Structure of the Silicate Minerals Comparing Crystal Structures






























































- Slides: 84

Structure of the Silicate Minerals Comparing Crystal Structures to Visible Mineral Properties

Silicon, Silicates, Silicones How to Tell the Difference Silicon: One of 92 naturally occurring chemical elements

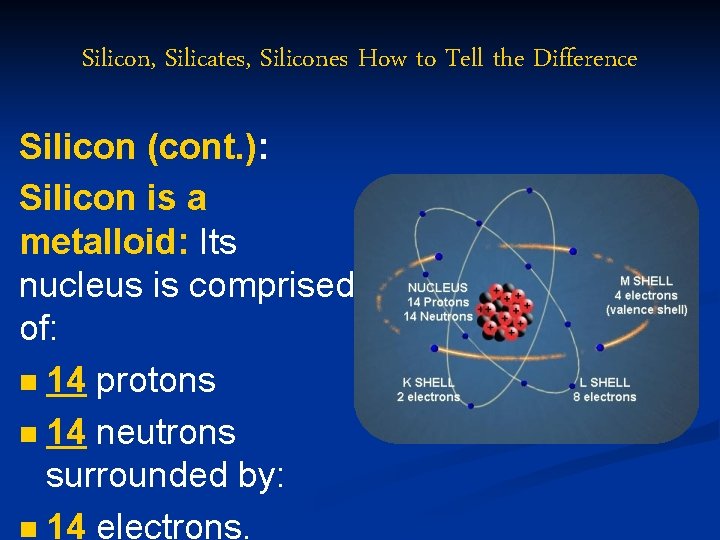
Silicon, Silicates, Silicones How to Tell the Difference Silicon (cont. ): Silicon is a metalloid: Its nucleus is comprised of: n 14 protons n 14 neutrons surrounded by: n 14 electrons.

Silicon

Silicon, Silicates, Silicones How to Tell the Difference Silicon (cont. ): n Rarely found as a pure substance n Silicon shines with luster. Silicon Wafer

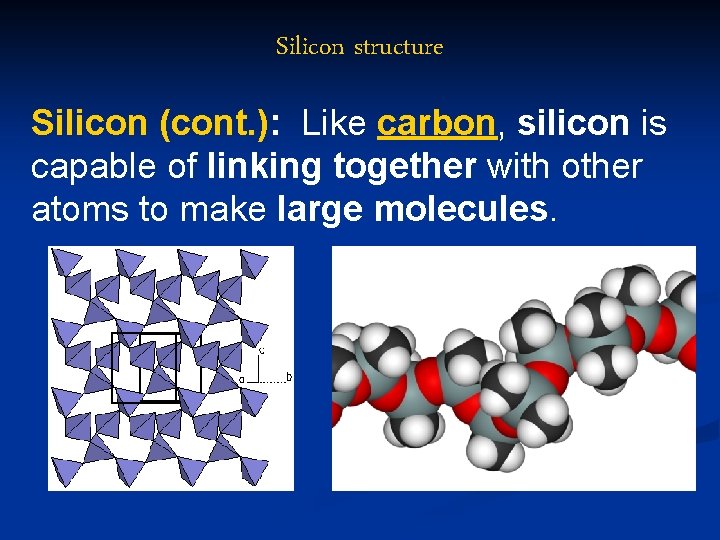
Silicon structure Silicon (cont. ): Like carbon, silicon is capable of linking together with other atoms to make large molecules.

Silicon, Silicates, Silicones How to Tell the Difference Silicates: Atoms join together to make molecules. Each silicate mineral is made of molecules. 90 % of Earth’s rocks are made of silicate minerals.

Silicon, Silicates, Silicones How to Tell the Difference Silicates (cont. ): Quartz Sand the simplest silicate molecule. Si. O - 2 n formed by joining one silicon atom with two oxygen atoms. n Sand Grains

Silica Ge. L Silica Gel is man-made. It is used to soak up moisture.

Silicates

Silicates Metallic Silicon combine to make… + Oxygen Gas

Silicates …glassy silicate gemstones.

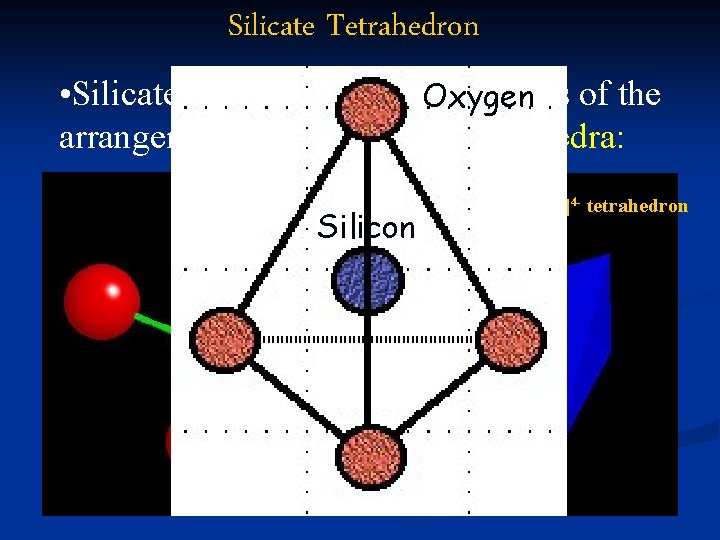
Silicate Tetrahedron • Silicates are classified on. Oxygen the basis of the arrangement of their silicate tetrahedra: The culprit: the [Si. O 4]4 - tetrahedron Silicon

Silicon, Silicates, Silicones How to Tell the Difference Silicones: n long, rubbery molecules n quite different from silicates n not like minerals at all.

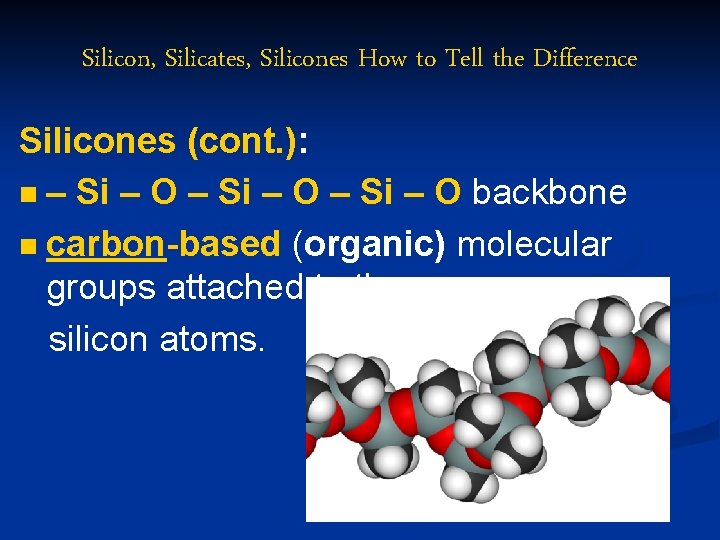
Silicon, Silicates, Silicones How to Tell the Difference Silicones (cont. ): n – Si – O backbone n carbon-based (organic) molecular groups attached to the silicon atoms.

Silicones: Used for: n Silicone caulk n Silicone cement or glue

Silicones: Used for: n Lubrication and n Waterproof coatings

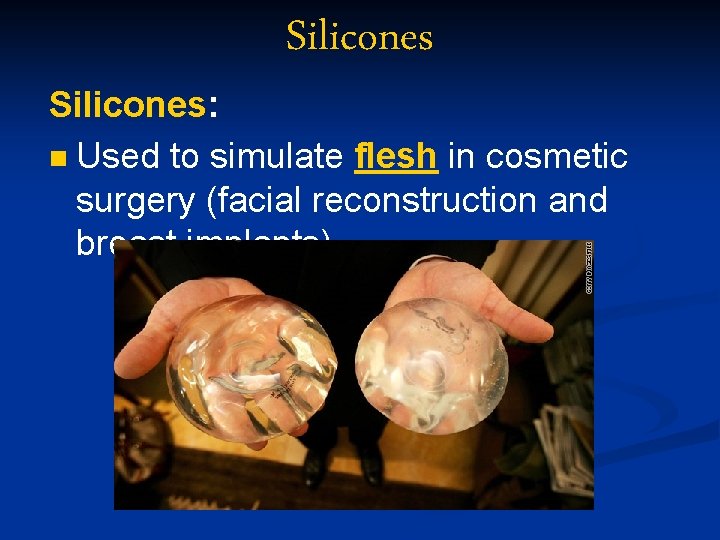
Silicones: n Used to simulate flesh in cosmetic surgery (facial reconstruction and breast implants).

Silicones: n Used to simulate flesh in cosmetic surgery (facial reconstruction and breast implants).

Structure of the Silicate Minerals Comparing Crystal Structures to Visible Mineral Properties

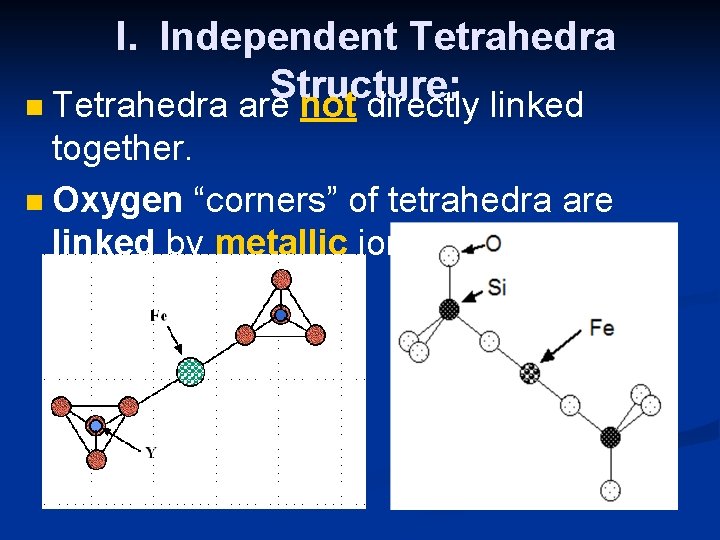
I. Independent Tetrahedra Structure: n Tetrahedra are not directly linked together. n Oxygen “corners” of tetrahedra are linked by metallic ions such as iron (Fe).

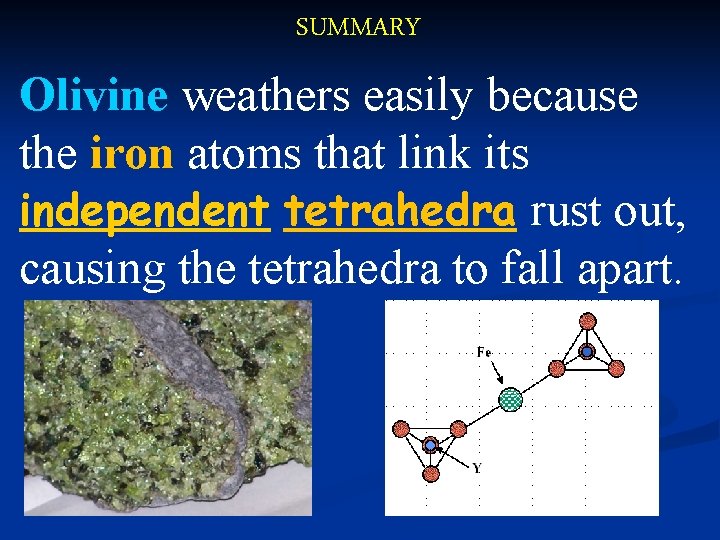
I. Independent Tetrahedra Structure: Olivine, a olive-green silicate mineral in basalt n Tetrahedra are linked by iron atoms, which rust out easily n Causes the tetrahedra to fall apart.

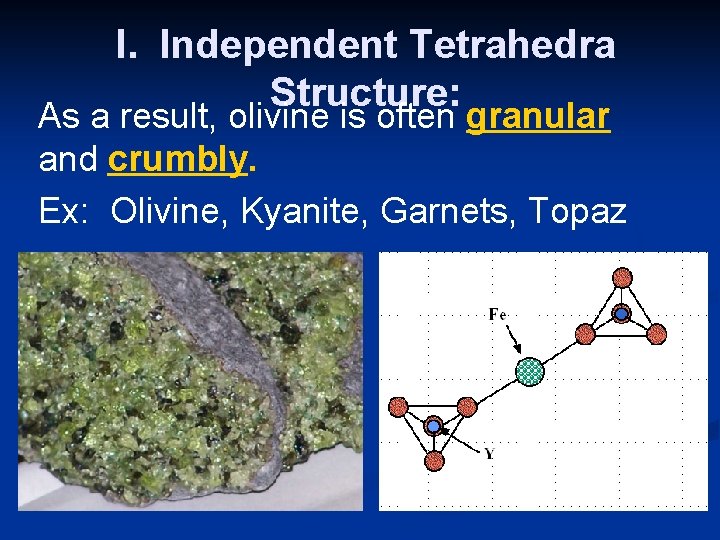
I. Independent Tetrahedra Structure: As a result, olivine is often granular and crumbly. Ex: Olivine, Kyanite, Garnets, Topaz

Other Independent Tetrahedra Minerals Kyanite

Other Independent Tetrahedra Minerals Garnet

Other Independent Tetrahedra Minerals Topaz / Staurolite

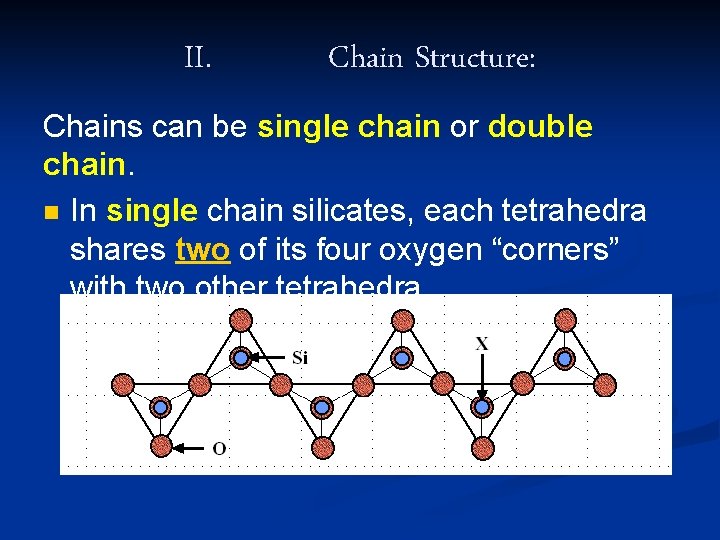
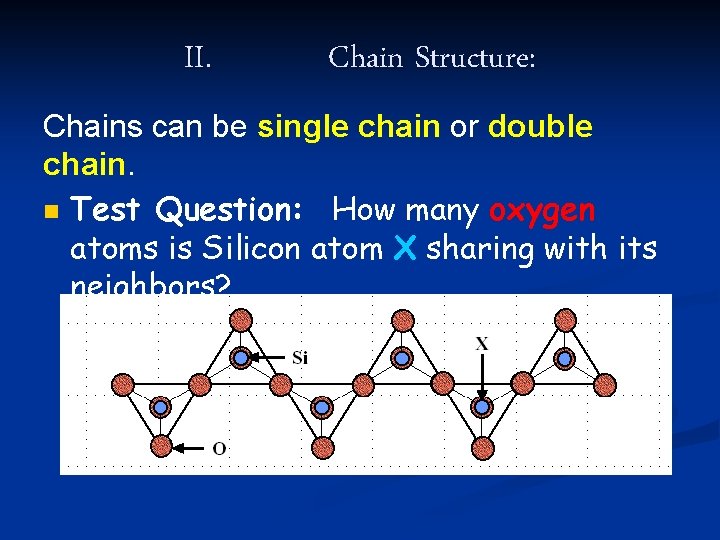
II. Chain Structure: Chains can be single chain or double chain. n In single chain silicates, each tetrahedra shares two of its four oxygen “corners” with two other tetrahedra.

II. Chain Structure: Chains can be single chain or double chain. n Test Question: How many oxygen atoms is Silicon atom X sharing with its neighbors?

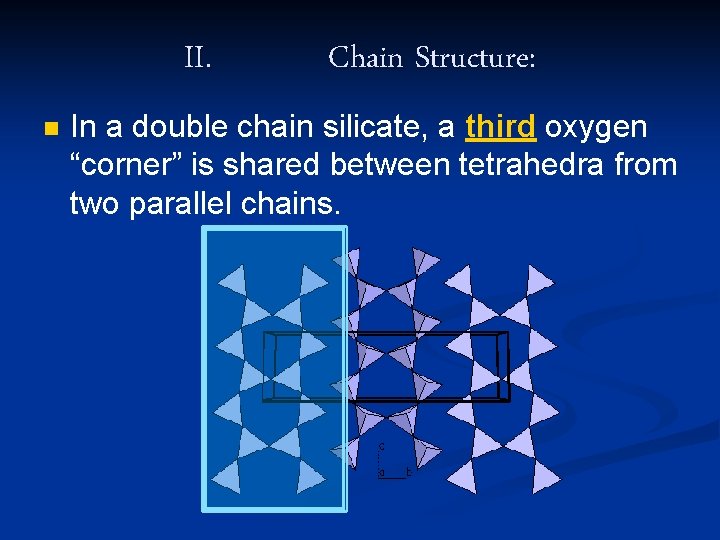
II. n Chain Structure: In a double chain silicate, a third oxygen “corner” is shared between tetrahedra from two parallel chains.

II. Chain Structure: Chains of tetrahedra are held together by linking metallic ions: n Linking bonds are much weaker than the Si–O bonds of the tetrahedra, n causes chain silicates to have a stringy or fibrous texture.

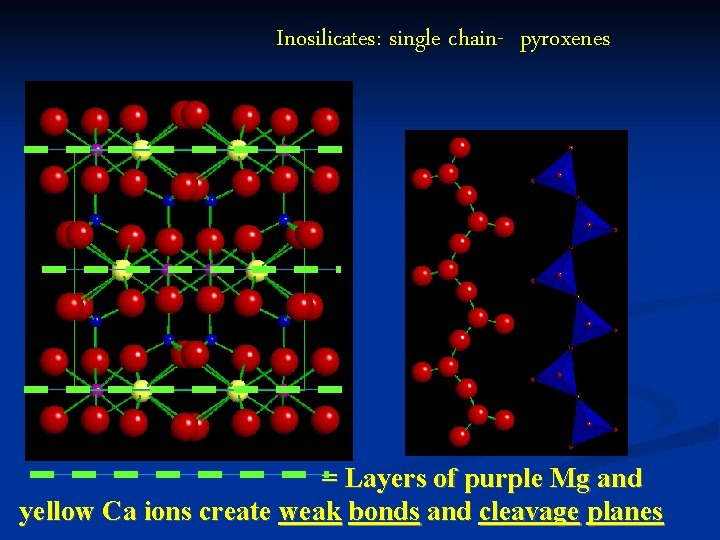
Inosilicates: single chain- pyroxenes = Layers of purple Mg and yellow Ca ions create weak bonds and cleavage planes

II. n Actinolite Chain Structure: is a good example of a fibrous chain silicate.

II. n Hornblende Chain Structure: and Augite are common Chain Structure minerals.

Inosilicates: single chain STRUCTURE - pyroxenes – Pectolite & Wollastonite Typical stringy, fibrous chain silicates

Inosilicates: single chain- pyroxenes – Kunzite A chain silicate gemstone

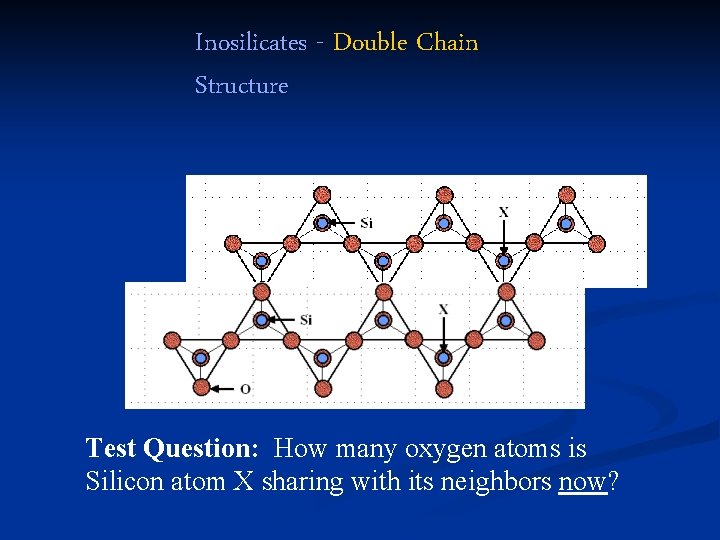
Inosilicates - Double Chain Structure Test Question: How many oxygen atoms is Silicon atom X sharing with its neighbors now?

Inosilicates - Double Chain Structure – Actinolite & Tremolite Typical stringy, fibrous chain silicates

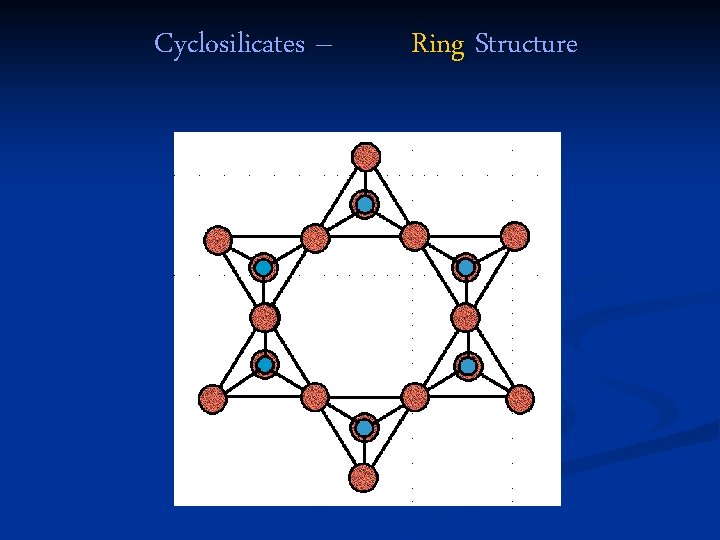
Cyclosilicates – Ring Structure

Cyclosilicates – Ring Structure • Two oxygen “corners” are shared with other silicate tetrahedra. • The tetrahedra joined in rings of 3, 4, or 6 tetrahedra. • Ring structures are not common. • Ring structure - usually long barrelshaped crystals (hexagonal prisms).

Cyclosilicates – Ring Structure Emerald

Cyclosilicates: Ring Structure Aquamarine

Cyclosilicates – Ring Structure Red Beryl

Cyclosilicates – Ring Structure Tourmaline

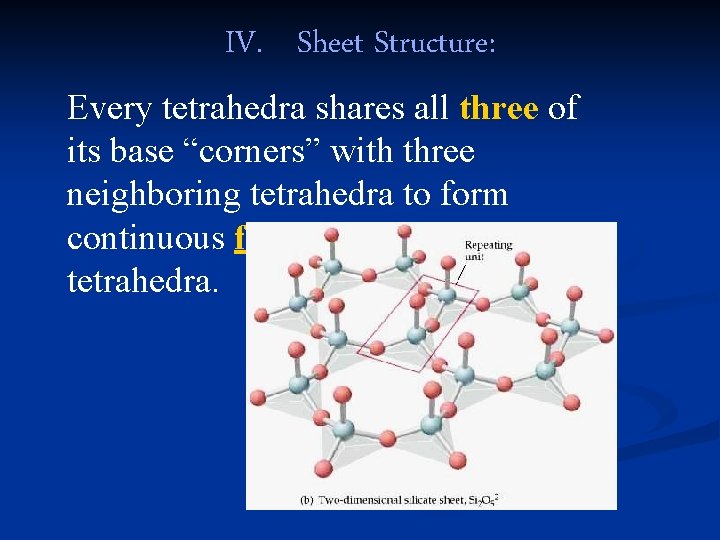
IV. Sheet Structure: Every tetrahedra shares all three of its base “corners” with three neighboring tetrahedra to form continuous flat sheets of tetrahedra.

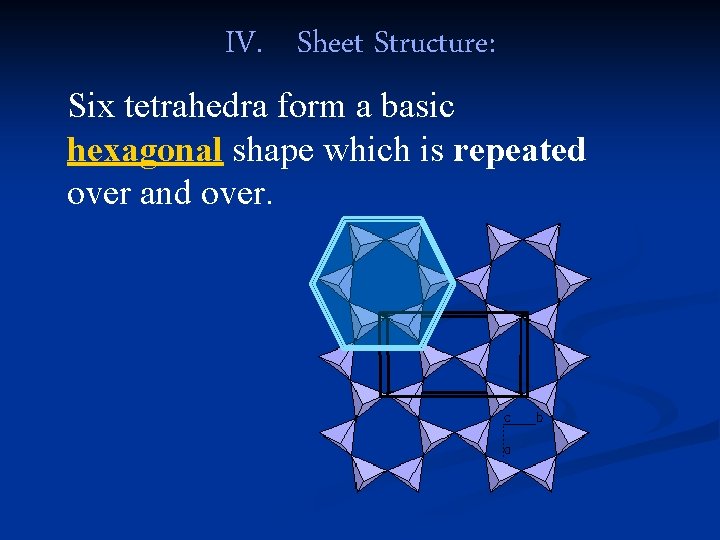
IV. Sheet Structure: Six tetrahedra form a basic hexagonal shape which is repeated over and over.

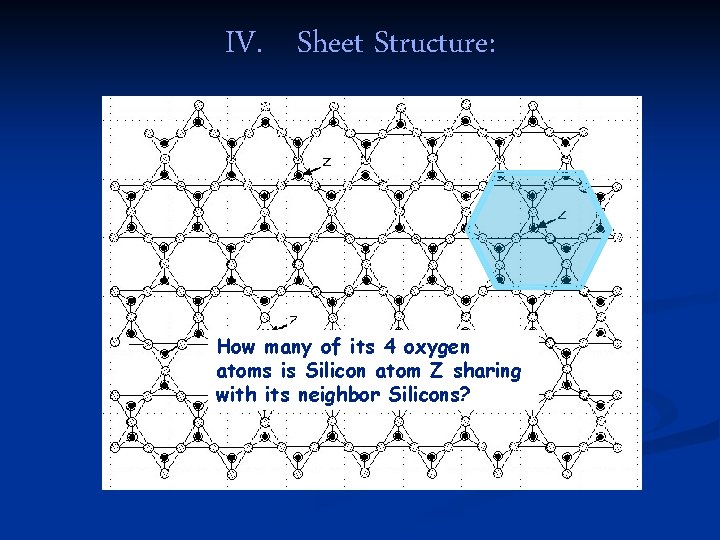
IV. Sheet Structure: How many of its 4 oxygen atoms is Silicon atom Z sharing with its neighbor Silicons?

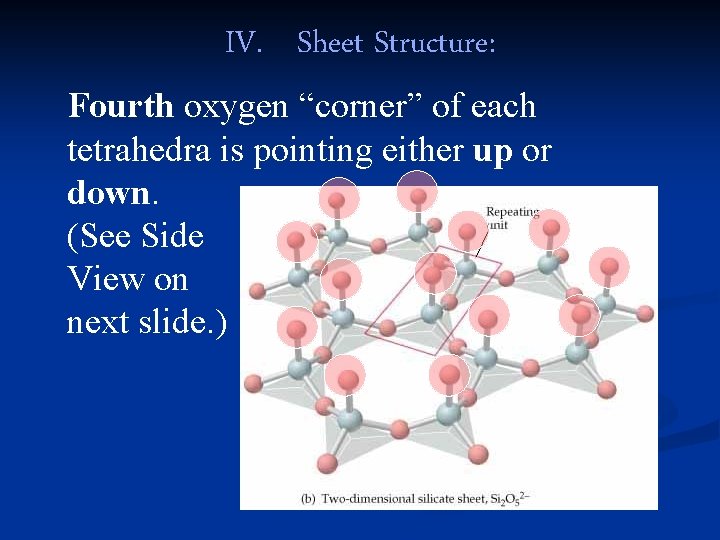
IV. Sheet Structure: Fourth oxygen “corner” of each tetrahedra is pointing either up or down. (See Side View on next slide. )

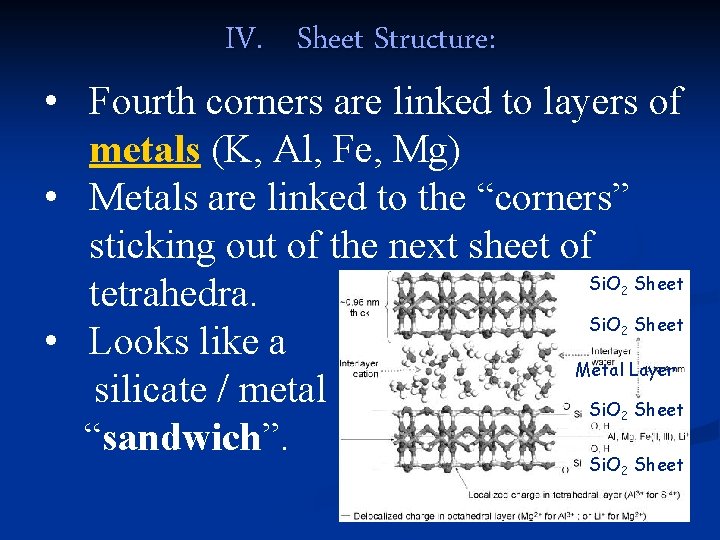
IV. Sheet Structure: • Fourth corners are linked to layers of metals (K, Al, Fe, Mg) • Metals are linked to the “corners” sticking out of the next sheet of Si. O Sheet tetrahedra. Si. O Sheet • Looks like a Metal Layer silicate / metal Si. O Sheet “sandwich”. 2 2 2 Si. O 2 Sheet

IV. Sheet Structure: Metallic linking bonds: • are much weaker than the Si – O bonds within each sheet, so they • break easily.

IV. Sheet Structure: Muscovite and Biotite Mica: • Are sheet silicates • Split easily into paper-thin sheets • Have perfect one direction cleavage.

Phyllosilicates - Sheet Structure Muscovite Mica

Phyllosilicates - Sheet Structure Biotite Mica

Phyllosilicates - Sheet Structure LEPIDOLITE Mica

Phyllosilicates - Sheet Structure Chrysacolla is a sheet silicate, but it is made of microscopic flat flakes.

Phyllosilicates - Sheet Structure Chrysacolla Kaolinite (clay) is a sheet silicate, but it is made of microscopic flat flakes.

Phyllosilicates - Sheet Structure Talc is made of small, flat flakes that slide over each other easily – causes talc’s “soapy” feel.

Phyllosilicates - Sheet Structure Talc

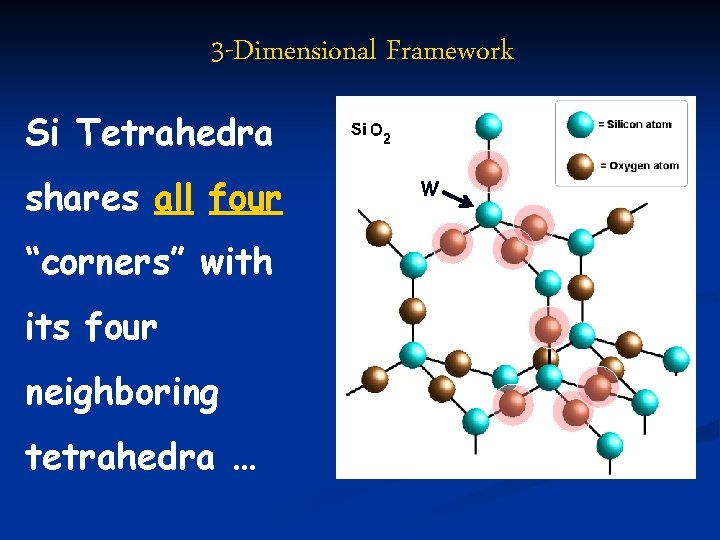
3 -Dimensional Framework Si Tetrahedra shares all four “corners” with its four neighboring tetrahedra … W

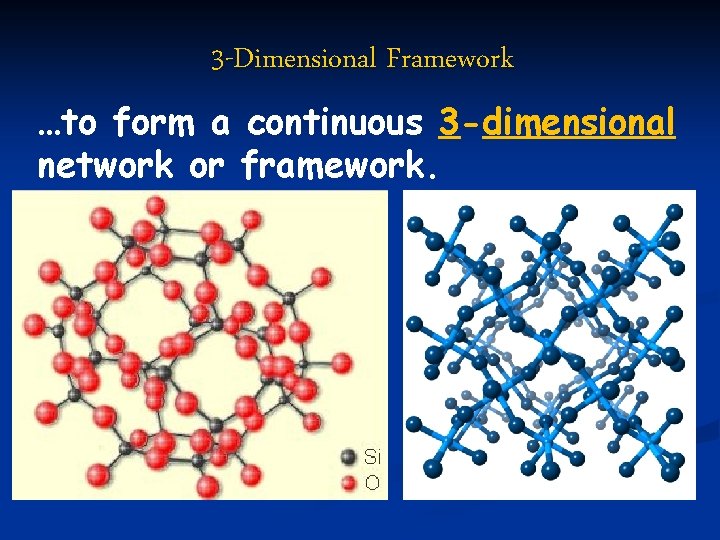
3 -Dimensional Framework …to form a continuous 3 -dimensional network or framework.

3 -Dimensional Framework

3 -Dimensional Framework Bonds are equally strong in all directions. n Produce minerals that are exceptionally hard.

3 -Dimensional Framework Bonds are equally strong in all directions: n Minerals do not break with cleavage n no layers of weak bonds to give way. Rose Quartz Milky Quartz

3 -Dimensional Framework Structure - Quartz

3 -Dimensional Framework Structure - Quartz Rock Crystal Quartz

3 -Dimensional Framework Structure - Quartz Citrine Rose Quartz

3 -Dimensional Framework Structure - Quartz Jasper Chert

modified Framework Some of the silicon ions are replaced by metal ions (K, Al, Na, and Ca). Na Feldspar K Feldspar

modified Framework Start with a regular framework mineral (Milky Quartz): Si. O 2 Milky Quartz

modified Framework Keep the Si: O ratio at 1: 2 and it’s still quartz. Si 2 O 4 Milky Quartz

modified Framework Keep the Si: O ratio at 1: 2 and it’s still quartz. Si 4 O 8 Milky Quartz

modified Framework Remove one of the Silicons (Si 4+)… Si 4 O 8 K Feldspar

modified Framework Remove one of the Silicons (Si 4+)… Si 3 O 8 K Feldspar

modified Framework …and replace it with an Aluminum ion (Al 3+) Al. Si O 3 8 K Feldspar

modified Framework Still need another +1 metal ion to balance out the charges ( K+ or Na+ ) KAl. Si O 3 8 Et voilà– le’ Framework Modifiée!! (Modified Framework Structure) K Feldspar

3 -Dimensional Framework n Layers of metal ions create layers of weak bonds n Modified Framework minerals do have cleavage planes. Na Feldspar K Feldspar

3 -Dimensional Framework Structure - Feldspars Amazonite Feldspar

3 -Dimensional Framework Structure - Feldspars Albite Feldspar Labradorit e Feldspar

3 -Dimensional Framework Structure – Sodalite

SUMMARY Many visible physical properties of minerals are reflections of their atomic structure:

SUMMARY The scaly micas split along parallel cleavage planes due to their sheet structure:

SUMMARY Some amphiboles such as actinolite are fibrous (stringy) due to their chain structure.

SUMMARY Olivine weathers easily because the iron atoms that link its independent tetrahedra rust out, causing the tetrahedra to fall apart.

SUMMARY Quartz is hard and shows no cleavage because its tetrahedra form a 3 – Dimensional Framework which is equally strong in all directions.

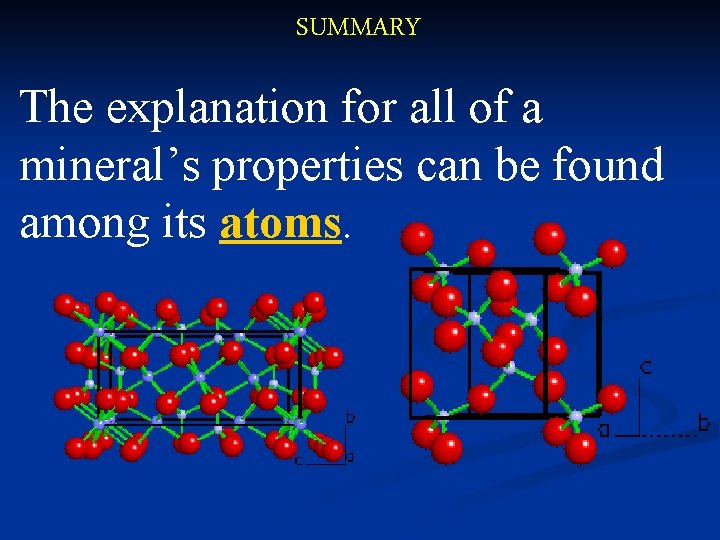
SUMMARY The explanation for all of a mineral’s properties can be found among its atoms.