Section 3 Periodic Trends Nuclear Charge Nuclear charge




- Slides: 17

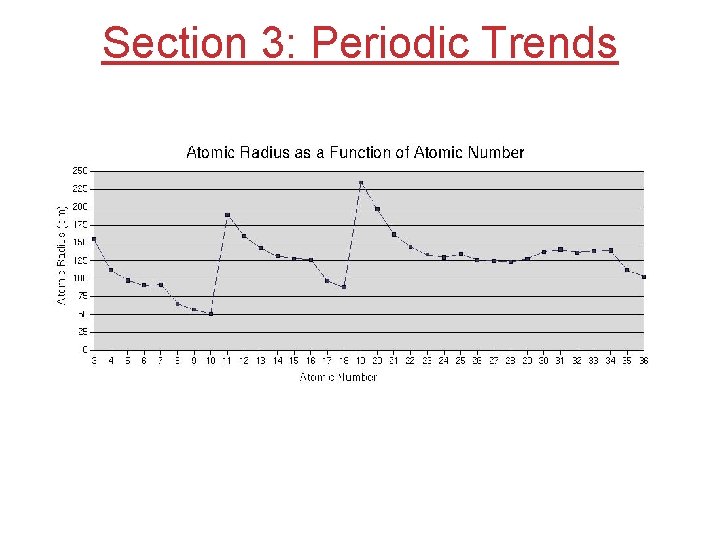
Section 3: Periodic Trends

Nuclear Charge • Nuclear charge is the pull that an electron “feels” from the nucleus. • The closer an electron is to the nucleus, the more pull it feels. • As nuclear charge increases, the electron cloud is pulled in tighter. • Increases across a period.

Shielding • As more energy levels are added to atoms, the inner layers of electrons shield the outer electrons from the nucleus. • Outer electrons are less tightly held. • Constant across a period. • Increases down a group.

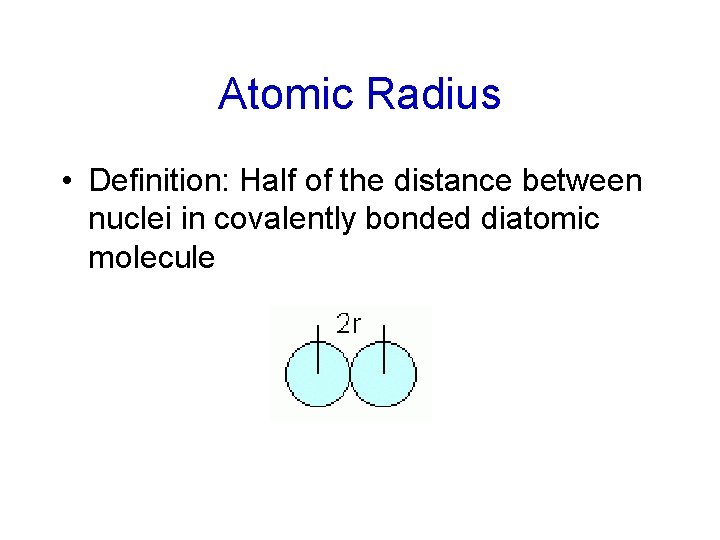
Atomic Radius • Definition: Half of the distance between nuclei in covalently bonded diatomic molecule

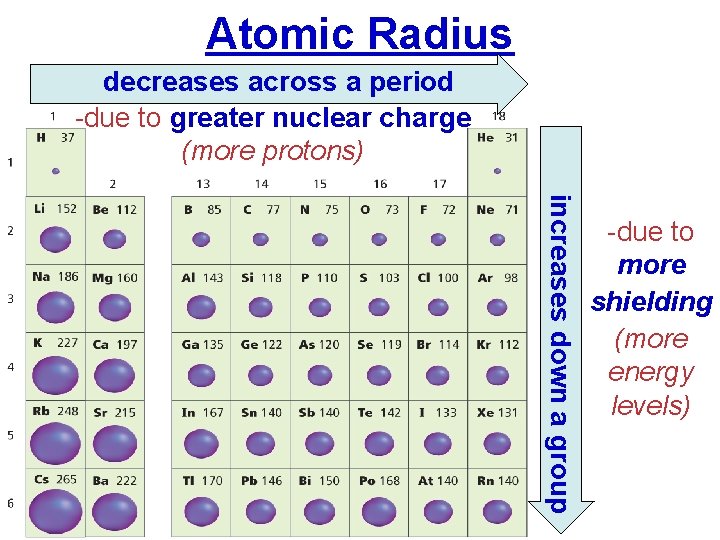
Atomic Radius decreases across a period -due to greater nuclear charge (more protons) increases down a group -due to more shielding (more energy levels) Electronic Structure of Atoms

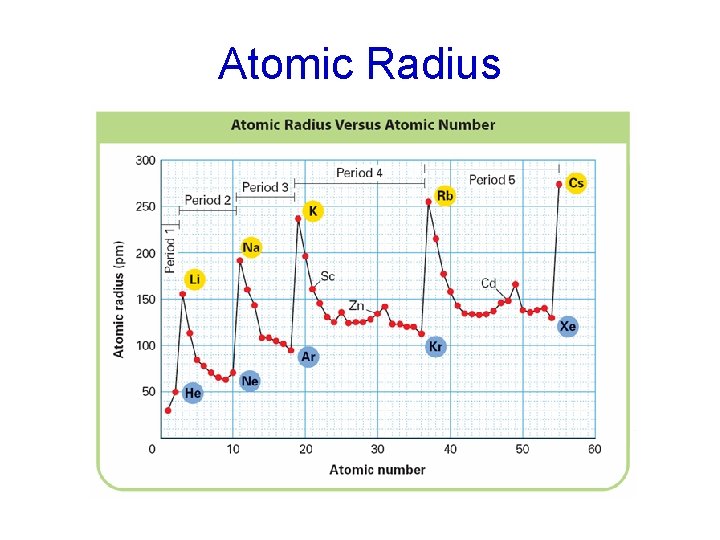
Atomic Radius

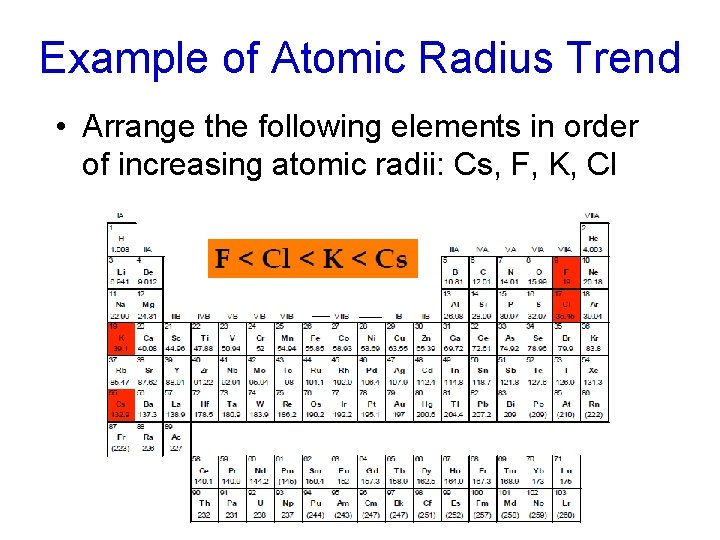
Example of Atomic Radius Trend • Arrange the following elements in order of increasing atomic radii: Cs, F, K, Cl

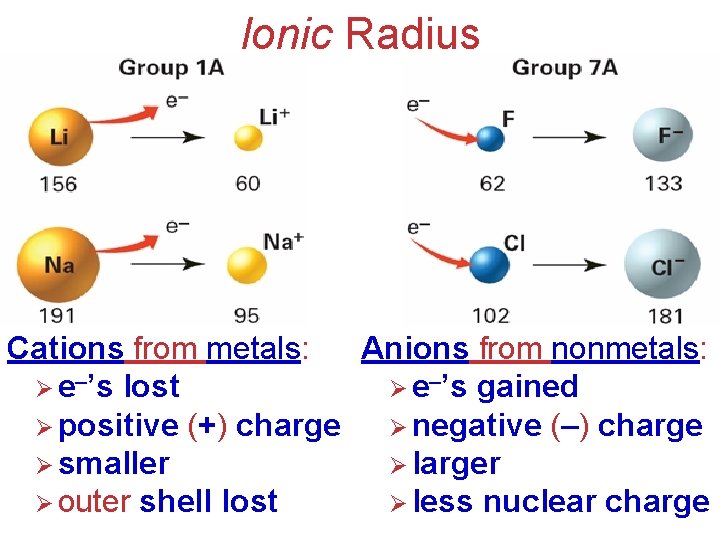
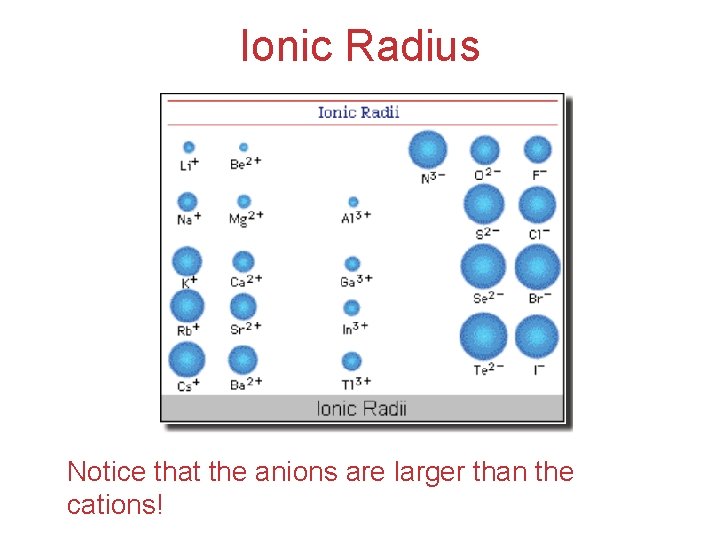
Ionic Radius Cations from metals: Anions from nonmetals: Ø e–’s lost Ø e–’s gained Ø positive (+) charge Ø negative (–) charge Ø smaller Ø larger Electronic Structure of Atoms Ø outer shell lost Ø less nuclear charge

Ions • Here is a simple way to remember which is the cation and which the anion: + This is Ann Ion. She’s unhappy and negative. + This is a cat-ion. He’s a “plussy” cat!

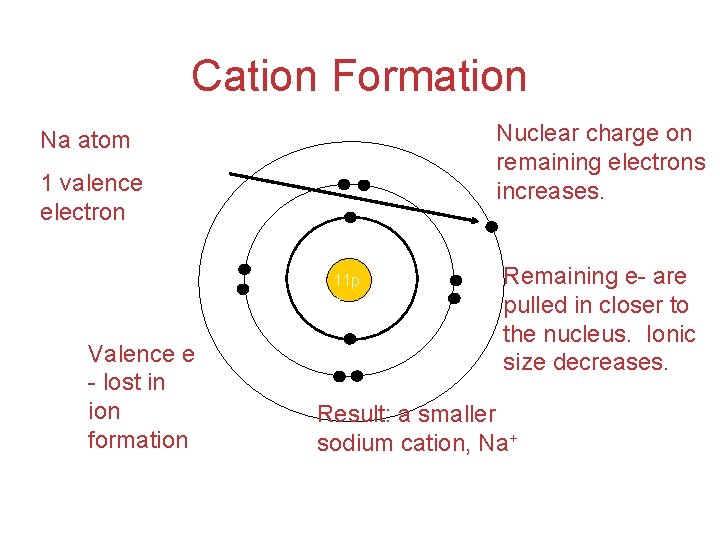
Cation Formation Nuclear charge on remaining electrons increases. Na atom 1 valence electron 11 p + Valence e - lost in ion formation Remaining e- are pulled in closer to the nucleus. Ionic size decreases. Result: a smaller sodium cation, Na+

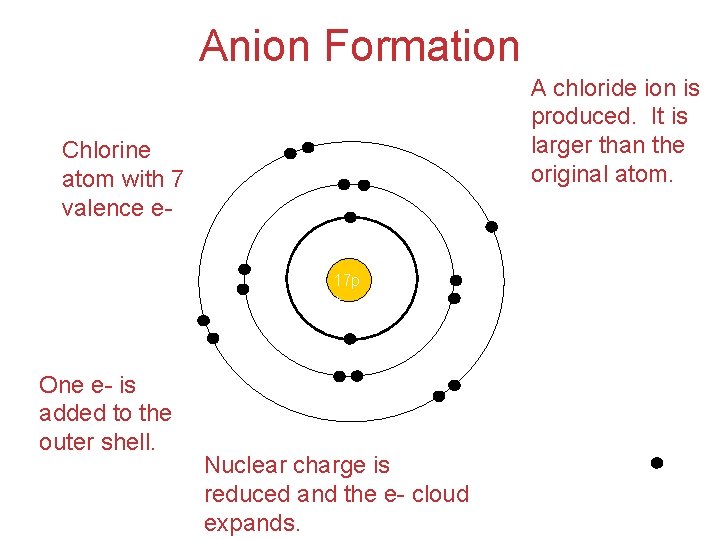
Anion Formation A chloride ion is produced. It is larger than the original atom. Chlorine atom with 7 valence e 17 p + One e- is added to the outer shell. Nuclear charge is reduced and the e- cloud expands.

Ionic Radius Notice that the anions are larger than the cations!

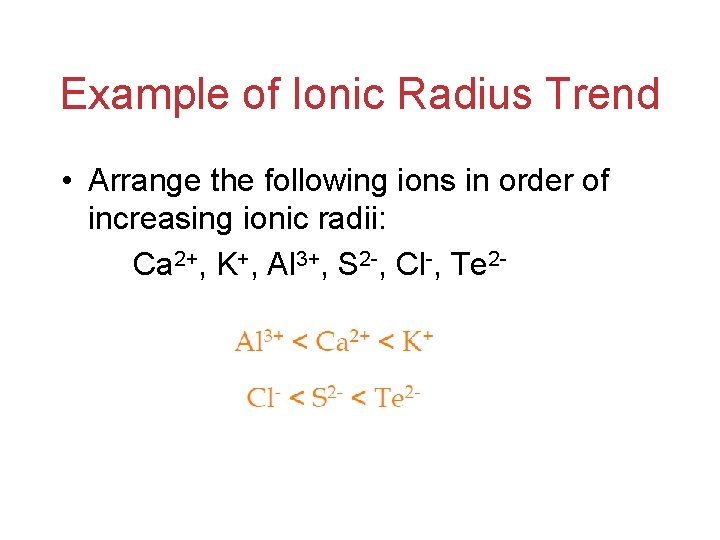
Example of Ionic Radius Trend • Arrange the following ions in order of increasing ionic radii: Ca 2+, K+, Al 3+, S 2 -, Cl-, Te 2 -

Quick Quiz! 1. Atoms get ___ down a group due to ___. A. B. C. D. bigger, greater nuclear charge (protons) smaller, less nuclear charge (protons) bigger, more shielding (levels) smaller, less shielding (levels) Electronic Structure of Atoms

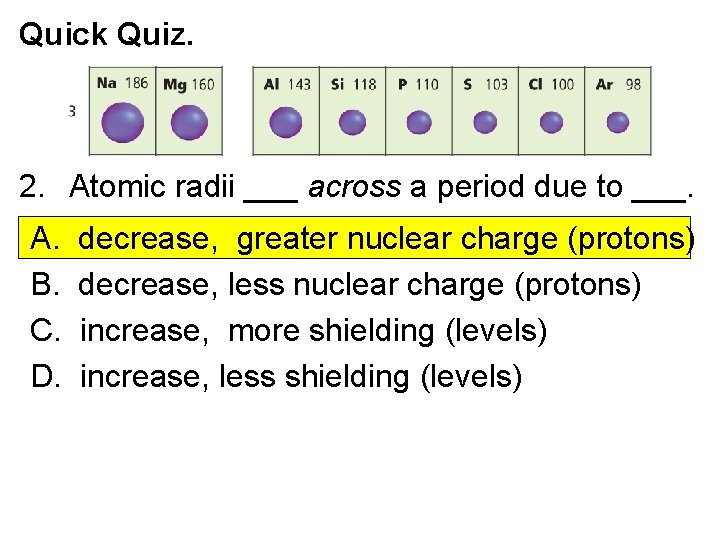
Quick Quiz. 2. Atomic radii ___ across a period due to ___. A. B. C. D. decrease, greater nuclear charge (protons) decrease, less nuclear charge (protons) increase, more shielding (levels) increase, less shielding (levels) Electronic Structure of Atoms

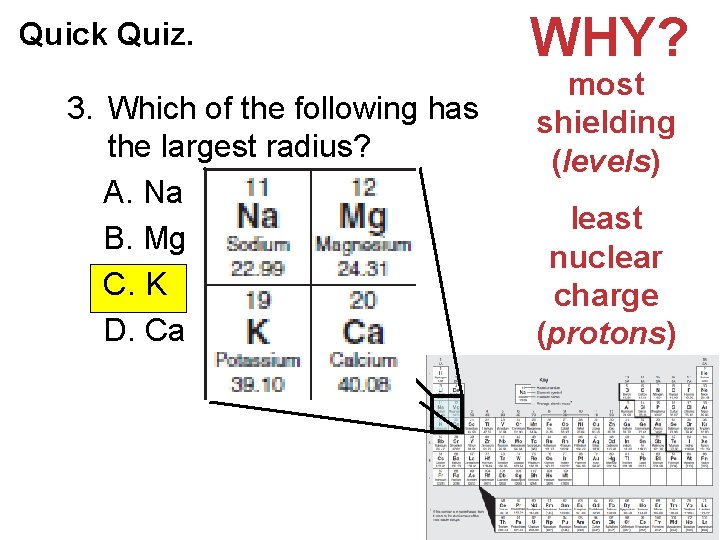
Quick Quiz. 3. Which of the following has the largest radius? A. Na B. Mg C. K D. Ca WHY? most shielding (levels) least nuclear charge (protons) Electronic Structure of Atoms

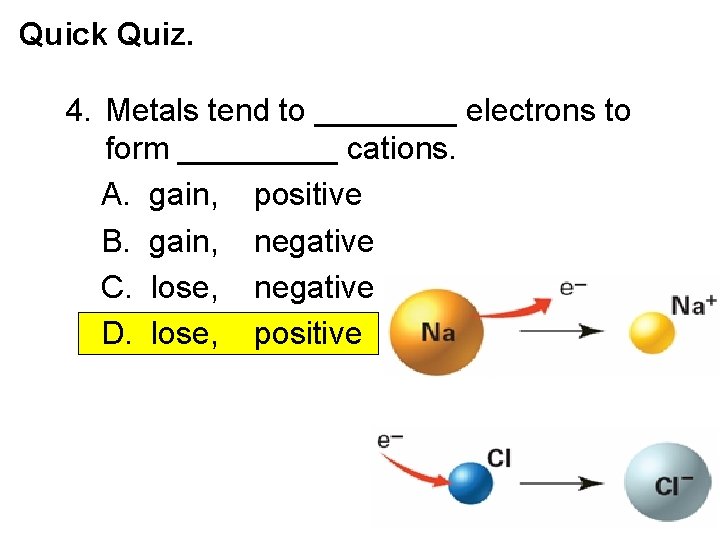
Quick Quiz. 4. Metals tend to ____ electrons to form _____ cations. A. gain, positive B. gain, negative C. lose, negative D. lose, positive Electronic Structure of Atoms