Nuclear Chemistry Chemical Nuclear Involve electrons Affected external


















- Slides: 32

Nuclear Chemistry

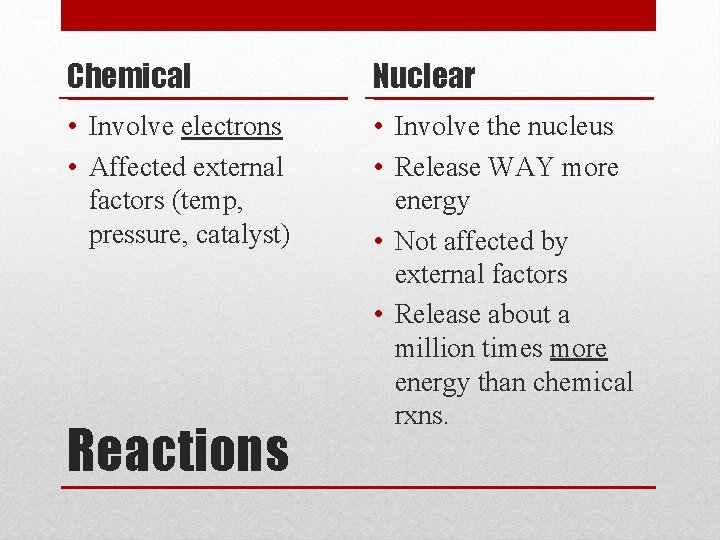
Chemical Nuclear • Involve electrons • Affected external factors (temp, pressure, catalyst) • Involve the nucleus • Release WAY more energy • Not affected by external factors • Release about a million times more energy than chemical rxns. Reactions

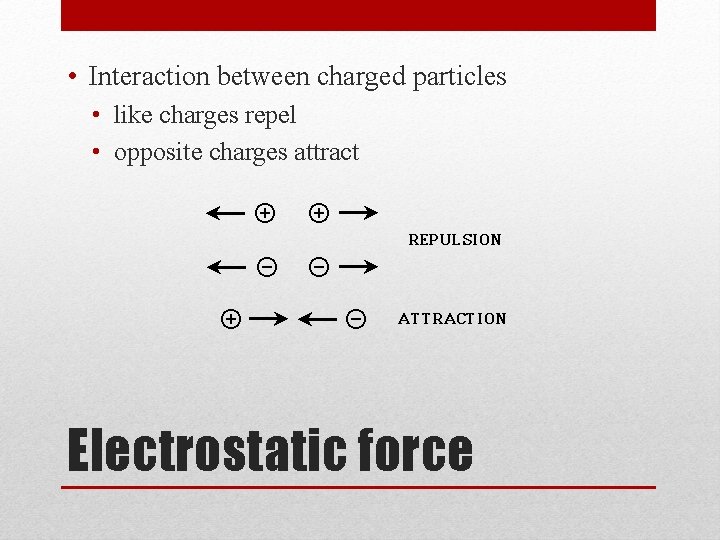
• Interaction between charged particles • like charges repel • opposite charges attract Electrostatic force

How does the nucleus stay together?

• Attractive force that acts between all nuclear particles that are extremely close together • Keeps the nucleus together • Much stronger than electrostatic force! Strong Nuclear Force

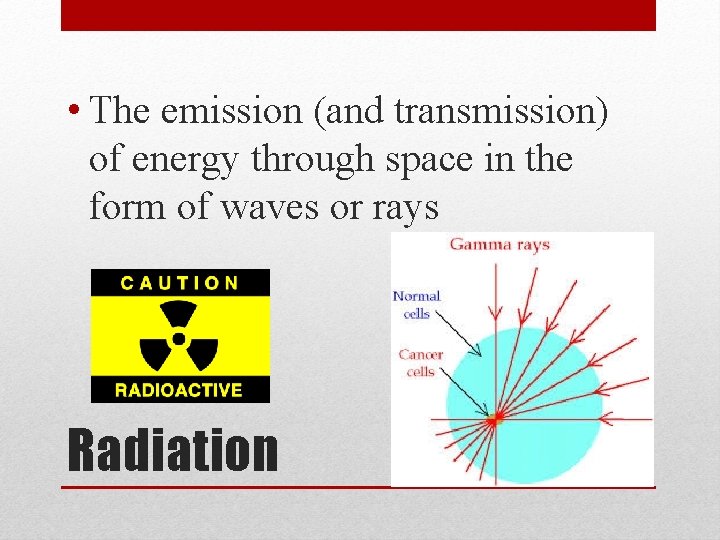
• The emission (and transmission) of energy through space in the form of waves or rays Radiation

Alpha 4α 2 Beta 0 β -1 (electron) Radioactivity Gamma 0 0 γ Neutron 1 0 n Proton 1 p 1

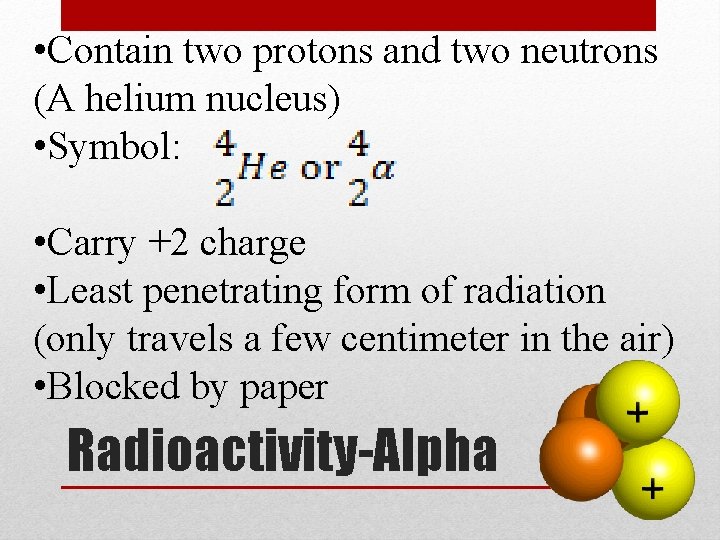
• Contain two protons and two neutrons (A helium nucleus) • Symbol: • Carry +2 charge • Least penetrating form of radiation (only travels a few centimeter in the air) • Blocked by paper Radioactivity-Alpha

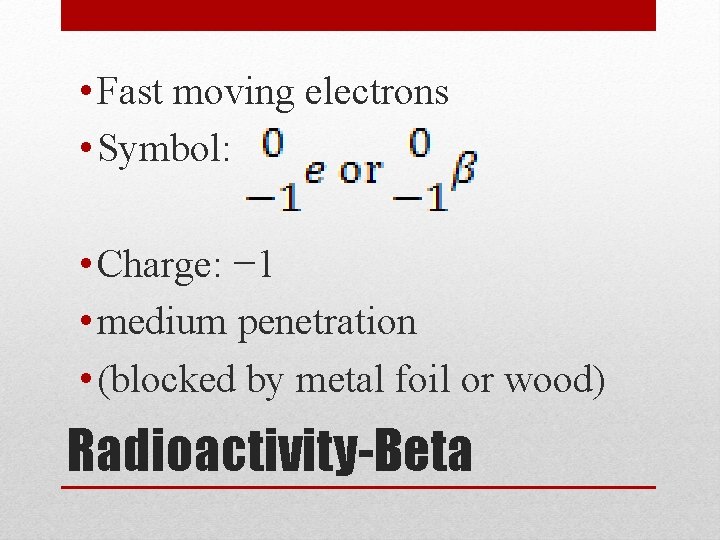
• Fast moving electrons • Symbol: • Charge: − 1 • medium penetration • (blocked by metal foil or wood) Radioactivity-Beta

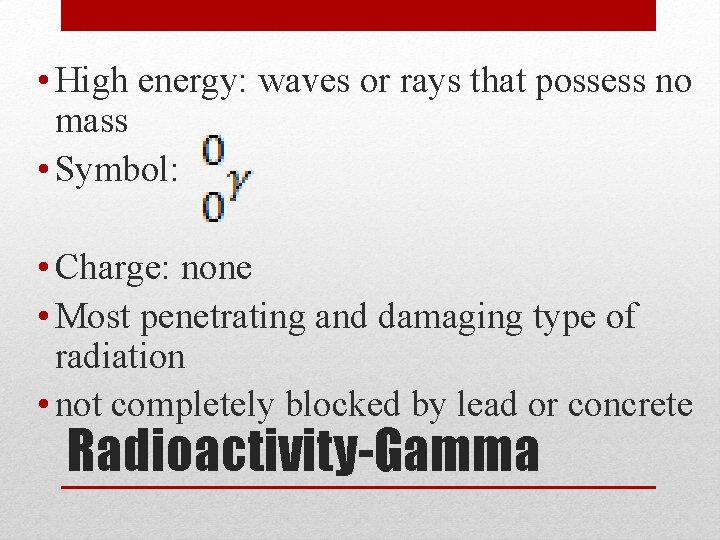
• High energy: waves or rays that possess no mass • Symbol: • Charge: none • Most penetrating and damaging type of radiation • not completely blocked by lead or concrete Radioactivity-Gamma

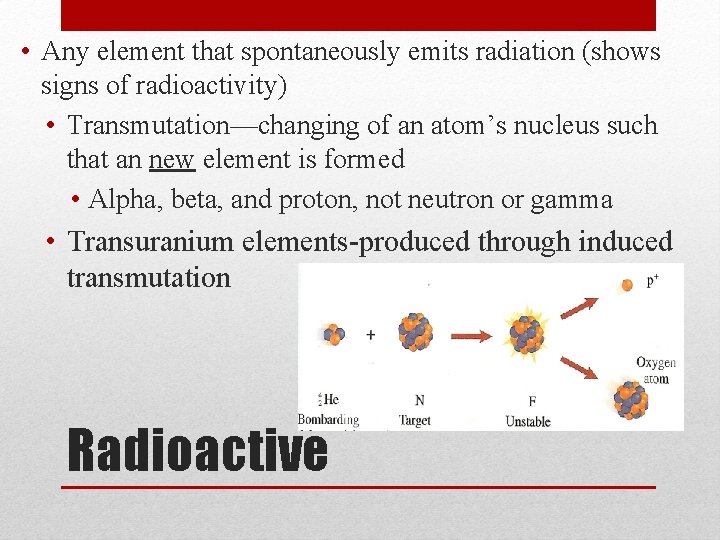
• Any element that spontaneously emits radiation (shows signs of radioactivity) • Transmutation—changing of an atom’s nucleus such that an new element is formed • Alpha, beta, and proton, not neutron or gamma • Transuranium elements-produced through induced transmutation Radioactive

Why does an element go undergo transmutation?

• Before 1919, the only way to change the nucleus, or cause transmutation, was to wait for nature. In 1919, Rutherford was the first to induce transmutation which proved that nuclear reactions could be produced artificially. • Can occur by bombarding an atom with alpha particles, protons, or electrons Induced Transmutation

• Unstable (Radioactive) nuclei are found outside the band of stability • The stability of the nucleus depends on the neutron to proton ratio • If a nucleus is unstable, it will emit radiation (decay) to gain stability

• Unstable nuclei (those that can be found outside the band of stability) losing energy by emitting radiation in a spontaneous process Radioactive decay

• Isotopes of atoms with unstable nuclei and go through radioactive decay to obtain a more stable nuclei • Small nuclei—up to 20 protons usually stable • Exception: Carbon— 14 • Large nuclei—tend to be radioactive, based on the ratio of neutrons to protons; ALL nuclei with 83 protons or more are radioactive Radioisotope

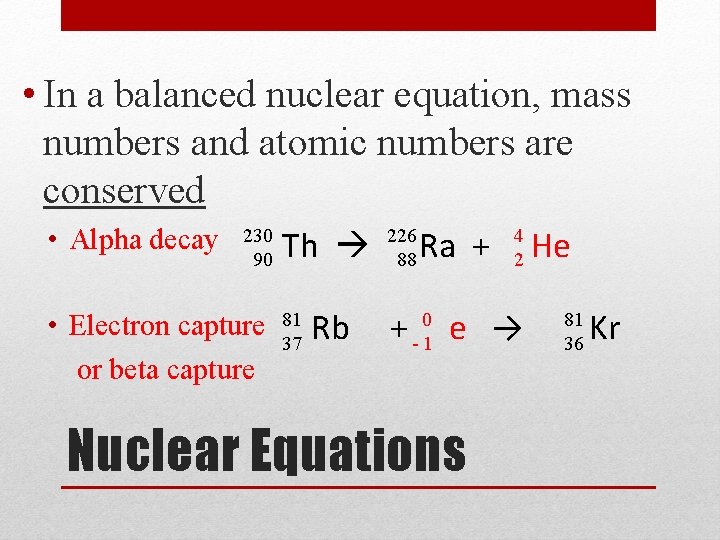
• In a balanced nuclear equation, mass numbers and atomic numbers are conserved • Alpha decay 230 90 • Electron capture or beta capture Th 81 37 Rb 226 88 Ra + 4 2 + - 01 e → Nuclear Equations He 81 36 Kr

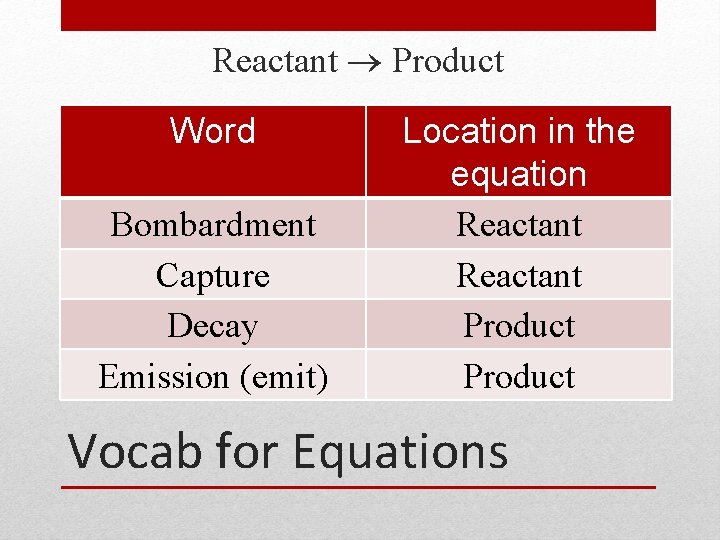
Reactant Product Word Bombardment Capture Decay Emission (emit) Location in the equation Reactant Product Vocab for Equations

97 40 1. 2. 218 84 Zr 3. ? 4. 5. 47 20 244 96 4 2 Po 222 86 0 -1 e + ? He + ? Rn + Ca Cm 0 -1 4 2 He e + ? He + ? Practice

Honors Only- write the equations and solve 6. Uranium-238 undergoing alpha emission 7. Krypton-81 undergoes beta capture 8. Cobalt-59 undergoes neutron bombardment, giving an alpha decay in addition to the new element 9. Would a)emission, b)capture, c) decay, d)bombardment be in the reactant or the product? Honors Practice with words

Day 2 of Nuclear Chemistry


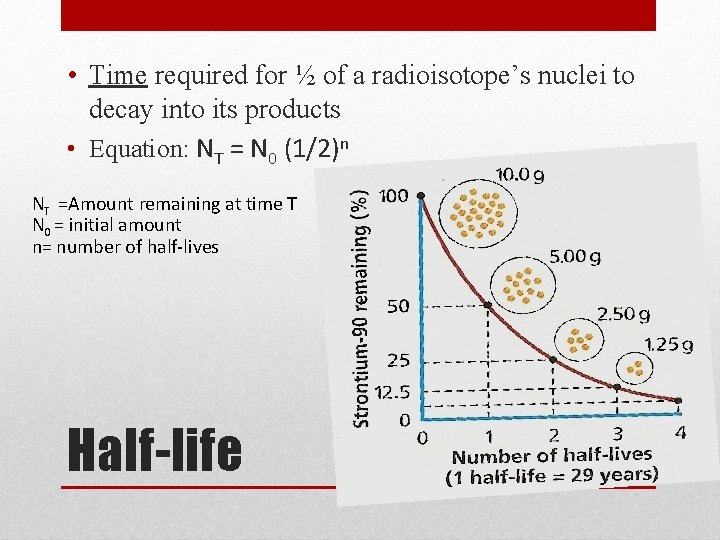
• Time required for ½ of a radioisotope’s nuclei to decay into its products • Equation: NT = N 0 (1/2)n NT =Amount remaining at time T N 0 = initial amount n= number of half-lives Half-life

Half-Life Formula Example 1 1. Scientists start with 50. 0 g sample of a radioisotope. How much is left after four halflives?

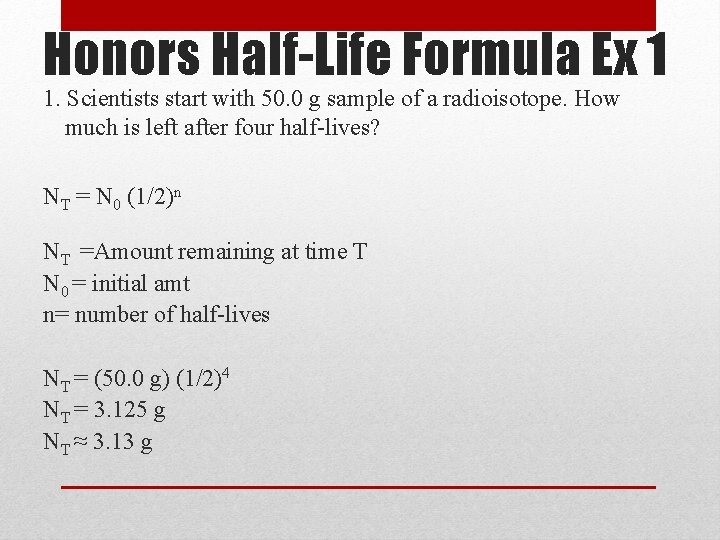
Honors Half-Life Formula Ex 1 1. Scientists start with 50. 0 g sample of a radioisotope. How much is left after four half-lives? NT = N 0 (1/2)n NT =Amount remaining at time T N 0 = initial amt n= number of half-lives NT = (50. 0 g) (1/2)4 NT = 3. 125 g NT ≈ 3. 13 g

CP Half-Life Formula Ex 1 1. Scientists start with 50. 0 g sample of a radioisotope. How much is left after four half-lives? Half-Life Skip count 50. 0 g 25. 0 g 12. 5 g 6. 25 g 3. 125 g ≈3. 13 g 1 2 3 4

Half-Life Formula Example 2 2. Iron-59 is used in medicine to diagnose blood circulation disorders. The half-life of iron-59 is 44. 5 days. How much of a 2. 000 mg sample will remain after 133. 5 days?

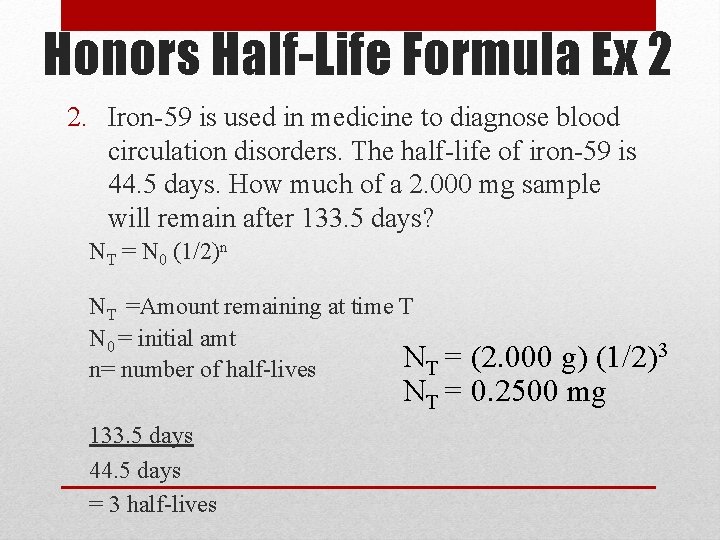
Honors Half-Life Formula Ex 2 2. Iron-59 is used in medicine to diagnose blood circulation disorders. The half-life of iron-59 is 44. 5 days. How much of a 2. 000 mg sample will remain after 133. 5 days? NT = N 0 (1/2)n NT =Amount remaining at time T N 0 = initial amt NT = n= number of half-lives (2. 000 g) (1/2)3 NT = 0. 2500 mg 133. 5 days 44. 5 days = 3 half-lives

CP Half-Life Formula Ex 2 2. Iron-59 is used in medicine to diagnose blood circulation disorders. The half-life of iron-59 is 44. 5 days. How much of a 2. 000 mg sample will remain after 133. 5 days? 133. 5 days 44. 5 days = 3 half-lives First figure out the number of half-lives Half-Life Skip count 2. 000 mg 1 0. 5000 mg 2 0. 2500 mg 3

• Carbon-14 is evenly spread in Earth’s biosphere • Carbon-14 is radioactive and undergoes beta decay; half-life of 5730 years • Dates carbon-bearing materials up to 62, 000 years Carbon-14 Dating

Honors-Using the graph, about what % of carbon-14 remains after 11, 400 years?

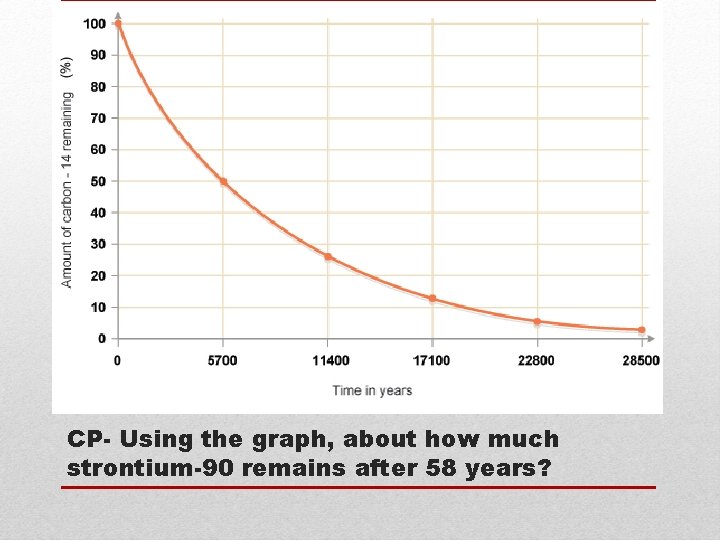
CP- Using the graph, about how much strontium-90 remains after 58 years?
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear chemistry webquest answer key
Nuclear chemistry webquest answer key Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave Applications of nuclear chemistry
Applications of nuclear chemistry T half life formula
T half life formula Nuclear fusion in real life
Nuclear fusion in real life Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Nuclear fusion
Nuclear fusion Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry External-external trips
External-external trips Neutron emission
Neutron emission Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Shorthand way of representing chemical reaction
Shorthand way of representing chemical reaction Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Equilibrium
Equilibrium