used in agriculture • Gamma radiation is used to sterilize male insects instead of killing them with pesticides. • Gamma-irradiation of food is used to kill microorganisms: – Irradiation of pork is used to kill the parasite that causes trichinosis. – Irradiation of fruits and vegetables is used to increase shelf life.

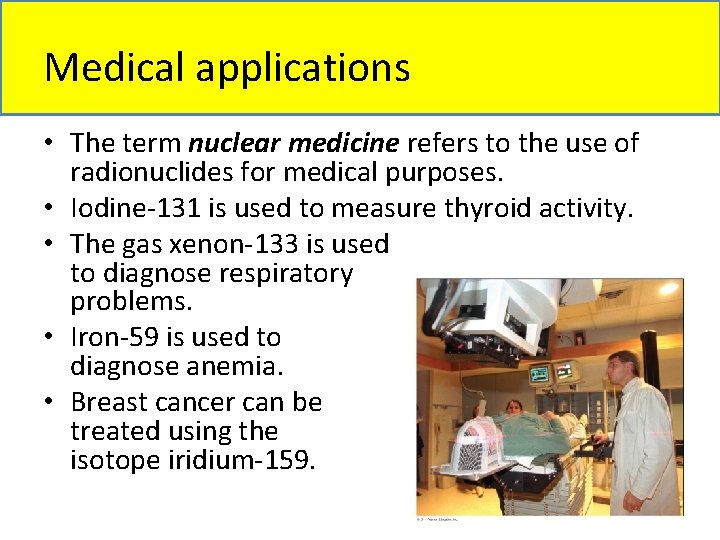
Medical applications • The term nuclear medicine refers to the use of radionuclides for medical purposes. • Iodine-131 is used to measure thyroid activity. • The gas xenon-133 is used to diagnose respiratory problems. • Iron-59 is used to diagnose anemia. • Breast cancer can be treated using the isotope iridium-159.
 Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Natural and artificial radioactivity
Natural and artificial radioactivity Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Natural and artificial radioactivity
Natural and artificial radioactivity Law of radioactive decay
Law of radioactive decay Natural radioactivity
Natural radioactivity Natural radioactivity
Natural radioactivity Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Mta
Mta Radioactivity
Radioactivity Unconformity
Unconformity Atoms and radioactivity
Atoms and radioactivity Environmental radioactivity
Environmental radioactivity Radioactivity phenomenon
Radioactivity phenomenon Units of radioactivity
Units of radioactivity Decay equation
Decay equation