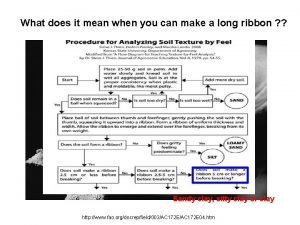
Properties of silicate minerals Controls on density hardness















- Slides: 28

Properties of silicate minerals Controls on density, hardness, cleavage and resistance to weathering in nesosilicates

Specific gravity (G) tends to decrease from nesosilicates to tectosilicates, as the degree of tetrahedral polymerization increases. G mineral formula (name) 3. 27 3. 58 3. 2 3. 0 2. 86 2. 54 2. 32 2. 65 Mg 2 Si. O 4 (olivine forsterite) Mg 3 Al 2(Si. O 4)3 (garnet pyrope) Ca. Mg. Si 2 O 6 (diopside pyroxene) Ca 2 Mg 7 Si 8 O 22(OH)2 (amphibole tremolite ) KMg 3(Al. Si 3)O 10(OH)2 (mica phlogopite) KAl. Si 3 O 8 (feldspar) Si. O 2 (cristobalite) Si. O 2 (quartz)

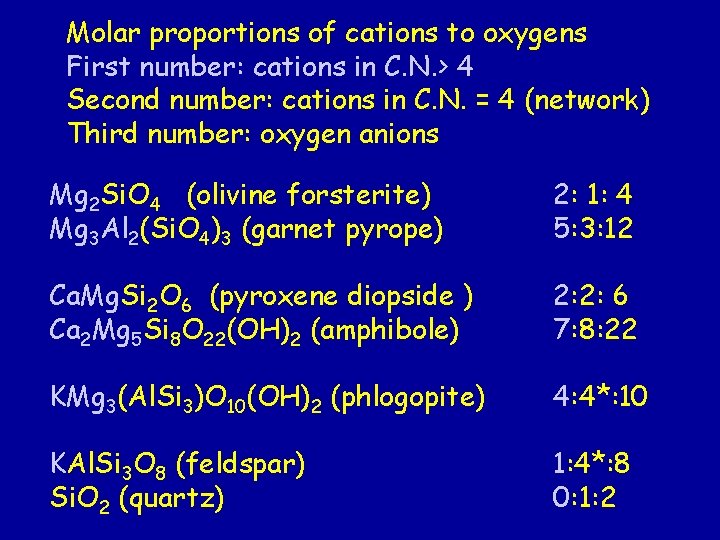
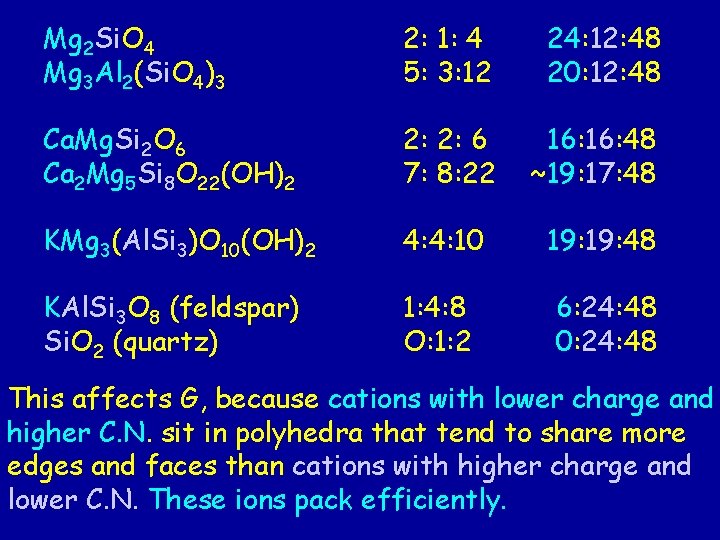
Molar proportions of cations to oxygens First number: cations in C. N. > 4 Second number: cations in C. N. = 4 (network) Third number: oxygen anions Mg 2 Si. O 4 (olivine forsterite) Mg 3 Al 2(Si. O 4)3 (garnet pyrope) 2: 1: 4 5: 3: 12 Ca. Mg. Si 2 O 6 (pyroxene diopside ) Ca 2 Mg 5 Si 8 O 22(OH)2 (amphibole) 2: 2: 6 7: 8: 22 KMg 3(Al. Si 3)O 10(OH)2 (phlogopite) 4: 4*: 10 KAl. Si 3 O 8 (feldspar) Si. O 2 (quartz) 1: 4*: 8 0: 1: 2

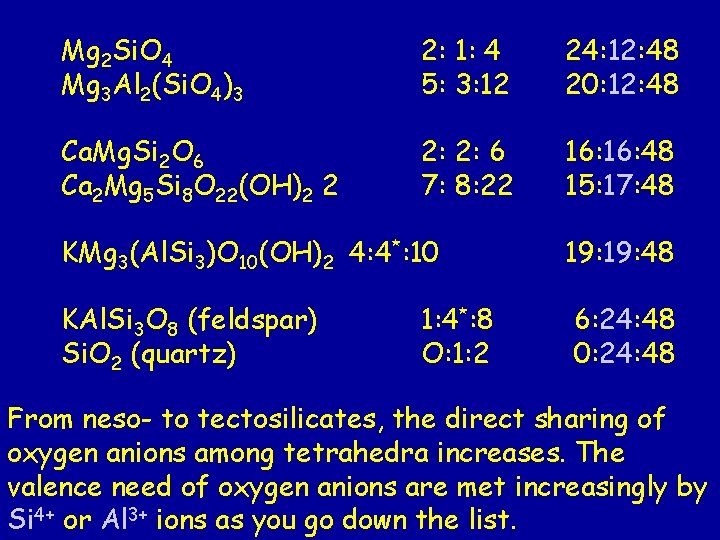
Mg 2 Si. O 4 Mg 3 Al 2(Si. O 4)3 2: 1: 4 = 24: 12: 48 5: 3: 12 = 20: 12: 48 Ca. Mg. Si 2 O 6 Ca 2 Mg 5 Si 8 O 22(OH)2 2: 2: 6 = 16: 48 7: 8: 22 = 15: 17: 48 KMg 3(Al. Si 3)O 10(OH)2* 4: 4*: 10 = 19: 48 KAl. Si 3 O 8 (feldspar) Si. O 2 (quartz) 1: 4*: 8 = 6: 24: 48 O: 1: 2 = 0: 24: 48 * * In these minerals, both Al and Si are found in tetrahedra that are sharing corners, so they are counted as network cations.

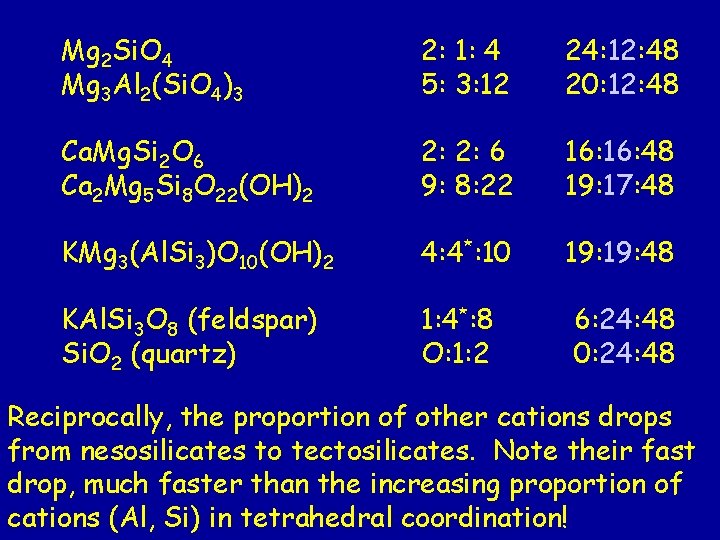
Mg 2 Si. O 4 Mg 3 Al 2(Si. O 4)3 2: 1: 4 5: 3: 12 24: 12: 48 20: 12: 48 Ca. Mg. Si 2 O 6 Ca 2 Mg 5 Si 8 O 22(OH)2 2 2: 2: 6 7: 8: 22 16: 48 15: 17: 48 KMg 3(Al. Si 3)O 10(OH)2 4: 4*: 10 19: 48 KAl. Si 3 O 8 (feldspar) Si. O 2 (quartz) 6: 24: 48 0: 24: 48 1: 4*: 8 O: 1: 2 From neso- to tectosilicates, the direct sharing of oxygen anions among tetrahedra increases. The valence need of oxygen anions are met increasingly by Si 4+ or Al 3+ ions as you go down the list.

Mg 2 Si. O 4 Mg 3 Al 2(Si. O 4)3 2: 1: 4 5: 3: 12 24: 12: 48 20: 12: 48 Ca. Mg. Si 2 O 6 Ca 2 Mg 5 Si 8 O 22(OH)2 2: 2: 6 9: 8: 22 16: 48 19: 17: 48 KMg 3(Al. Si 3)O 10(OH)2 4: 4*: 10 19: 48 KAl. Si 3 O 8 (feldspar) Si. O 2 (quartz) 1: 4*: 8 O: 1: 2 6: 24: 48 0: 24: 48 Reciprocally, the proportion of other cations drops from nesosilicates to tectosilicates. Note their fast drop, much faster than the increasing proportion of cations (Al, Si) in tetrahedral coordination!

vi. Mg 2 Si. O 4 viii. Mg vi. Al (Si. O ) 3 2 4 3 2: 1: 4 5: 3: 12 24: 12: 48 20: 12: 48 viii. Cavi. Mg. Si viii. Ca vi. Mg Si O (OH) 2 5 8 22 2 2: 2: 6 7: 8: 22 16: 48 ~19: 17: 48 x-xii. Kvi. Mg 4: 4: 10 2 O 6 3(Al. Si 3)O 10(OH)2 19: 48 KAl. Si 3 O 8 (feldspar) 1: 4: 8 6: 24: 48 Si. O 2 (quartz) O: 1: 2 0: 24: 48 Other cations have lower charges (and higher C. N. ) than Si 4+ or Al 3+ ions in the structure. More moles of these other cations are needed to meet the valence requirement of oxygen anions that are no longer shared by two Si 4+ or Al 3+ ions.

Mg 2 Si. O 4 Mg 3 Al 2(Si. O 4)3 2: 1: 4 5: 3: 12 24: 12: 48 20: 12: 48 Ca. Mg. Si 2 O 6 Ca 2 Mg 5 Si 8 O 22(OH)2 2: 2: 6 7: 8: 22 16: 48 ~19: 17: 48 KMg 3(Al. Si 3)O 10(OH)2 4: 4: 10 19: 48 KAl. Si 3 O 8 (feldspar) Si. O 2 (quartz) 1: 4: 8 O: 1: 2 6: 24: 48 0: 24: 48 This affects G, because cations with lower charge and higher C. N. sit in polyhedra that tend to share more edges and faces than cations with higher charge and lower C. N. These ions pack efficiently.

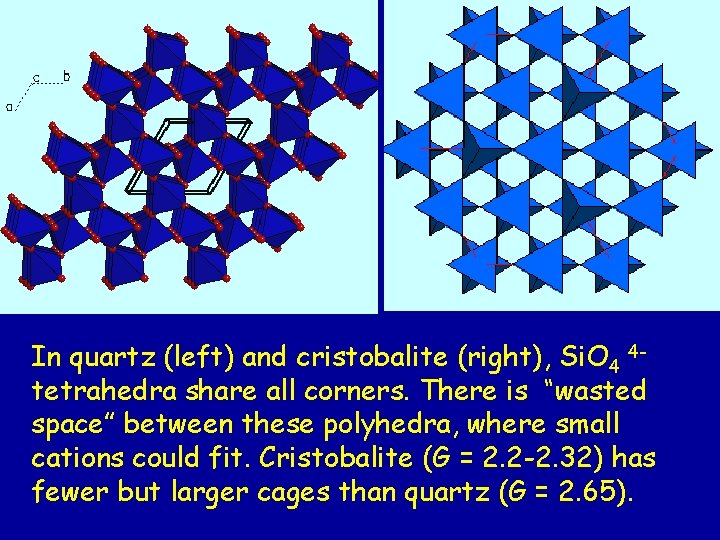
In quartz (left) and cristobalite (right), Si. O 4 4 tetrahedra share all corners. There is “wasted space” between these polyhedra, where small cations could fit. Cristobalite (G = 2. 2 -2. 32) has fewer but larger cages than quartz (G = 2. 65).

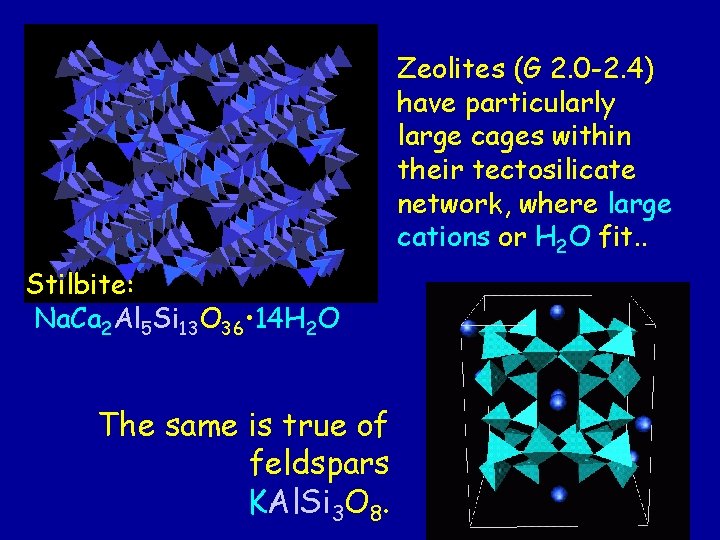
Zeolites (G 2. 0 -2. 4) have particularly large cages within their tectosilicate network, where large cations or H 2 O fit. . Stilbite: Na. Ca 2 Al 5 Si 13 O 36 • 14 H 2 O The same is true of feldspars KAl. Si 3 O 8.

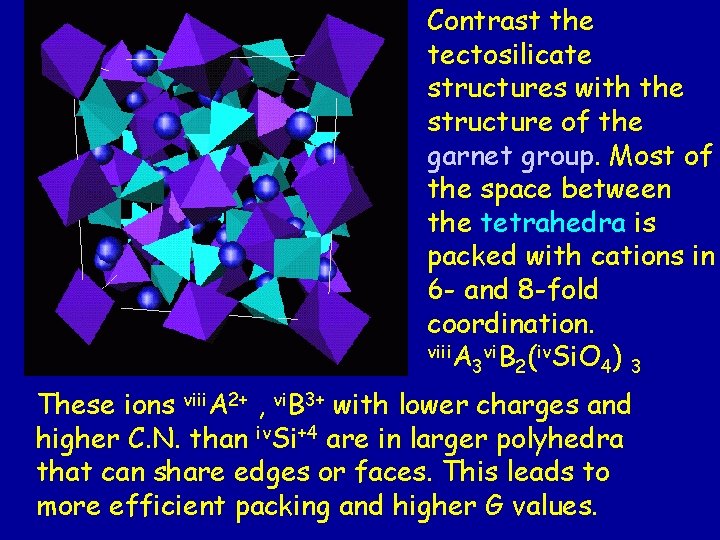
Contrast the tectosilicate structures with the structure of the garnet group. Most of the space between the tetrahedra is packed with cations in 6 - and 8 -fold coordination. viii. A vi. B (iv. Si. O ) 3 2 4 3 These ions viii. A 2+ , vi. B 3+ with lower charges and higher C. N. than iv. Si+4 are in larger polyhedra that can share edges or faces. This leads to more efficient packing and higher G values.

Specific gravity (G) tends to decrease from nesosilicates to tectosilicates, as cations with high C. N. are replaced by cations with lower C. N. G mineral formula (name) 3. 58 Mg 3 Al 2(Si. O 4)3 (garnet pyrope) 3. 27 Mg 2 Si. O 4 (olivine forsterite) 3. 2 Ca. Mg. Si 2 O 6 (diopside pyroxene) 3. 0 Ca 2 Mg 5 Si 8 O 22(OH)2 (amphibole tremolite ) 2. 86 KMg 3(Al. Si 3)O 10(OH)2 (mica phlogopite) 2. 54 KAl. Si 3 O 8 (feldspar) 2. 65 Si. O 2 (quartz) 2. 32 Si. O 2 (cristobalite) 4. 35 Si. O 2 (stishovite… but Si+4 has C. N. = 6)

HARDNESS and CLEAVAGE cannot be evaluated solely from a mineral formula. One must consider the strength of bonds and the directions along which strong bonds are dominantly oriented. Sometimes, hardness reflects the presence of the weakest bonds, but only these weaker bonds are concentrated along a given plane throughout the structure.

CLEAVAGE reflects either: • Planes across which the weakest bonds are found (in micas, K-O bonds between the sheets) • Planes held by a lower density of bonds (strong or weak doesn’t matter… as in diamond, where octahedral cleavage planes are held by a lower number of strong C-C bonds per area) AND • bonding character that is dominantly ionic or covalent (rather than metallic)

Some bonds that might be expected to be fairly ionic or covalent turn out to show a strong polarization of the electron cloud. This is often seen in bonding between a relatively small, highly charged ion next to a large, weakly charged ion. Na. Cl: ionic, good cleavage Na. I, Ag. Cl: sectile solids with poor cleavage. (Easier to cut it with a knife than to cleave… a bit like a metallic solid).

Most nesosilicates are rather hard (H = 6 or more) and show poor cleavage because: 1) their structures distributes the weaker bonds (e. g. , Mg-O) fairly evenly among stronger bonds (e. g. , Si-O, Al-O); 2) frequent edge sharing among the larger polyhedra gives high density of bonds across most planes.

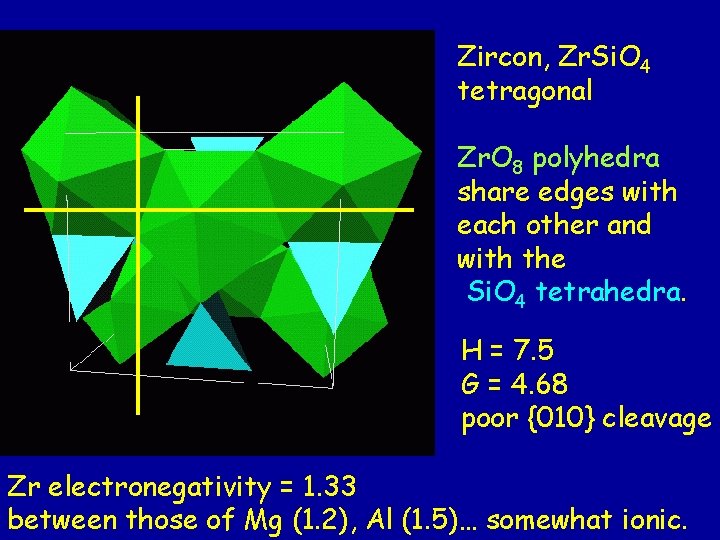
Zircon formula: Zr+4 Si+4 O 4 Zr+4 (high charge) (rather large cation, C. N. =8) forms a moderately strong Zr-O bond (e. v. = valence/C. N. = +4/8 = 0. 5 ) Si-O bonds are strong (e. v. = +4/4 = 1 ) Zr. O 8 polyhedra share many edges. Every direction meets a high density of bonds. How many cations does each oxygen bond to? Answer: one Si-O bond and two Zr-O bonds = 2 e. v.

Zircon, Zr. Si. O 4 tetragonal Zr. O 8 polyhedra share edges with each other and with the Si. O 4 tetrahedra. H = 7. 5 G = 4. 68 poor {010} cleavage Zr electronegativity = 1. 33 between those of Mg (1. 2), Al (1. 5)… somewhat ionic.

Minerals which, like zircon, are heavy and resistant to weathering, are found in the sandy sediment of streams. This includes several nesosilicates. Minerals which form at relatively high pressures, in the upper mantle (Mg-rich garnet), are used as indicators for the prospection of diamonds. This is how some of the diamond deposits that are now exploited in Nunavut and northern Quebec were discovered… There were grains of high-pressure minerals in sands that came from the kimberlite pipes.

These characteristics (H 6, poor cleavage) make many nesosilicates desirable gemstones, at least in specimens of remarkable colour and transparency. Peridot (gem-quality olivine) Garnet Topaz Zircon (careful, that one cleaves easily)

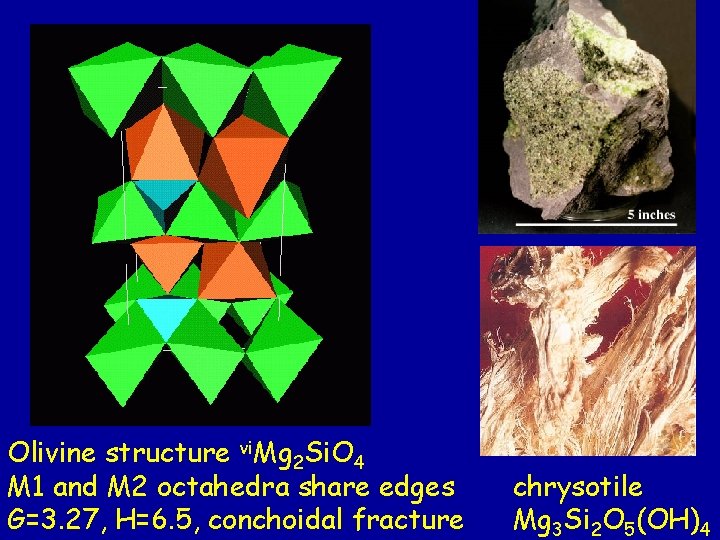
Olivine structure vi. Mg 2 Si. O 4 M 1 and M 2 octahedra share edges G=3. 27, H=6. 5, conchoidal fracture chrysotile Mg 3 Si 2 O 5(OH)4

Olivine in basalt is usually quite close to forsterite, Mg 2 Si. O 4… This makes it quite susceptible to weathering, or hydrolysis when it comes in contact with H 2 O. One common result is chrysotile, Mg 3 Si 2 O 5(OH)4 This is the origin of the chrysotile asbestos of Thetford Mines.

The garnets viii. A vi. B (iv. Si. O ) 3 2 4 3 have no specific plane of weakness: Conchoidal Fracture H = 6. 5 -7. 5 The weaker bonds are equally distributed among stronger bonds. In addition, the high density of packing (a lot of edge sharing) offsets the longer weaker bonds that one might expect from having viii. A 2+ ions (small charge, high C. N. ).

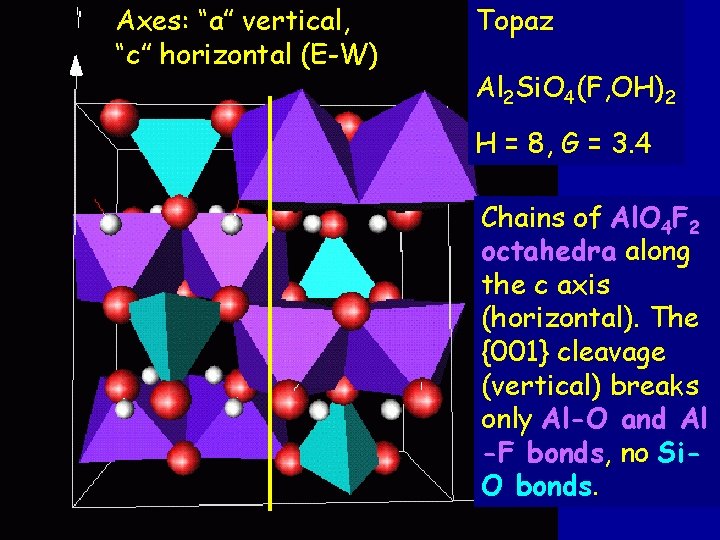
Axes: “a” vertical, “c” horizontal (E-W) Topaz Al 2 Si. O 4(F, OH)2 H = 8, G = 3. 4 Chains of Al. O 4 F 2 octahedra along the c axis (horizontal). The {001} cleavage (vertical) breaks only Al-O and Al -F bonds, no Si. O bonds.

Maany minerals including nesosilicates (* in list below) are used as abrasive materials. . . to clean graffitis off walls, strip old paint from surfaces prior to repainting, or polish wooden furniture. • silica sand (silica is cheap, but its dust is carcinogenic) • hematite • aluminum oxide (synthetic, slag) • staurolite* • olivine* • garnet* • diamond

Weak bonds affect the hardness of a mineral if they are predominantly holding planes in one dominant direction. K-O bonds, for example, are present in feldspars and in micas, two mineral groups with very different hardnesses. In each structure, K+-O bonds are the longest, weakest bonds (K+ cation is quite large and has a low charge).

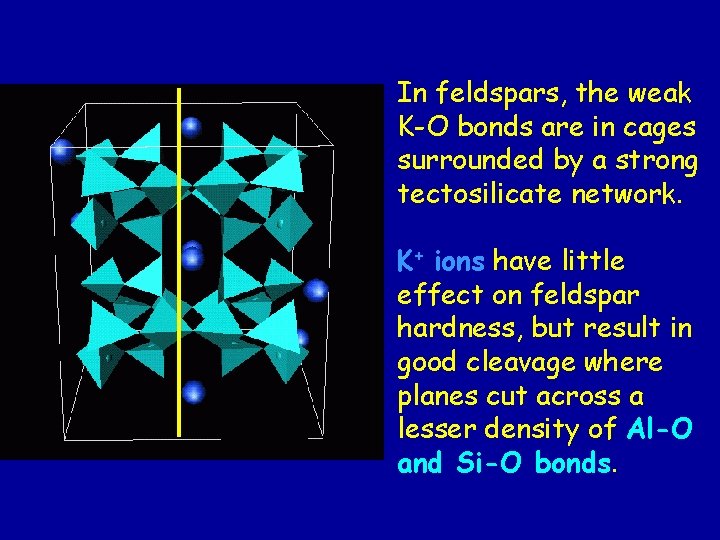
In feldspars, the weak K-O bonds are in cages surrounded by a strong tectosilicate network. K+ ions have little effect on feldspar hardness, but result in good cleavage where planes cut across a lesser density of Al-O and Si-O bonds.

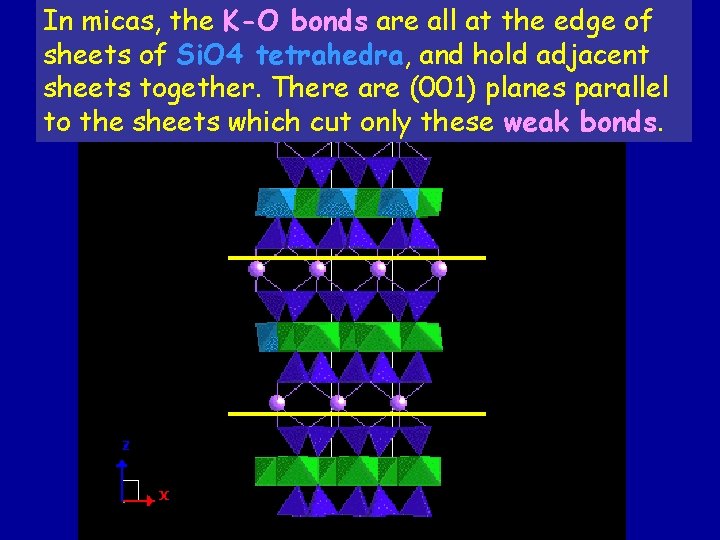
In micas, the K-O bonds are all at the edge of sheets of Si. O 4 tetrahedra, and hold adjacent sheets together. There are (001) planes parallel to the sheets which cut only these weak bonds.
 Silicate hardness
Silicate hardness Streak property of minerals
Streak property of minerals Luster
Luster The basic building block of the silicate minerals
The basic building block of the silicate minerals Graphite hardness mohs scale
Graphite hardness mohs scale General controls vs application controls
General controls vs application controls He who controls the past controls the future
He who controls the past controls the future Properties of matter definition
Properties of matter definition Zinc ethyl silicate primer
Zinc ethyl silicate primer Is muscovite a silicate
Is muscovite a silicate Dental silicate ceramic
Dental silicate ceramic Double chain silicate
Double chain silicate Crystalline silicate clays
Crystalline silicate clays Properties of minerals cleavage
Properties of minerals cleavage Properties of minerals
Properties of minerals Uses of minerals
Uses of minerals Optical properties of minerals
Optical properties of minerals Properties of minerals
Properties of minerals Physical properties of minerals graphic organizer
Physical properties of minerals graphic organizer Perpendicular
Perpendicular Site:slidetodoc.com
Site:slidetodoc.com Nda full dac
Nda full dac Derive linear density expressions for fcc 100 and 111
Derive linear density expressions for fcc 100 and 111 Physiological density egypt
Physiological density egypt What does arithmetic density tell us
What does arithmetic density tell us Linear density of atoms
Linear density of atoms Mass, volume and density are all properties of
Mass, volume and density are all properties of Zéolite danger
Zéolite danger Hardness test is the ability of a material to resist
Hardness test is the ability of a material to resist