IGCSE Physics Types of Energy 1 of 36









- Slides: 36

IGCSE Physics Types of Energy 1 of 36 © Boardworks Ltd 2005

Contents Types of Energy Different types of energy Nuclear energy Gravitational potential energy Kinetic energy Summary activities 2 of 36 © Boardworks Ltd 2005

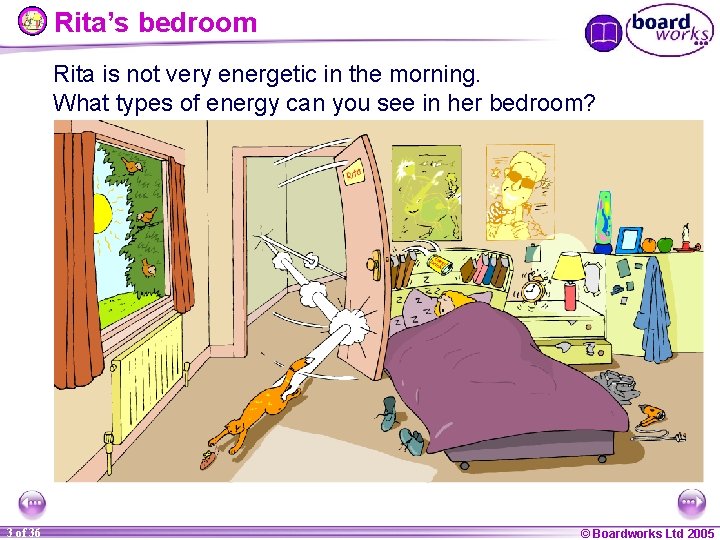
Rita’s bedroom Rita is not very energetic in the morning. What types of energy can you see in her bedroom? 3 of 36 © Boardworks Ltd 2005

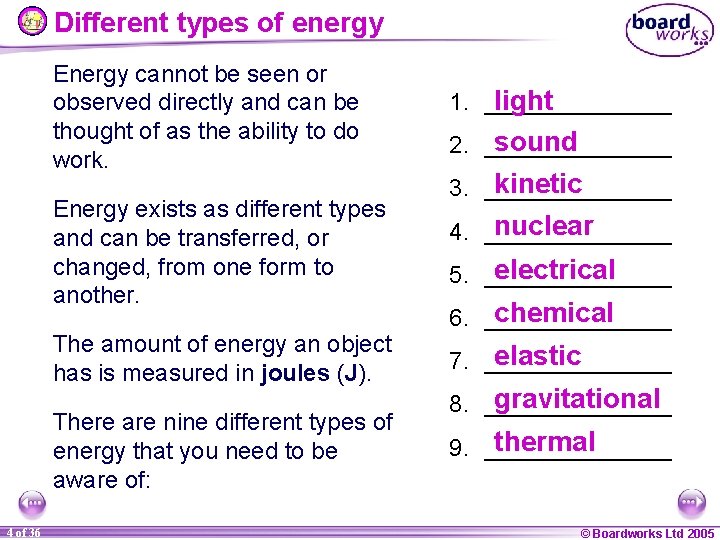
Different types of energy Energy cannot be seen or observed directly and can be thought of as the ability to do work. Energy exists as different types and can be transferred, or changed, from one form to another. The amount of energy an object has is measured in joules (J). There are nine different types of energy that you need to be aware of: 4 of 36 1. _______ light sound 2. _______ kinetic 3. _______ nuclear 4. _______ electrical 5. _______ chemical 6. _______ elastic 7. _______ gravitational 8. _______ thermal 9. _______ © Boardworks Ltd 2005

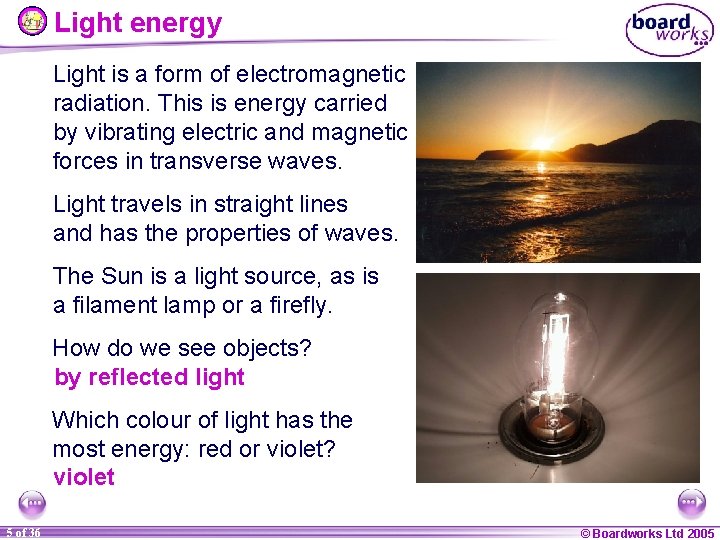
Light energy Light is a form of electromagnetic radiation. This is energy carried by vibrating electric and magnetic forces in transverse waves. Light travels in straight lines and has the properties of waves. The Sun is a light source, as is a filament lamp or a firefly. How do we see objects? by reflected light Which colour of light has the most energy: red or violet? violet 5 of 36 © Boardworks Ltd 2005

Sound energy needs a medium through which to travel. Sound energy is transmitted by vibrations of the particles in the medium through which it is travelling. Sound travels in longitudinal waves. The louder the sound, the more energy it transmits. The quieter the sound, the less energy it transmits. 6 of 36 © Boardworks Ltd 2005

Thermal energy All objects have thermal energy. It is the energy a substance has due to the kinetic energy (movement) of its particles. The more thermal energy a material has, the hotter it is. In which state of matter (solid, liquid or gas), do water molecules have the most thermal energy and why? 7 of 36 © Boardworks Ltd 2005

Thermal energy Ice (frozen water) has the least thermal energy. Its molecules have little kinetic energy and so are unable to move past each other. 8 of 36 Liquid water has more thermal energy. Its molecules have more kinetic energy. They can move past each other but are still held together. Water vapour has the most thermal energy. Its molecules have lots of kinetic energy and are moving so fast they are no longer held together. © Boardworks Ltd 2005

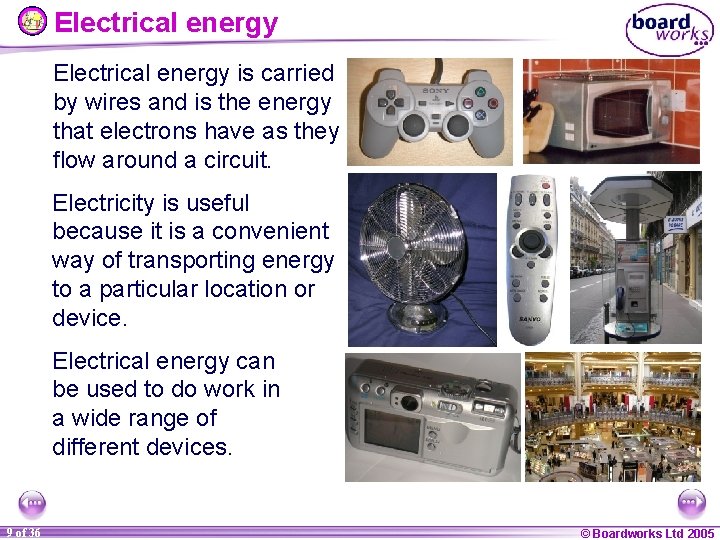
Electrical energy is carried by wires and is the energy that electrons have as they flow around a circuit. Electricity is useful because it is a convenient way of transporting energy to a particular location or device. Electrical energy can be used to do work in a wide range of different devices. 9 of 36 © Boardworks Ltd 2005

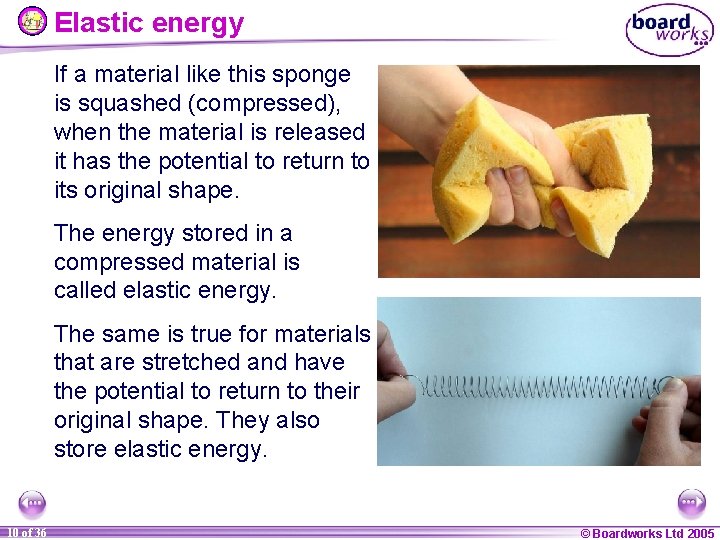
Elastic energy If a material like this sponge is squashed (compressed), when the material is released it has the potential to return to its original shape. The energy stored in a compressed material is called elastic energy. The same is true for materials that are stretched and have the potential to return to their original shape. They also store elastic energy. 10 of 36 © Boardworks Ltd 2005

Chemical energy is a type of stored energy that is released by chemical reactions. Fuels, food and batteries all contain chemical energy. When a fuel burns, energy is released by a chemical reaction with oxygen. Respiration is the process used by the body to release the chemical energy stored in digested food. 11 of 36 © Boardworks Ltd 2005

Contents Types of Energy Different types of energy Nuclear energy Gravitational potential energy Kinetic energy Summary activities 12 of 36 © Boardworks Ltd 2005

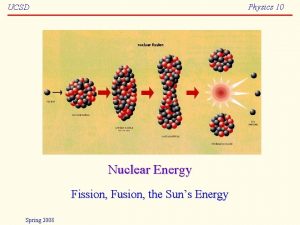
Nuclear fission The particles in the nucleus of an atom are held together by strong forces. Some atoms, such as uranium, have a very large unstable nucleus, which can split into two smaller nuclei. This splitting is called fission and releases huge amounts of nuclear energy. The release of nuclear energy is exploited in nuclear power stations and some atomic bombs. 13 of 36 © Boardworks Ltd 2005

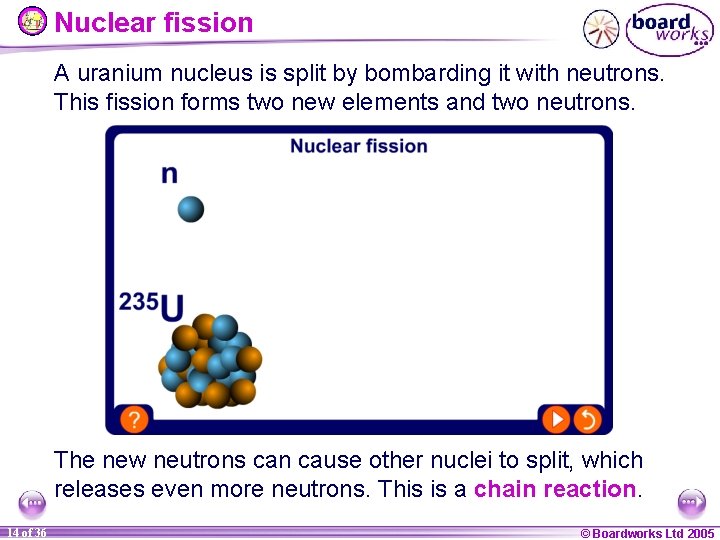
Nuclear fission A uranium nucleus is split by bombarding it with neutrons. This fission forms two new elements and two neutrons. The new neutrons can cause other nuclei to split, which releases even more neutrons. This is a chain reaction. 14 of 36 © Boardworks Ltd 2005

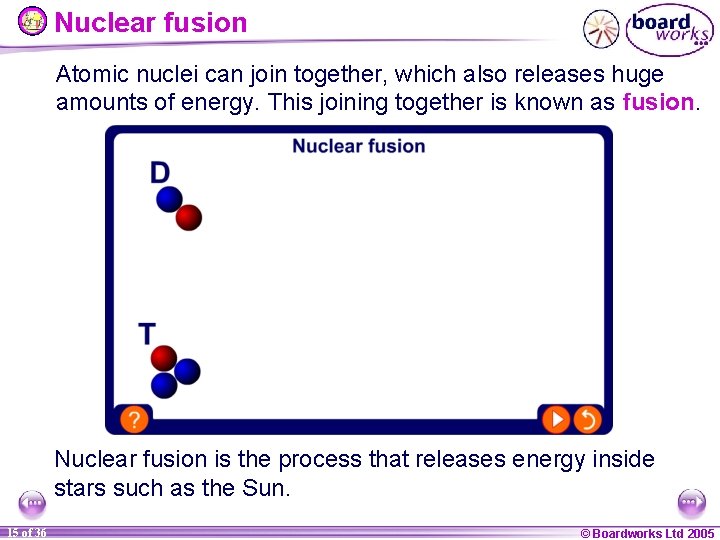
Nuclear fusion Atomic nuclei can join together, which also releases huge amounts of energy. This joining together is known as fusion. Nuclear fusion is the process that releases energy inside stars such as the Sun. 15 of 36 © Boardworks Ltd 2005

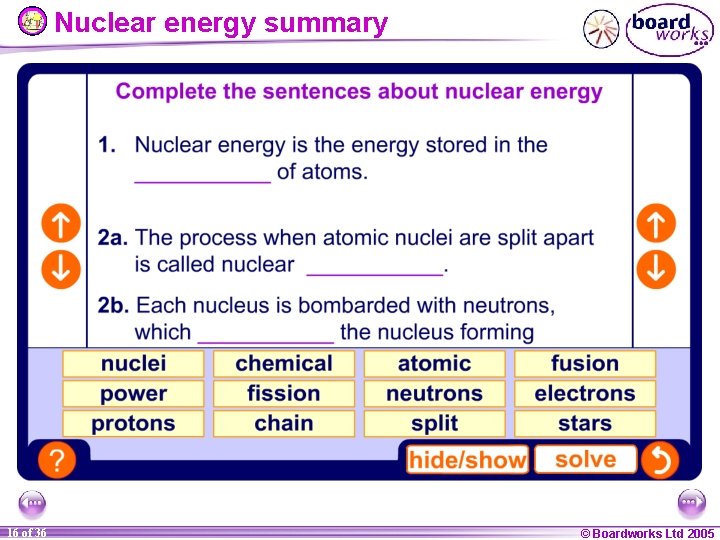
Nuclear energy summary 16 of 36 © Boardworks Ltd 2005

Contents Types of Energy Different types of energy Nuclear energy Gravitational potential energy Kinetic energy Summary activities 17 of 36 © Boardworks Ltd 2005

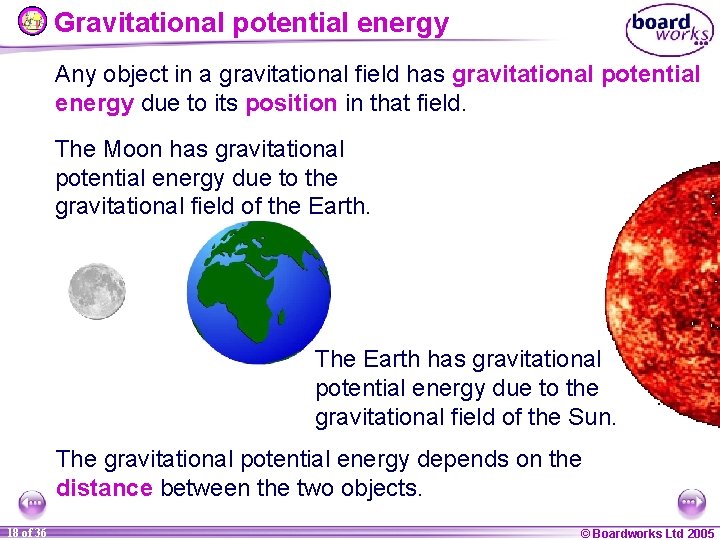
Gravitational potential energy Any object in a gravitational field has gravitational potential energy due to its position in that field. The Moon has gravitational potential energy due to the gravitational field of the Earth. The Earth has gravitational potential energy due to the gravitational field of the Sun. The gravitational potential energy depends on the distance between the two objects. 18 of 36 © Boardworks Ltd 2005

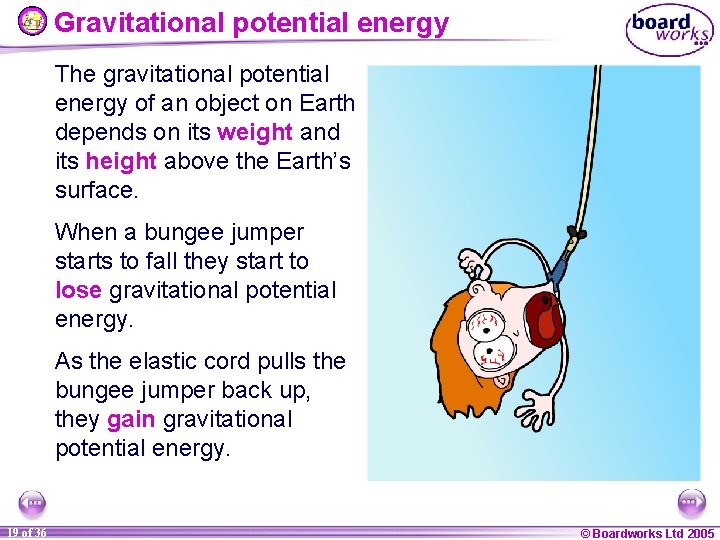
Gravitational potential energy The gravitational potential energy of an object on Earth depends on its weight and its height above the Earth’s surface. When a bungee jumper starts to fall they start to lose gravitational potential energy. As the elastic cord pulls the bungee jumper back up, they gain gravitational potential energy. 19 of 36 © Boardworks Ltd 2005

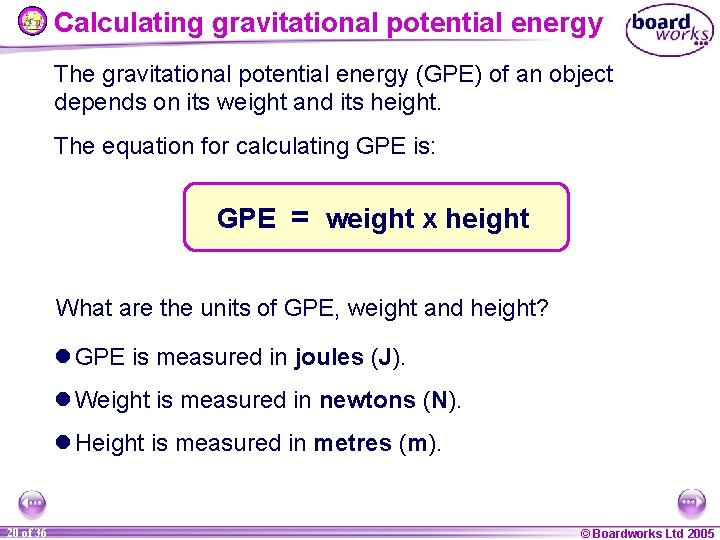
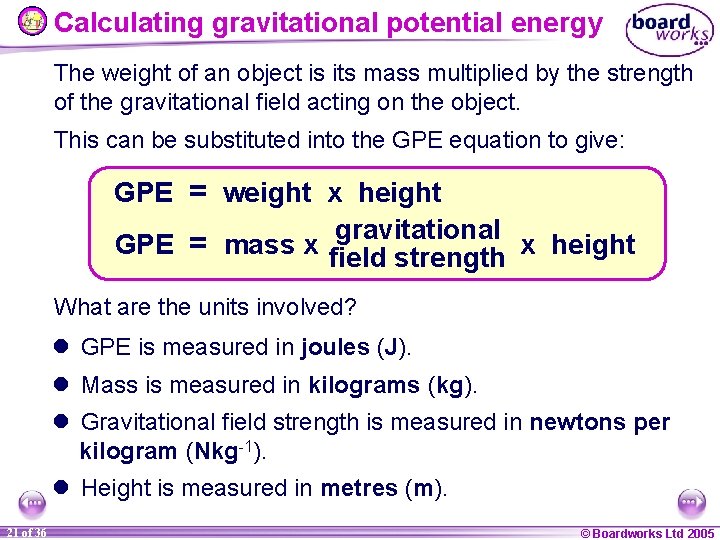
Calculating gravitational potential energy The gravitational potential energy (GPE) of an object depends on its weight and its height. The equation for calculating GPE is: GPE = weight x height What are the units of GPE, weight and height? l GPE is measured in joules (J). l Weight is measured in newtons (N). l Height is measured in metres (m). 20 of 36 © Boardworks Ltd 2005

Calculating gravitational potential energy The weight of an object is its mass multiplied by the strength of the gravitational field acting on the object. This can be substituted into the GPE equation to give: GPE = weight x height gravitational GPE = mass x field strength x height What are the units involved? l GPE is measured in joules (J). l Mass is measured in kilograms (kg). l Gravitational field strength is measured in newtons per kilogram (Nkg-1). l Height is measured in metres (m). 21 of 36 © Boardworks Ltd 2005

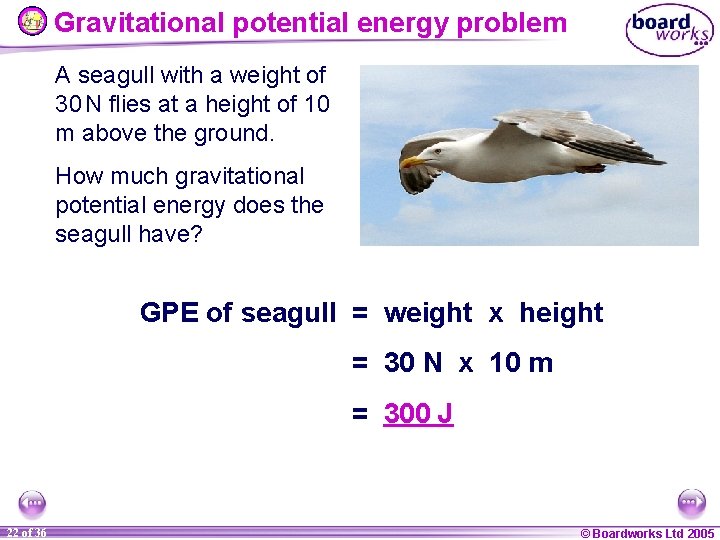
Gravitational potential energy problem A seagull with a weight of 30 N flies at a height of 10 m above the ground. How much gravitational potential energy does the seagull have? GPE of seagull = weight x height = 30 N x 10 m = 300 J 22 of 36 © Boardworks Ltd 2005

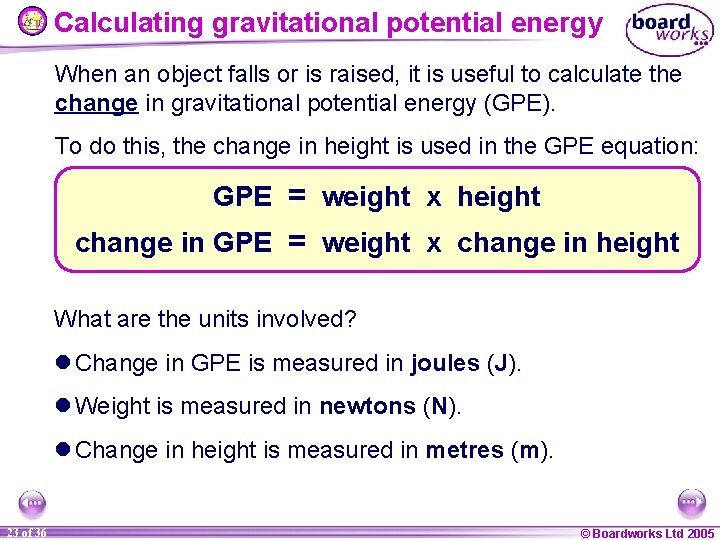
Calculating gravitational potential energy When an object falls or is raised, it is useful to calculate the change in gravitational potential energy (GPE). To do this, the change in height is used in the GPE equation: GPE = weight x height change in GPE = weight x change in height What are the units involved? l Change in GPE is measured in joules (J). l Weight is measured in newtons (N). l Change in height is measured in metres (m). 23 of 36 © Boardworks Ltd 2005

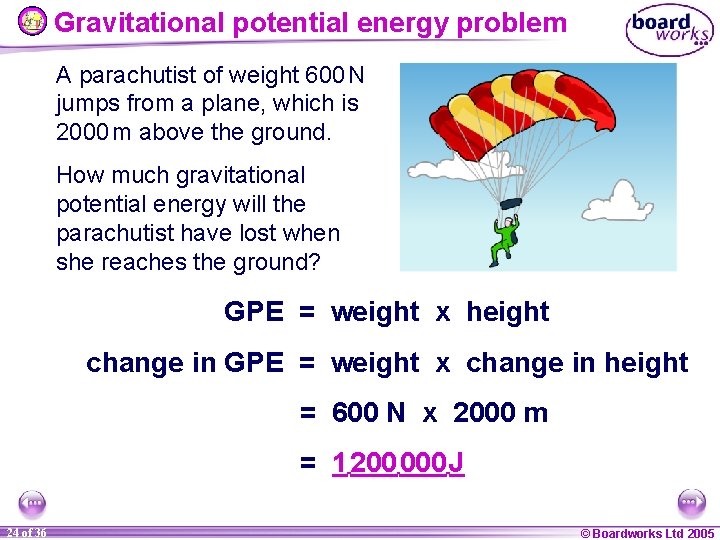
Gravitational potential energy problem A parachutist of weight 600 N jumps from a plane, which is 2000 m above the ground. How much gravitational potential energy will the parachutist have lost when she reaches the ground? GPE = weight x height change in GPE = weight x change in height = 600 N x 2000 m = 1 200 000 J 24 of 36 © Boardworks Ltd 2005

Gravitational potential energy problems 25 of 36 © Boardworks Ltd 2005

Contents Types of Energy Different types of energy Nuclear energy Gravitational potential energy Kinetic energy Summary activities 26 of 36 © Boardworks Ltd 2005

Kinetic energy is the energy an object has because it is moving. l The greater the mass of a moving object, the greater its kinetic energy. If the mass is doubled, the kinetic energy is doubled. l The greater the velocity of a moving object, the greater its kinetic energy. If the velocity is doubled, the kinetic energy is quadrupled! So, if the velocity of a car is slightly above the speed limit, its kinetic energy is much greater than it would be at the speed limit. This means it is more difficult to stop the and car there is more chance of an accident. 27 of 36 © Boardworks Ltd 2005

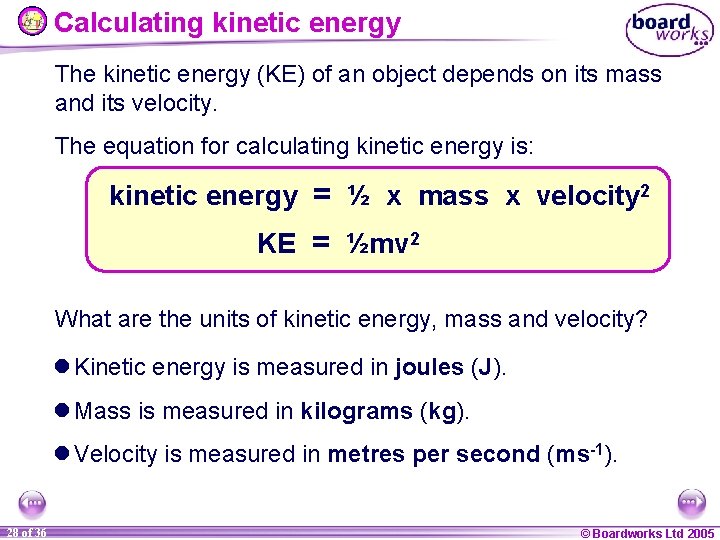
Calculating kinetic energy The kinetic energy (KE) of an object depends on its mass and its velocity. The equation for calculating kinetic energy is: kinetic energy = ½ x mass x velocity 2 KE = ½mv 2 What are the units of kinetic energy, mass and velocity? l Kinetic energy is measured in joules (J). l Mass is measured in kilograms (kg). l Velocity is measured in metres per second (ms-1). 28 of 36 © Boardworks Ltd 2005

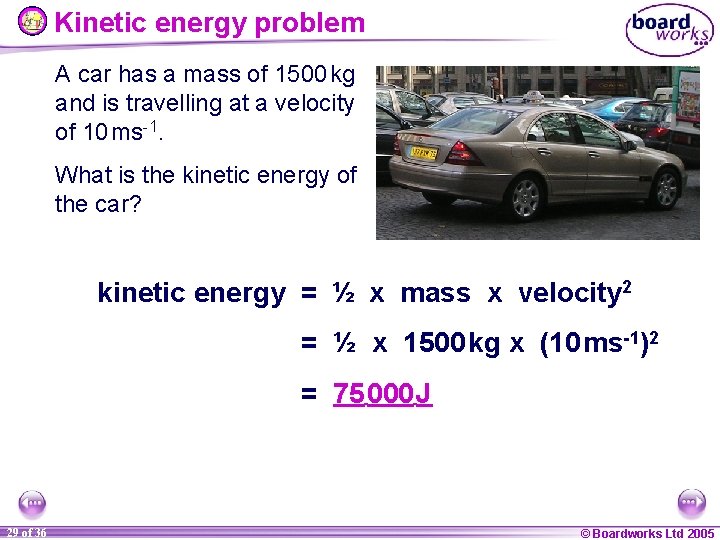
Kinetic energy problem A car has a mass of 1500 kg and is travelling at a velocity of 10 ms-1. What is the kinetic energy of the car? kinetic energy = ½ x mass x velocity 2 = ½ x 1500 kg x (10 ms-1)2 = 75 000 J 29 of 36 © Boardworks Ltd 2005

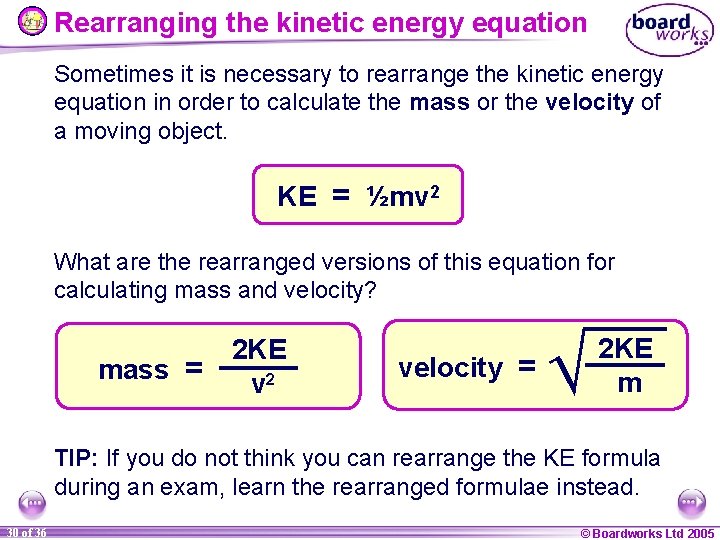
Rearranging the kinetic energy equation Sometimes it is necessary to rearrange the kinetic energy equation in order to calculate the mass or the velocity of a moving object. KE = ½mv 2 What are the rearranged versions of this equation for calculating mass and velocity? mass = 2 KE v 2 velocity = Ö 2 KE m TIP: If you do not think you can rearrange the KE formula during an exam, learn the rearranged formulae instead. 30 of 36 © Boardworks Ltd 2005

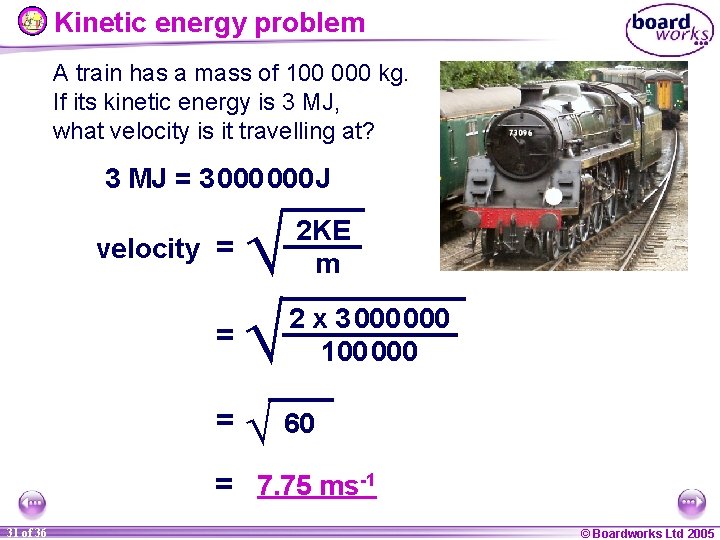
Kinetic energy problem A train has a mass of 100 000 kg. If its kinetic energy is 3 MJ, what velocity is it travelling at? 3 MJ = 3 000 J Ö = Ö velocity = 2 KE m 2 x 3 000 100 000 = Ö 60 = 7. 75 ms-1 31 of 36 © Boardworks Ltd 2005

Kinetic energy problems activity 32 of 36 © Boardworks Ltd 2005

Contents Types of Energy Different types of energy Nuclear energy Gravitational potential energy Kinetic energy Summary activities 33 of 36 © Boardworks Ltd 2005

Glossary l chemical energy – The energy stored in chemicals and which is released when they react. l elastic energy – The energy stored in a material because it is being stretched or compressed. l gravitational potential energy – The energy an object has because of its position in a gravitational field. l kinetic energy – The energy an object has because it is moving. l nuclear energy – The energy stored in an atom’s nucleus. l nuclear fission – The splitting of a large nucleus into two nuclei, which releases very large amounts of energy. l nuclear fusion – The joining together of two atomic nuclei, which releases very large amounts of energy. l thermal energy – The energy of a substance due to the movement of its particles. 34 of 36 © Boardworks Ltd 2005

Anagrams 35 of 36 © Boardworks Ltd 2005

Multiple-choice quiz 36 of 36 © Boardworks Ltd 2005
 Efficiency igcse physics
Efficiency igcse physics 5 types of energy
5 types of energy Igcse physics forces and motion
Igcse physics forces and motion Mains electricity examples
Mains electricity examples Igcse physics edexcel
Igcse physics edexcel Movement and position physics igcse
Movement and position physics igcse Mass density
Mass density State hooke's law in physics
State hooke's law in physics Define pressure igcse
Define pressure igcse Joules to volts
Joules to volts Igcse astronomy
Igcse astronomy Physics igcse
Physics igcse Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Why does it happen
Why does it happen University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Ib physics ia ideas thermal physics
Ib physics ia ideas thermal physics What is energy in physics
What is energy in physics Definition of work in physics
Definition of work in physics What is energy in simple words
What is energy in simple words Rotational inertia ap physics c
Rotational inertia ap physics c Regents physics work power energy
Regents physics work power energy Nuclear fission
Nuclear fission Energy physics definition
Energy physics definition Thermal energy equation physics
Thermal energy equation physics Change in heat formula
Change in heat formula Strain energy formula
Strain energy formula How many kinds of energy are there
How many kinds of energy are there Physics 03-06 impulse and momentum answer key
Physics 03-06 impulse and momentum answer key F= ma units
F= ma units Regents physics work power energy
Regents physics work power energy Physics 2204 unit 3: work, power, energy
Physics 2204 unit 3: work, power, energy Work and energy physics
Work and energy physics Formula kinetic energy
Formula kinetic energy Sound energy
Sound energy Define energy in physics
Define energy in physics Equation for energy
Equation for energy