Nuclear Chemistry Nuclear Reactions Chemical reactions w What










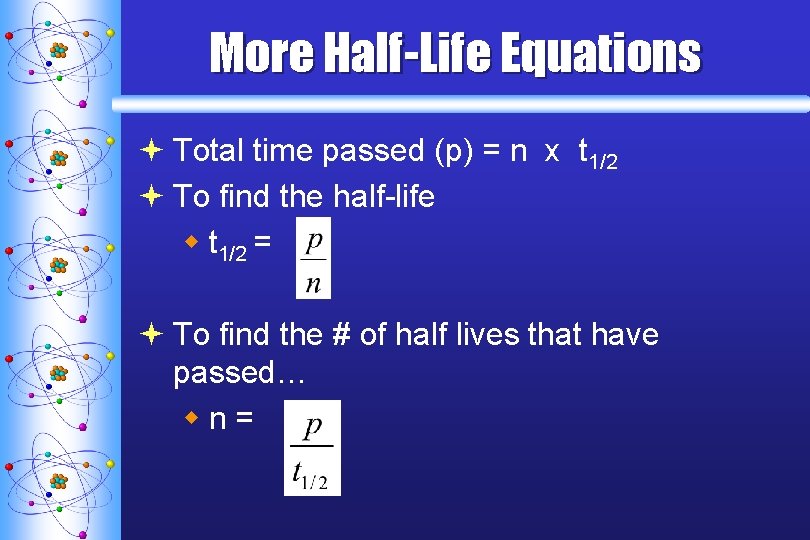



- Slides: 24

Nuclear Chemistry

Nuclear Reactions ª Chemical reactions w What can NOT change in a chemical reaction? ª Nuclear Reactions: changes that occur in the nucleus of an atom w The nucleus is unstable!! ª Most atoms have unstable nuclei.

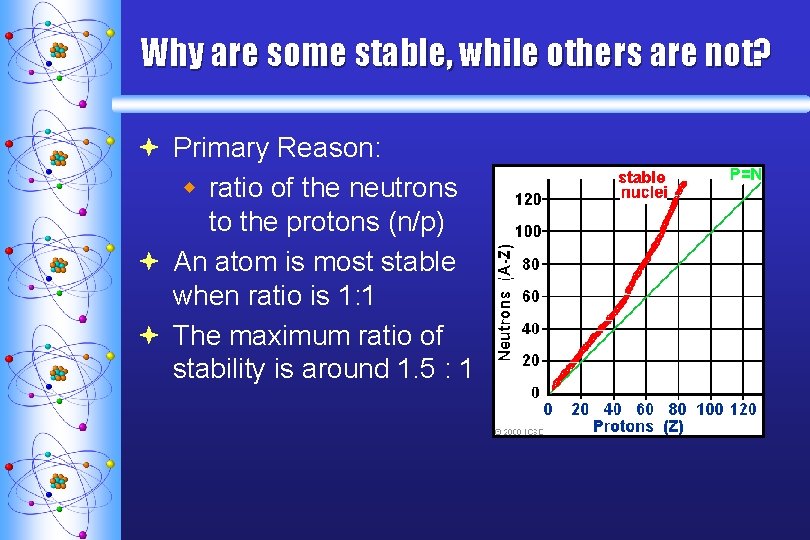
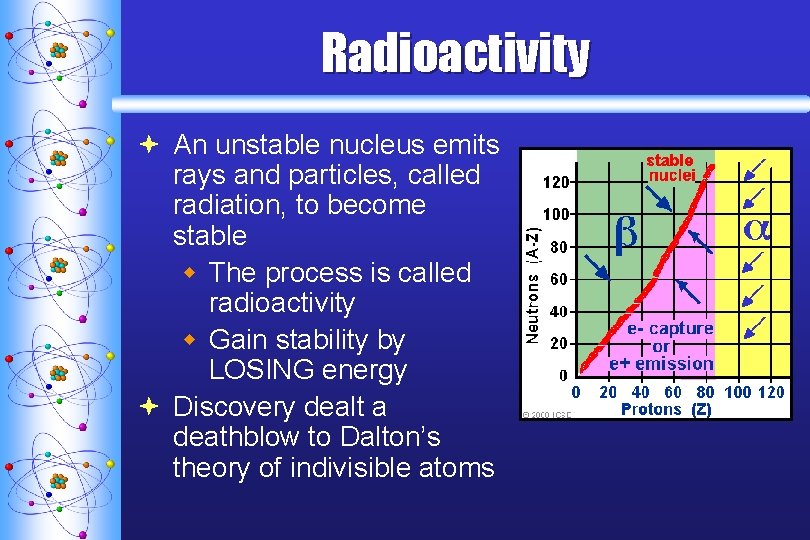
Why are some stable, while others are not? ª Primary Reason: w ratio of the neutrons to the protons (n/p) ª An atom is most stable when ratio is 1: 1 ª The maximum ratio of stability is around 1. 5 : 1

Radioactivity ª An unstable nucleus emits rays and particles, called radiation, to become stable w The process is called radioactivity w Gain stability by LOSING energy ª Discovery dealt a deathblow to Dalton’s theory of indivisible atoms

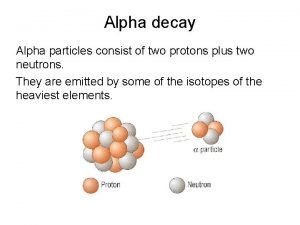
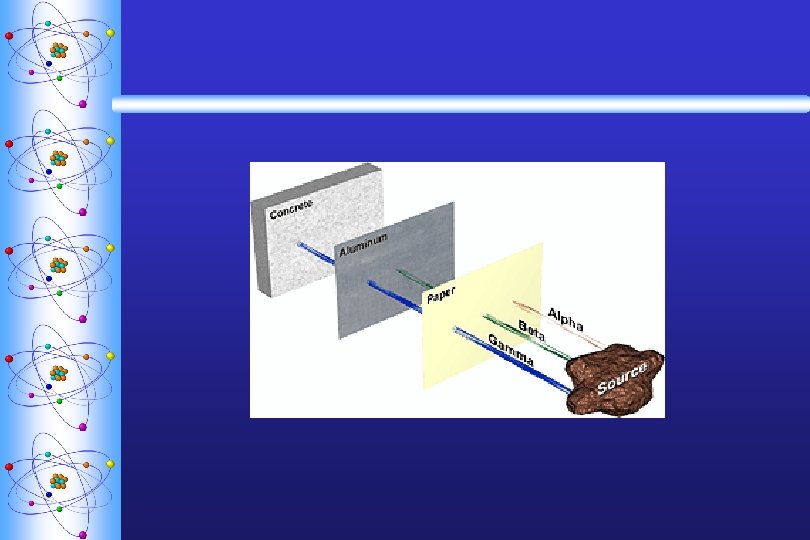
Types of Radiation ª Alpha particle ( ) w helium nucleus ª Beta particle ( -) w electron ª Gamma ( ) w high-energy photon 2+ paper 10 lead


3 Radioactive Particles ª 1. Alpha Particle (+) w It travels about 1/10 the speed of light (slowest) • It is the largest, most massive particle w It is the most dangerous if ingested w It has the least penetrating ability paper can stop this particle

3 Radioactive Particles ª 2. Beta Particle (-) w Fast accelerated electron w Ejected when a neutron is converted to a proton in the nucleus w Travels 1/4 the speed of light. w It is lighter and faster than the alpha particle. w Average penetrating ability - can be stopped by heavy clothing

3 Radioactive Particles ª 3. Gamma Ray w Not a particle; it is high energy electromagnetic radiation w Has no mass and no charge. w Always accompanies either beta or alpha radiation. w Travels at the speed of light. w Highest penetrating ability - can be stopped by heavy shielding such as lead.

Radioactive Particles Summary

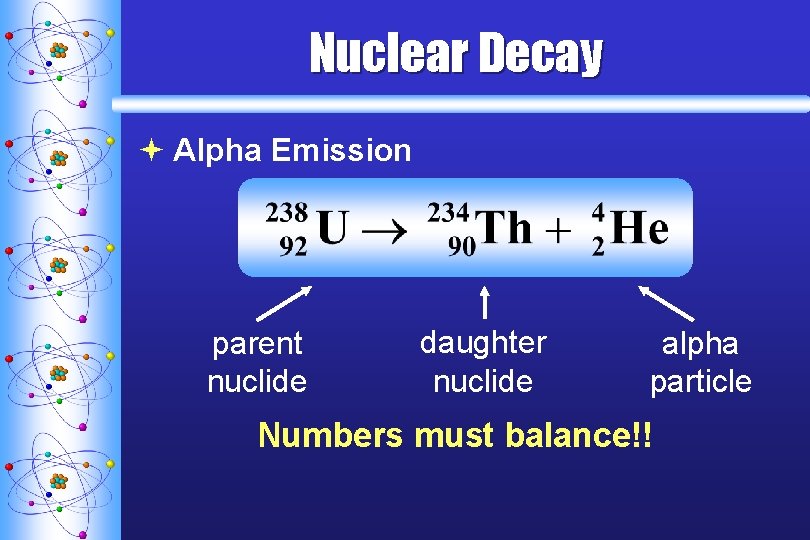
Nuclear Decay ª Alpha Emission parent nuclide daughter nuclide alpha particle Numbers must balance!!

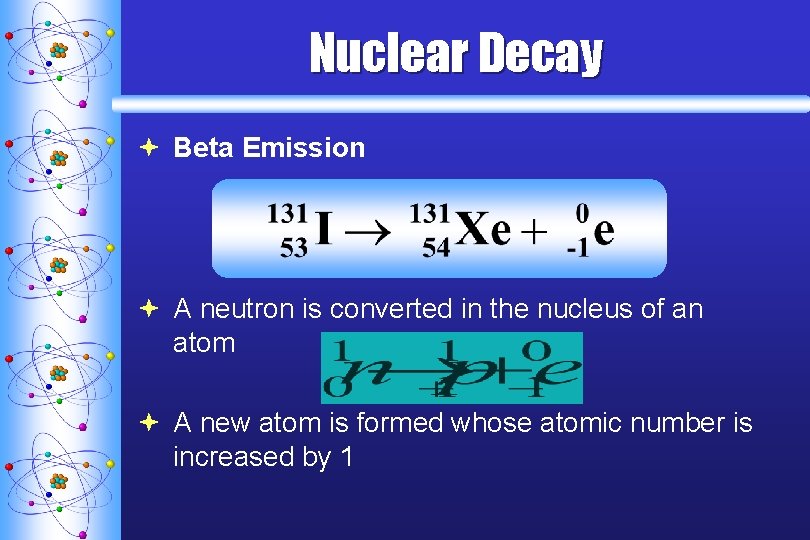
Nuclear Decay ª Beta Emission ª A neutron is converted in the nucleus of an atom ª A new atom is formed whose atomic number is increased by 1

Nuclear Decay ª Gamma Emission w Always accompanies alpha and beta emissions. ª Transmutation w One element becomes another.


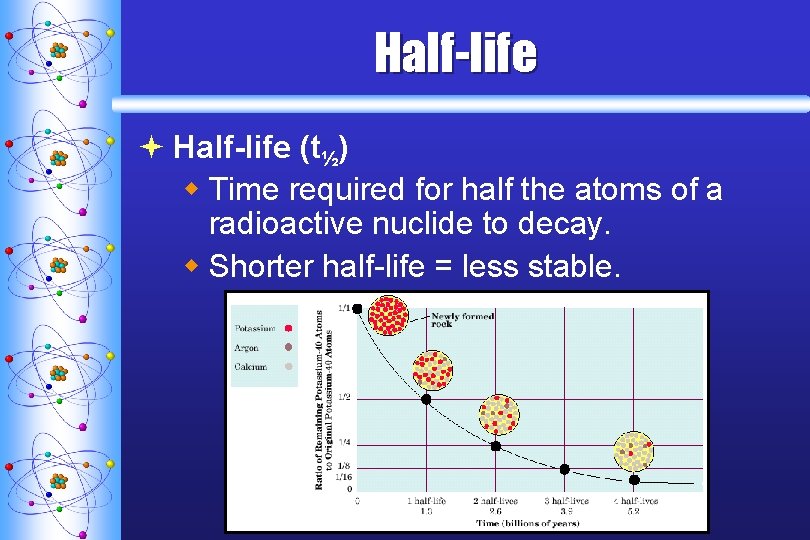
Half-life ª Half-life (t½) w Time required for half the atoms of a radioactive nuclide to decay. w Shorter half-life = less stable.

So, what does this mean? ª Let’s say you have 100 g of radioactive C 14. The half-life of C-14 is 5730 years. w How many grams are left after one halflife? 50 g w How many grams are left after two halflives? 25 g

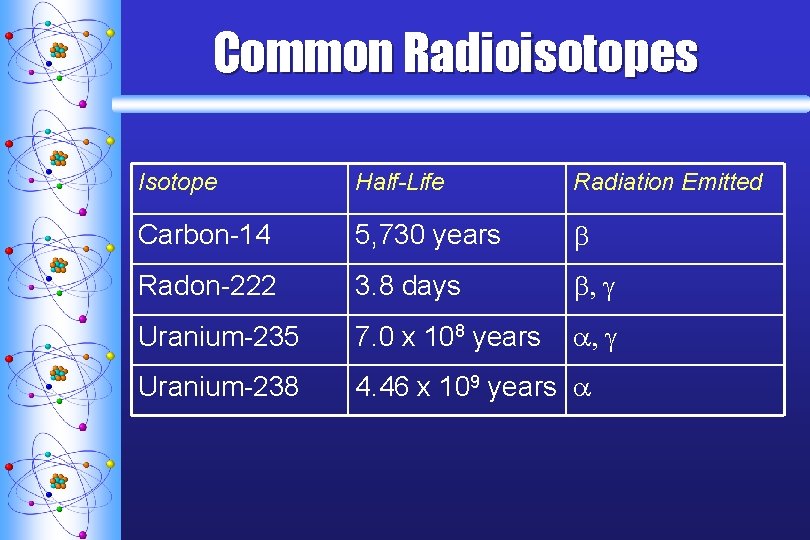
Common Radioisotopes Isotope Half-Life Radiation Emitted Carbon-14 5, 730 years b Radon-222 3. 8 days b, g Uranium-235 7. 0 x 108 years a, g Uranium-238 4. 46 x 109 years a

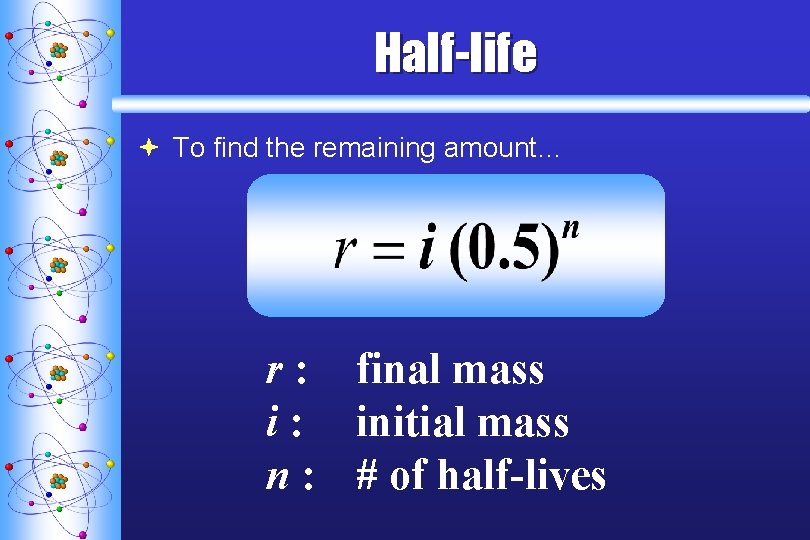
Half-life ª To find the remaining amount… r : final mass i : initial mass n : # of half-lives
More Half-Life Equations ª Total time passed (p) = n x t 1/2 ª To find the half-life w t 1/2 = ª To find the # of half lives that have passed… wn=

Half-life ª Fluorine-21 has a half-life of 5. 0 seconds. If you start with 25 g of fluorine-21, how many grams would remain after 60. 0 s? GIVEN: WORK: t½ = 5. 0 s r = i (0. 5 )n i = 25 g r = (25 g)(0. 5)12 r=? r = 0. 0061 g p = 60. 0 s n = 60. 0 s ÷ 5. 0 s =12

You try it! ª If the half life element A is 3 hours and you have 90 grams of it, how many grams would be left after 9 hours?

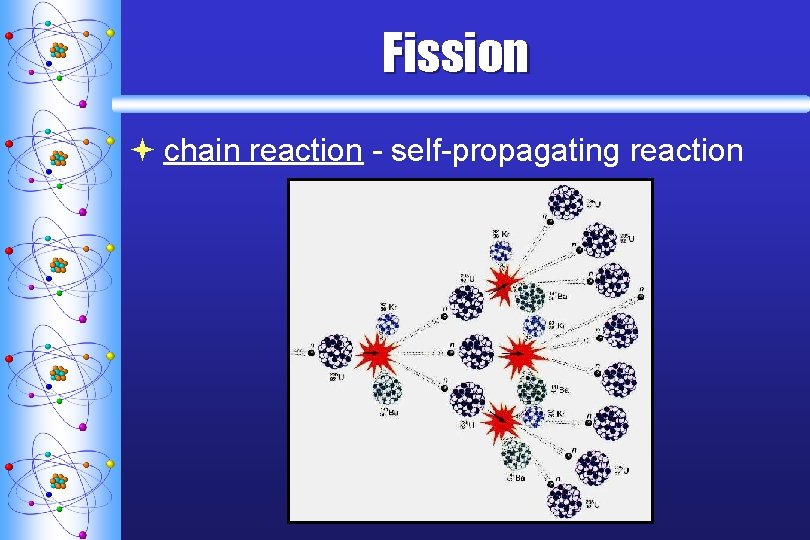
Fission ª splitting a nucleus into two or more smaller nuclei ª 1 g of 235 U = 3 tons of coal

Fission ª chain reaction - self-propagating reaction

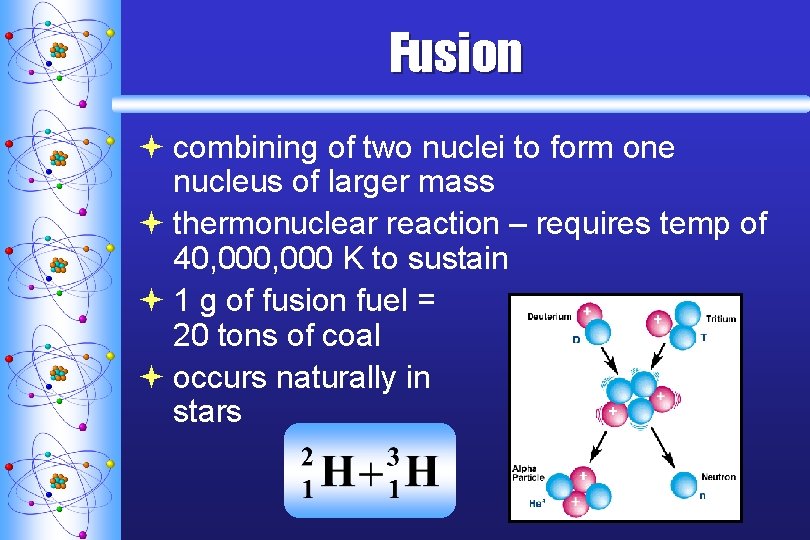
Fusion ª combining of two nuclei to form one nucleus of larger mass ª thermonuclear reaction – requires temp of 40, 000 K to sustain ª 1 g of fusion fuel = 20 tons of coal ª occurs naturally in stars
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Fission equation
Fission equation Natural transmutation example
Natural transmutation example Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Alpha decay of uranium
Alpha decay of uranium Two types of nuclear reactions
Two types of nuclear reactions Nuclear bombardment reactions
Nuclear bombardment reactions Nuclear reactions are at
Nuclear reactions are at Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions 20 examples of redox reaction
20 examples of redox reaction Nuclear chemistry webquest
Nuclear chemistry webquest Nuclear chemistry
Nuclear chemistry