Mineralogy Presentation Minerals origin occurrence and associations Rock



































- Slides: 61

Mineralogy Presentation Minerals, origin, occurrence and associations Rock forming minerals Classification, Description and Uses

References. The following books are also useful compilations of mineral data and descrip tions. W. A. Deer, R. A. Howie and J. Zussman (1962, 1974, 1980) Rock Forming Minerals (Seven Volumes) Longmans, London. R. W. G. Wyckoff (1964) Crystal Structures. Wiley (New York) (Eight Volumes) (Vols 1, 2, 3 and 4 contain mineral structures). T. Zoltai and J. M. Stout (1985) Mineralogy: Concepts and Principles. Burgess (Minneapolis). C. Klein and C. S. Hurlbut, Jr. (1993) Manual of Mineralogy (After J. D. Dana, 21 st edition). Wiley, (New York L. G. Berry, B. Mason, and R. V. Dietrich (1983) Mineralogy (Second Edition) Freeman (San Francisco). W. H. Balckburn and W. H. Dennen (1988) Principles of Mineralogy. W. C. Brown, K. Frye (1974) Modern Mineralogy. Prentice-Hall. Deer, Howie, Zussman (1992) An Introduction to the Rock Forming Minerals (Second Edition) London Longmans ISBN 0 -582 -30094 -0. 696 pp.

Mineralogy �It is the study of the chemistry, physics and crystal structure of natural, solid, crystalline materials known as mineral i. e. is the study of minerals. It also includes the study of origin, processes of mineral formation, their geographical distribution, classification, as well as utilization.

Importance of Mineralogy • • · · · Minerals are the building blocks of the planet. The study of the earth materials depends on an understanding of minerals making up the rocks and sediments Mineral resources: Ore minerals are the source of valuable metals (e. g. Cu, Au) and provide energy resources like uranium. Certain forms of minerals, gems, delight the eye as jewelry. Industrial / agro minerals serve as the raw materials for manufacturing chemicals, dimension stones, aggregates (metals) for road and concrete , etc. Soil formation processes Unfortunately, not all minerals are beneficial; some pose environmental hazards (geological hazards)

DEFINITION �A mineral is a naturally occurring homogeneous solid with a definite chemical composition and ordered atomic arrangement formed usually by inorganic processes.

Explanation of Definition 1 Naturally Occurring �Minerals are not synthetic (made in a laboratory) �Synthetic equivalents of minerals have the qualifier “syn”after their names

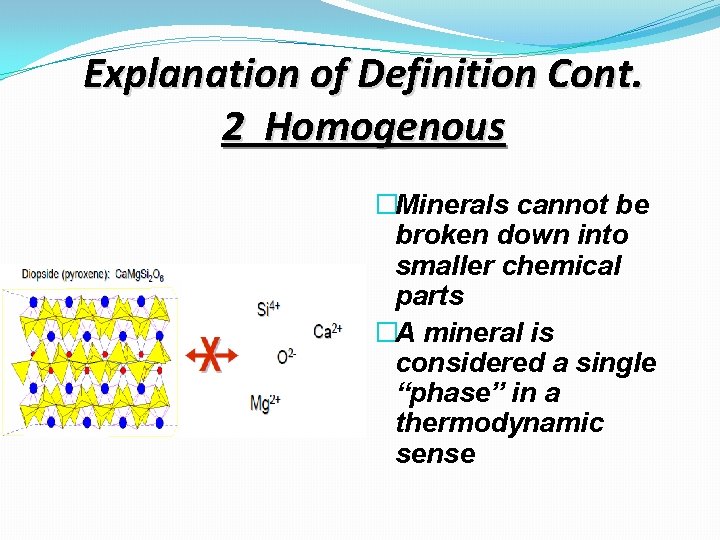
Explanation of Definition Cont. 2 Homogenous �Minerals cannot be broken down into smaller chemical parts �A mineral is considered a single “phase” in a thermodynamic sense

Explanation of Definition Cont. 3 Solid �Minerals are not gases or liquids �The exception is liquid mercury (Hg)

Explanation of Definition Cont. 4 Definite chemical composition �Minerals are made up of elements which are present as atoms or ions or radicals Examples: silicates: Si 4++ O 2 - → Si. O 44 carbonates: C 4+ + O 2 - → CO 32 phosphates: P 5+ + O 2 - → PO 43 Carbon: C

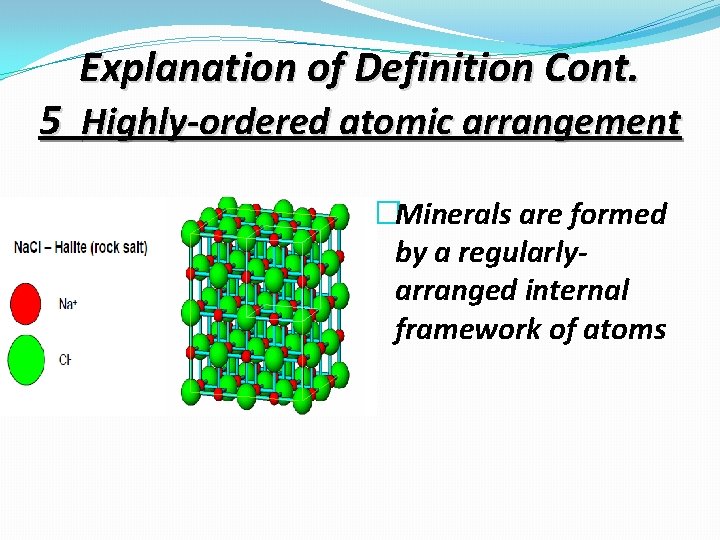
Explanation of Definition Cont. 5 Highly-ordered atomic arrangement �Minerals are formed by a regularlyarranged internal framework of atoms

Explanation of Definition Cont. 6 Inorganic �Minerals are not organic compounds (i. e. composed solely of C, H, N & O) and are not biological in origin

Mineraloid �Natural materials which resemble minerals in all ways except for one of the 6 criteria e. g. volcanic glass –amorphous (only shortrange order) e. g. Metamict minerals: minerals which have lost their internal order (destroyed) due to self-inflicted radiation damage (radioactive decay of some elements) e. g. Uranium

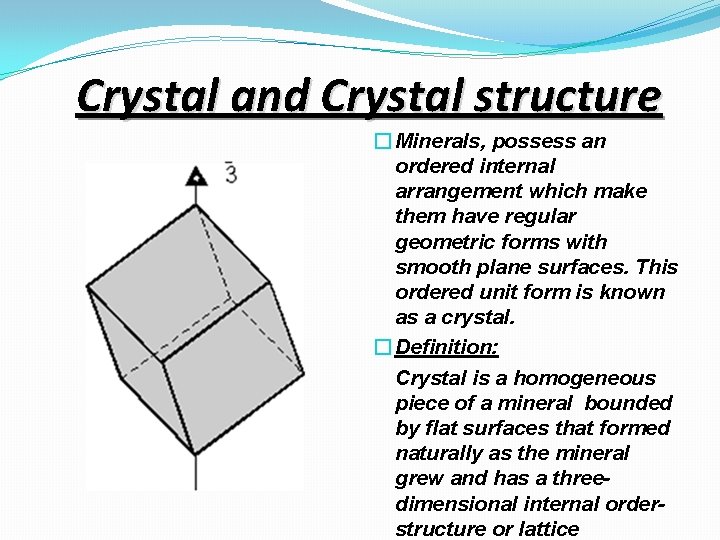
Crystal and Crystal structure � Minerals, possess an ordered internal arrangement which make them have regular geometric forms with smooth plane surfaces. This ordered unit form is known as a crystal. � Definition: Crystal is a homogeneous piece of a mineral bounded by flat surfaces that formed naturally as the mineral grew and has a threedimensional internal orderstructure or lattice

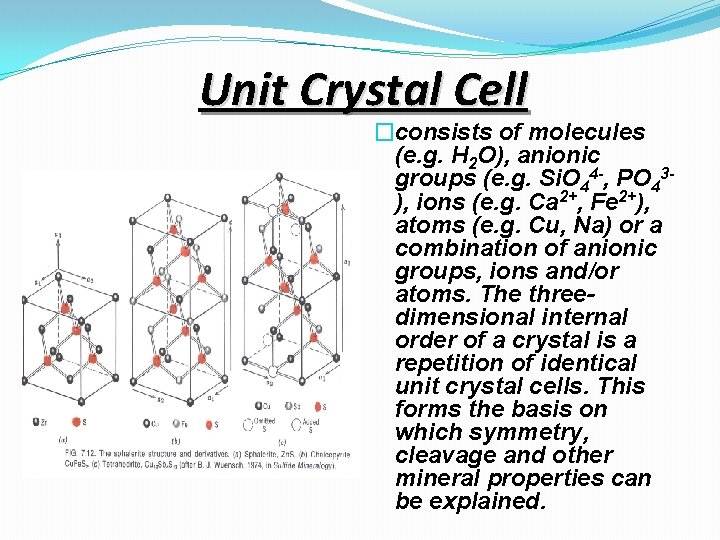
Unit Crystal Cell �consists of molecules (e. g. H 2 O), anionic groups (e. g. Si. O 44 -, PO 43), ions (e. g. Ca 2+, Fe 2+), atoms (e. g. Cu, Na) or a combination of anionic groups, ions and/or atoms. The threedimensional internal order of a crystal is a repetition of identical unit crystal cells. This forms the basis on which symmetry, cleavage and other mineral properties can be explained.

TYPES OF CRYSTALS

Chemical Formula �Calcite Ca. CO 3 �Each mineral has a �Dolomite Ca. Mg(CO 3)2 unique arrangement and the number of �Kyanite Al 2 Si. O 5 elements contained in �Orthoclase its crystal structure. KAl. Si 3 O 8 �All minerals have a �Olivine (Mg. Fe)2 Si. O 4 chemical formula, �Apatite which is an analysis of Ca 5(PO 4)3(F, Cl, OH) the types and amounts of elements present in a mineral.

Naming of Minerals �Minerals are mostly given a name that reflects their major chemical component (oxides, sulphides, silicates, carbonates, phosphates etc. Also serves as the basis for their classification �Minerals may be given names on the basis of some physical property or chemical aspect, or they may be named after a locality, public figure, a mineralogist etc. �Examples �Albite (Na. Al. Si 3 O 8) form Latin albus (white) due to its colour.

Naming of Minerals Cont. �Chromite (Fe. Cr 2 O 4) because of the presence of a large amount of chromium in the mineral. �Magnetite (Fe 3 O 4) because of its magnetic properties. �Silimanite (Al 2 Si. O 5) after Professor Benjamin Siliman of Yale University (179 -1864). �Franklinite (Zu. Fe 2 O 4) after a locality Franklin, New jersey where it occurs as the dominant zinc mineral. �Rhodonite (Mn. Si. O 3) from Greek rhodon (a rose) due to its characteristically pink colour.

Mineral Formation �The origin of chemical elements: Cooling of gaseous elements precipitated first heavy elements in a form of solid particles dust according to “Big Bang” theory. �Formation Processes: 1 Precipitation 2 Sublimation 3 Crystallization 4 Solid - Solid reactions (Recrystallization)

Mineral Formation Cont. �Precipitation (settling or fall out) from a fluid like H 2 O. Within the Earth: by hydrothermal processes, diagenesis, and metamorphism, At or near the Earth's surface: by evaporation, weathering, or biological activity. �Sublimation from a vapour. This process is somewhat rarer, but can take place at a volcanic vent, or deep in space where the pressure is near vacuum. �Crystallization from a liquid. This takes place during crystallization of molten rock (magma) either below or at the Earth's surface. �Solid - Solid reactions. This process involves minerals reacting with other minerals in the solid state to produce one or more new minerals. Such processes take place during metamorphism and diagenesis due to changing temperature and pressure conditions.

Environments �The environments of mineral formation geologically are highly varied; within the Earth's crust, different temperatures and depths result in varied minerals and on the Earth’s Surface, low temperature lead to precipitation from saline brine (slightly salty water). � Based on energy sources environment is groups as: 1 Endogenetic (hypogene)- deep-seated processes in the interior of the earth. 2 Exogenetic (hypergene)- surface processes (at or near the earth’s surface as well as in the atmosphere and hydrosphere).

Mode of Occurrence �Magmatic- crystallization from a magma or lava �Pegmatitic – final stage crystallization of magma �Puematolitic - sublimation from volcanic gases �Hydrothermal – deposition from hot water and steam �Metamorphic and Metasomatic – recrystallization of existing minerals �Evaporites - sun evaporates water and leaves salt �Crystallization during diagenesis of sediments �Formation by oxidation and weathering

q. In geologic environments where mineral formation is taking place, the kinds of minerals that form depend on various factors such as: (a) Temperature (b) Pressure (c) The chemical activity of the water present (d) The mobility and relative abundance of chemical element q The mineral formed is defined by two fundamental properties: crystal structure – the geometric arrangement of the ions (atoms) composing the minerals. chemical composition- the proportions of different chemical elements contained. .

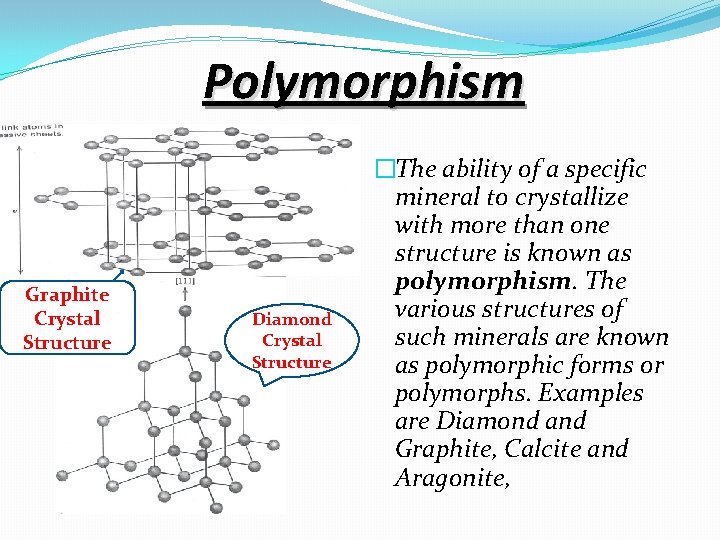
Polymorphism Graphite Crystal Structure Diamond Crystal Structure �The ability of a specific mineral to crystallize with more than one structure is known as polymorphism. The various structures of such minerals are known as polymorphic forms or polymorphs. Examples are Diamond and Graphite, Calcite and Aragonite,

Pseudomorphism �If a crystal of a mineral is altered so that the internal structure or chemical composition is changed but the external form is preserved it is called a pseudomorph. Thus pseudomorphs are formed as a result of slow replacement of a crystal substance by another substance without any change in the external form and occasionally also in the internal structure of the original crystal. Example Goethite having cubic shape after Pyrite.

Physical Properties �The elemental composition and crystal structure of mineral are not readily visible, therefore the field identification and description of mineral species is done on the basis of its physical properties (external aspects). However, physical properties are governed by the chemical composition and the crystal structure of the mineral. �Minerals physical properties can be determined by inspection with a hand lens or by relatively simple tests on hand specimens

Physical Properties Cont. �The physical properties of minerals include : Colour Streak Luster Density (Specific Gravity) Hardness Cleavage Fracture Tenacity Crystal habit Crystal Form, etc.

Streak �It refers to the colour of a powder produced by pulverizing the mineral. It is obtained by scraping or rubbing the mineral against an unglazed ceramic plate (porcelain)

Hardness �It is a measure of the relative ability of a mineral to resist scratching (breaking of lattice structure). The stronger the binding force between the atoms, the harder the mineral. �A series of 10 common minerals were chosen as scale (F. Mohs) by comparison with which the relative hardness of any mineral can be told.

Hardness Cont. 3 1 6 4 5 9 10 2 7 8 �Moh Scale of Hardness Talc – 1 Gypsum – 2 Calcite – 3 Flourite – 4 Apatite – 5 Orthoclase – 6 Quartz – 7 Topaz – 8 Corundum – 9 Diamond - 10

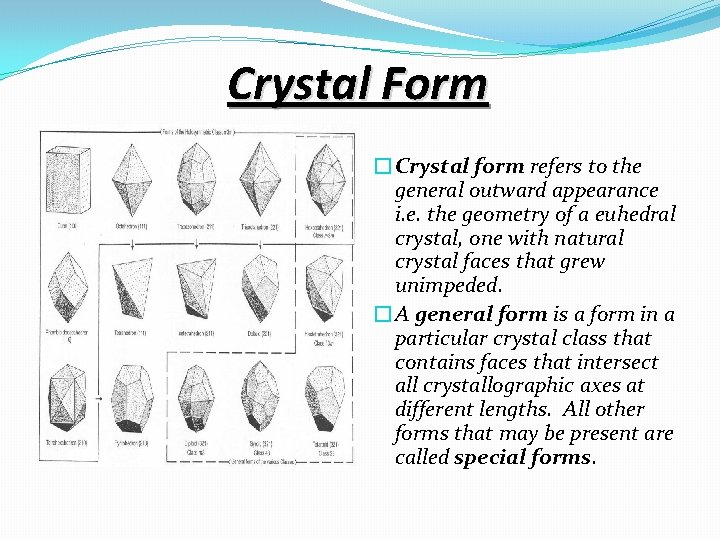
Crystal Form �Crystal form refers to the general outward appearance i. e. the geometry of a euhedral crystal, one with natural crystal faces that grew unimpeded. �A general form is a form in a particular crystal class that contains faces that intersect all crystallographic axes at different lengths. All other forms that may be present are called special forms.

Mineral Classification �Reasons for this classification are: 1. Minerals having the same anion or anionic group (oxides, silicates, sulphides halides etc. ) dominant in their composition have unmistakable family resemblances, than minerals containing the same dominant cation. 2. Minerals related by dominance of the same anion tend to occur together or in the same or similar geologic environment. 3. The scheme agrees well with the chemical practice in the naming and classification of inorganic compounds.

Mineral Classification Cont. �By the character of bonding between atoms the following types of chemical compounds in minerals can be distinguished: 1. Free atoms elements. These are minerals that occur in nature in the native state. 2. Compounds of cations with simple anions (sulphides, halides and oxides). 3. Compounds of cations with complex anions

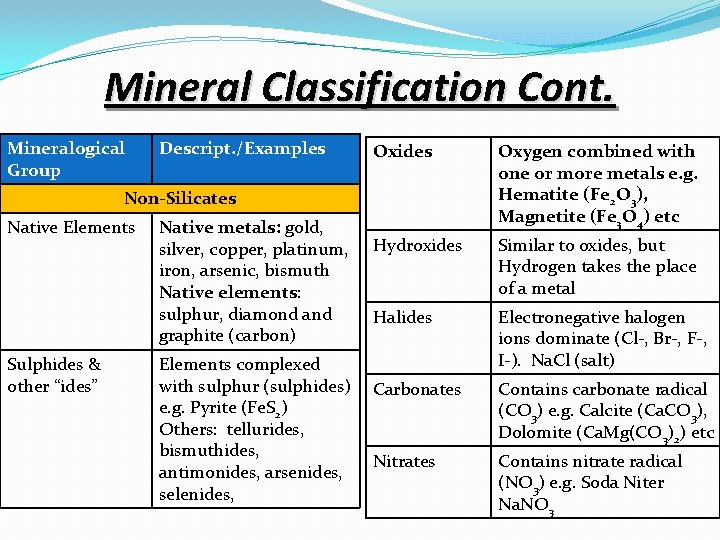
Mineral Classification Cont. Mineralogical Group Descript. /Examples Oxides Oxygen combined with one or more metals e. g. Hematite (Fe 2 O 3), Magnetite (Fe 3 O 4) etc Hydroxides Similar to oxides, but Hydrogen takes the place of a metal Halides Electronegative halogen ions dominate (Cl-, Br-, F-, I-). Na. Cl (salt) Non-Silicates Native Elements Sulphides & other “ides” Native metals: gold, silver, copper, platinum, iron, arsenic, bismuth Native elements: sulphur, diamond and graphite (carbon) Elements complexed with sulphur (sulphides) Carbonates e. g. Pyrite (Fe. S 2) Others: tellurides, bismuthides, Nitrates antimonides, arsenides, selenides, Contains carbonate radical (CO 3) e. g. Calcite (Ca. CO 3), Dolomite (Ca. Mg(CO 3)2) etc Contains nitrate radical (NO 3) e. g. Soda Niter Na. NO 3

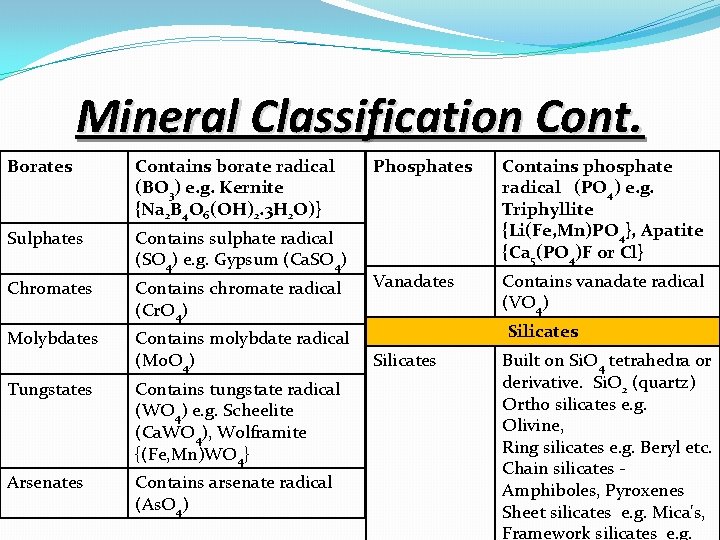
Mineral Classification Cont. Borates Contains borate radical (BO 3) e. g. Kernite {Na 2 B 4 O 6(OH)2. 3 H 2 O)} Sulphates Contains sulphate radical (SO 4) e. g. Gypsum (Ca. SO 4) Chromates Contains chromate radical (Cr. O 4) Molybdates Contains molybdate radical (Mo. O 4) Silicates Tungstates Contains tungstate radical (WO 4) e. g. Scheelite (Ca. WO 4), Wolframite {(Fe, Mn)WO 4} Arsenates Contains arsenate radical (As. O 4) Phosphates Contains phosphate radical (PO 4) e. g. Triphyllite {Li(Fe, Mn)PO 4}, Apatite {Ca 5(PO 4)F or Cl} Vanadates Contains vanadate radical (VO 4) Silicates Built on Si. O 4 tetrahedra or derivative. Si. O 2 (quartz) Ortho silicates e. g. Olivine, Ring silicates e. g. Beryl etc. Chain silicates - Amphiboles, Pyroxenes Sheet silicates e. g. Mica's, Framework silicates e. g.

Megascopic Mineral Identification �Determining the name of a mineral involves testing of the physical properties of an unknown mineral for identification purposes. The observer needs to systematically note as many properties as possible for the unknown mineral before consulting the mineral tables. Below are the key properties to note: �Colour �Luster: Three main types are metallic, submetallic and nonmetallic �Streak Colour �Cleavage

Cont. • Fracture • Crystal Habit • Hardness • Specific gravity • Magnetism • Optical Properties: • Fluorescence • Effervescence • Luminescence

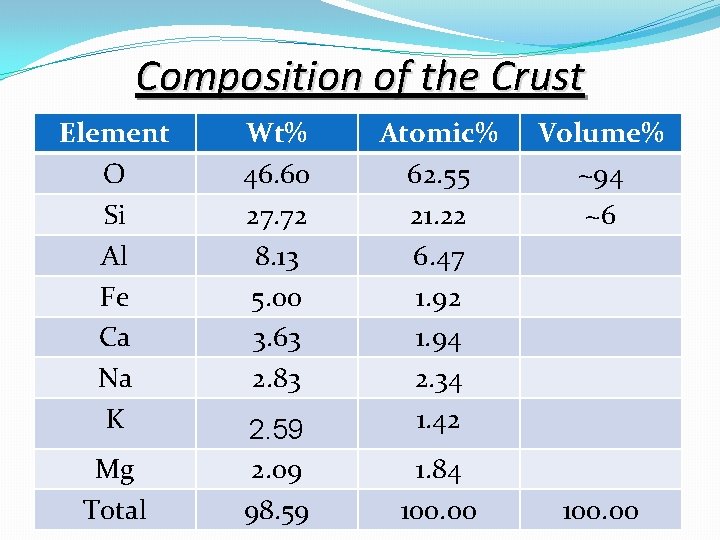
Composition of the Crust Element O Si Al Fe Ca Na K Mg Total Wt% 46. 60 27. 72 8. 13 5. 00 3. 63 2. 83 2. 59 2. 09 98. 59 Atomic% 62. 55 21. 22 6. 47 1. 92 1. 94 2. 34 1. 42 1. 84 100. 00 Volume% ~94 ~6 100. 00

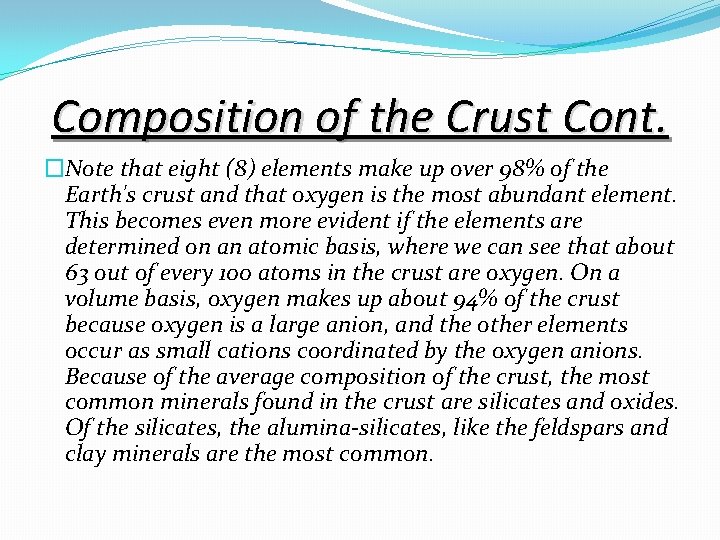
Composition of the Crust Cont. �Note that eight (8) elements make up over 98% of the Earth's crust and that oxygen is the most abundant element. This becomes even more evident if the elements are determined on an atomic basis, where we can see that about 63 out of every 100 atoms in the crust are oxygen. On a volume basis, oxygen makes up about 94% of the crust because oxygen is a large anion, and the other elements occur as small cations coordinated by the oxygen anions. Because of the average composition of the crust, the most common minerals found in the crust are silicates and oxides. Of the silicates, the alumina-silicates, like the feldspars and clay minerals are the most common.

Rock-forming Minerals �Oxygen and silicon are the most abundant elements (make up about 74% of the earth's crust), thus the silicate minerals are the most common and the largest group and of greater importance than any other rock-forming minerals. �Almost all the igneous rock-forming minerals are silicates and they therefore constitute over 90% of the earth’s crust.

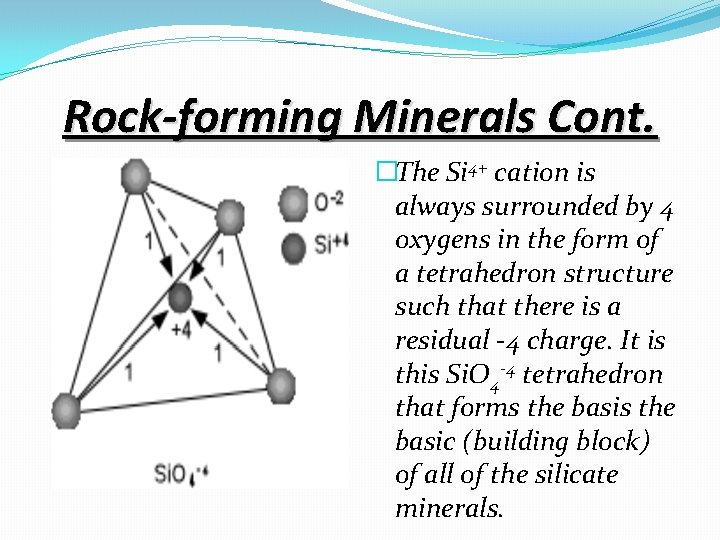
Rock-forming Minerals Cont. �The Si 4+ cation is always surrounded by 4 oxygens in the form of a tetrahedron structure such that there is a residual -4 charge. It is this Si. O 4 -4 tetrahedron that forms the basic (building block) of all of the silicate minerals.

Rock-forming Minerals Cont. �When these Si. O 4 -4 tetrahedrons are linked together, only corner oxygens will be shared with other Si. O 4 -4 groups. Several possibilities i. e. polymerizations of the silica (Si. O 4) tetrahedral exist and give rise to the different silicate groups as: � Nesosilicates, Sorosilicates, � Cyclosilicates, Inosilicates, � Phyllosilicates and Tectosilicates.

Nesosilicates (Ortho or Island Silicates) �The Si. O 4 -4 tetrahedra are isolated and bound to each other only by ionic bonds from interstitial cations. Their structure depend chiefly on the size and charge of the interstitial cations. Olivine is a good example: (Mg, Fe)2 Si. O 4.

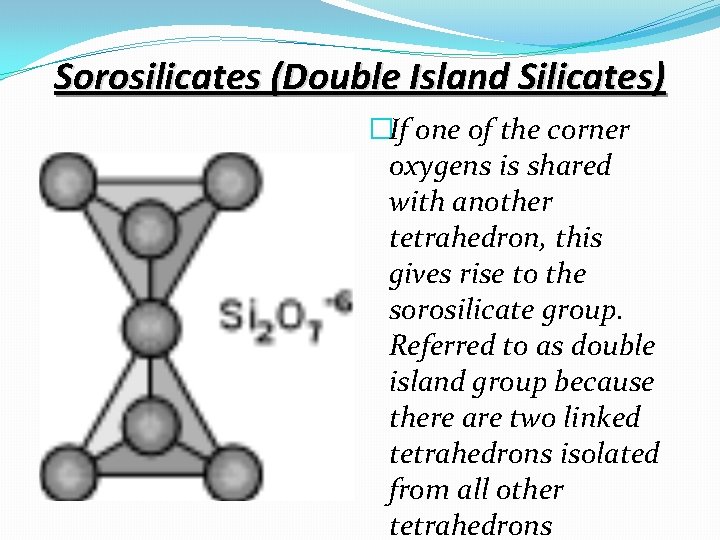
Sorosilicates (Double Island Silicates) �If one of the corner oxygens is shared with another tetrahedron, this gives rise to the sorosilicate group. Referred to as double island group because there are two linked tetrahedrons isolated from all other tetrahedrons

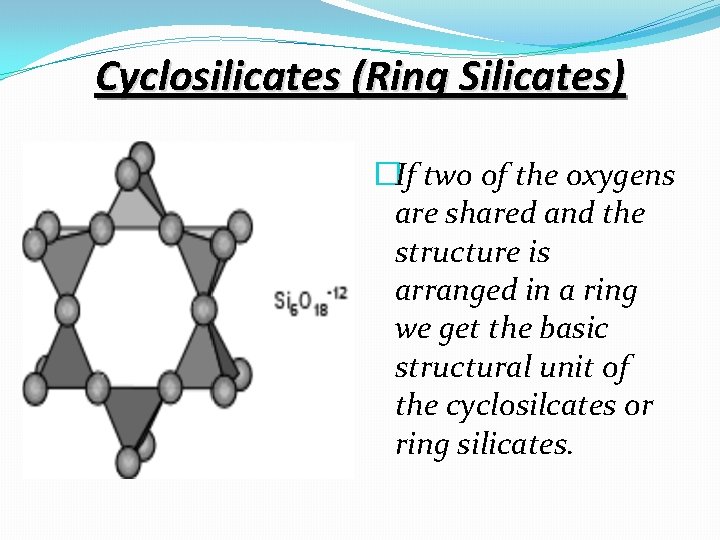
Cyclosilicates (Ring Silicates) �If two of the oxygens are shared and the structure is arranged in a ring we get the basic structural unit of the cyclosilcates or ring silicates.

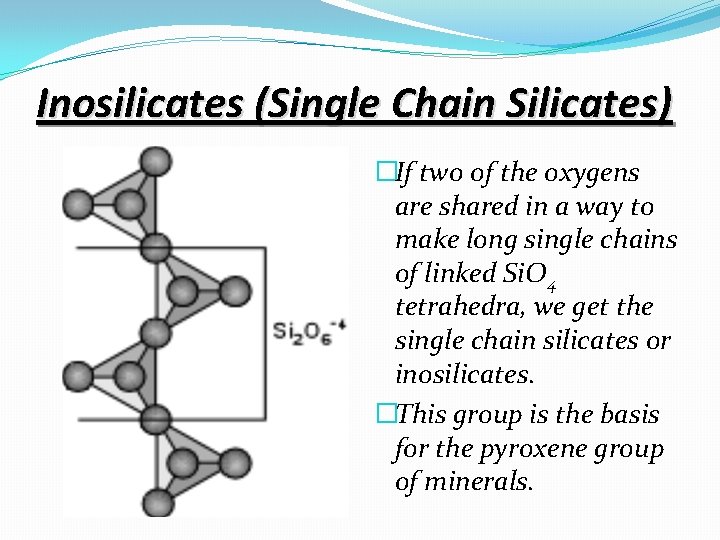
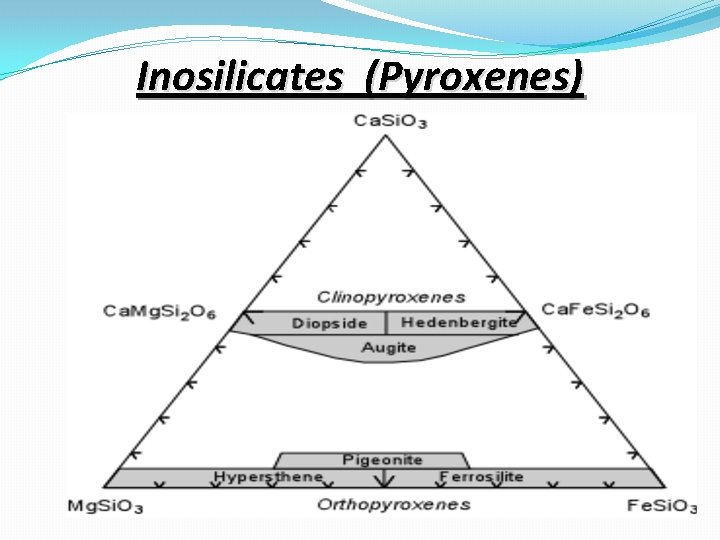
Inosilicates (Single Chain Silicates) �If two of the oxygens are shared in a way to make long single chains of linked Si. O 4 tetrahedra, we get the single chain silicates or inosilicates. �This group is the basis for the pyroxene group of minerals.

Phyllosilicates (Sheet Silicates) �If three of the oxygens from each tetrahedral group are shared such that an infinite sheet of Si. O 4 tetrahedra are shared we get the basis for the phyllosilicates or sheet silicates. The basic structural group is Si 2 O 52 -. The micas, clay minerals, chlorite, talc, and serpentine minerals are all based on this structure.

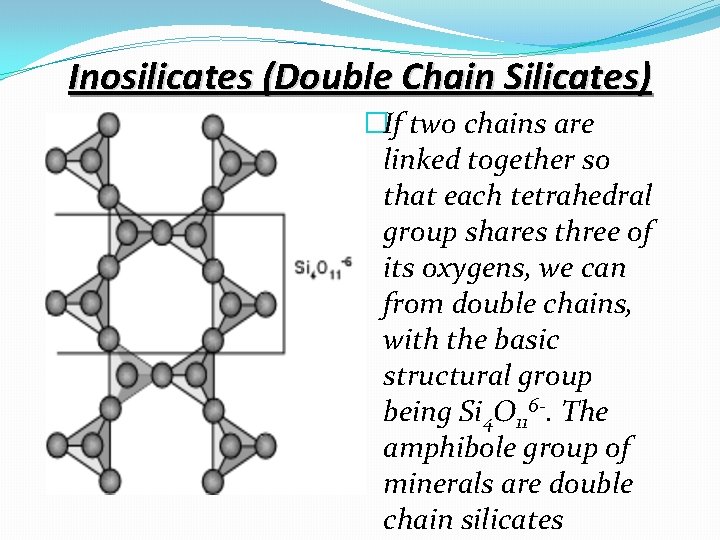
Inosilicates (Double Chain Silicates) �If two chains are linked together so that each tetrahedral group shares three of its oxygens, we can from double chains, with the basic structural group being Si 4 O 116 -. The amphibole group of minerals are double chain silicates

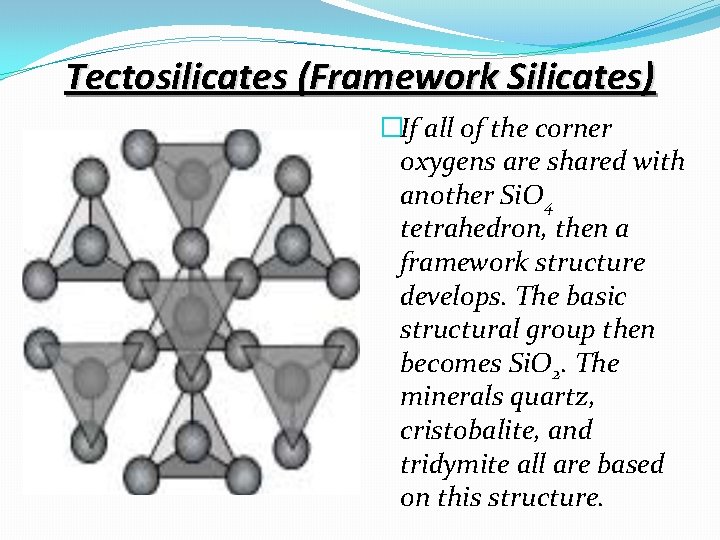
Tectosilicates (Framework Silicates) �If all of the corner oxygens are shared with another Si. O 4 tetrahedron, then a framework structure develops. The basic structural group then becomes Si. O 2. The minerals quartz, cristobalite, and tridymite all are based on this structure.

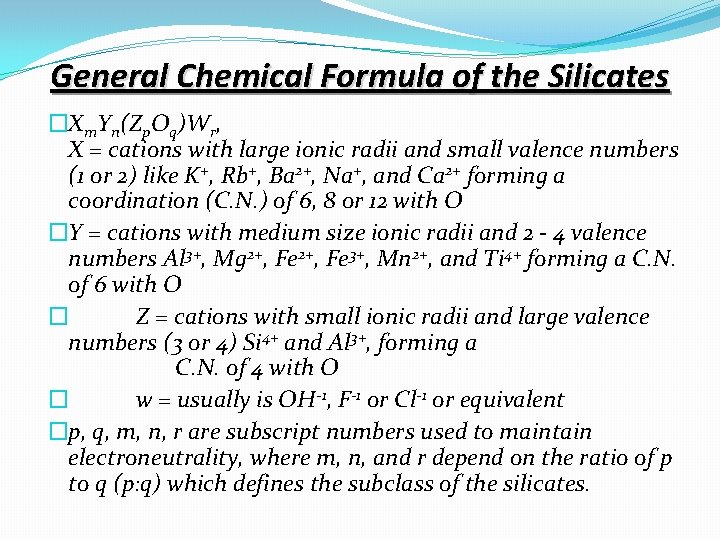
General Chemical Formula of the Silicates �Xm. Yn(Zp. Oq)Wr, X = cations with large ionic radii and small valence numbers (1 or 2) like K+, Rb+, Ba 2+, Na+, and Ca 2+ forming a coordination (C. N. ) of 6, 8 or 12 with O �Y = cations with medium size ionic radii and 2 - 4 valence numbers Al 3+, Mg 2+, Fe 3+, Mn 2+, and Ti 4+ forming a C. N. of 6 with O � Z = cations with small ionic radii and large valence numbers (3 or 4) Si 4+ and Al 3+, forming a C. N. of 4 with O � w = usually is OH-1, F-1 or Cl-1 or equivalent �p, q, m, n, r are subscript numbers used to maintain electroneutrality, where m, n, and r depend on the ratio of p to q (p: q) which defines the subclass of the silicates.

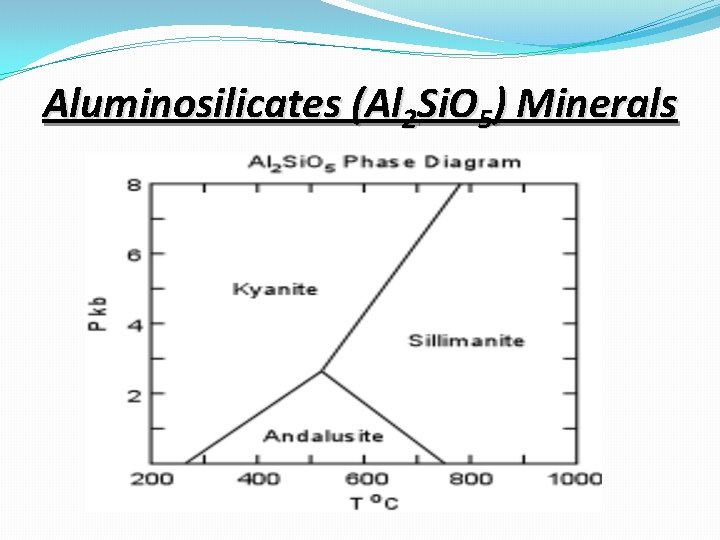
Aluminosilicates (Al 2 Si. O 5) Minerals

Inosilicates (Pyroxenes)

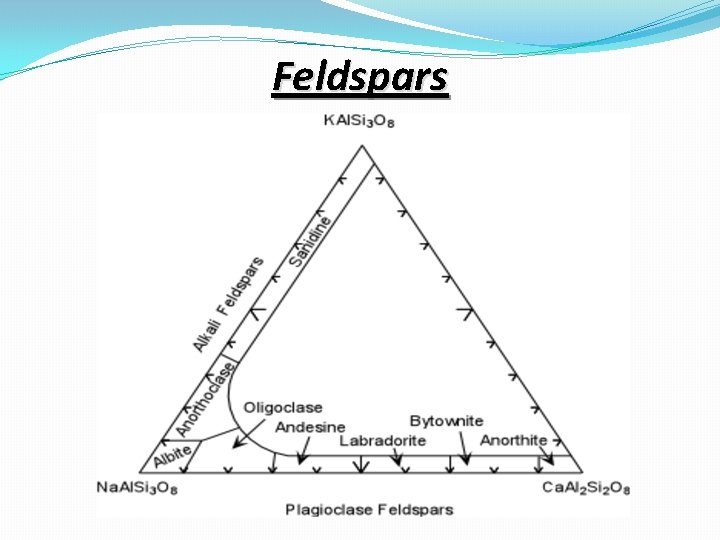
Feldspars

Accessory Minerals �An accessory mineral is a mineral comprising less than 10% (<10%) 0 f a rock which makes it insignificant to classification as compared to essential mineral. Though there a good number of them, few will be considered here. They include: �Zircon (Zr. Si. O 4) �Sphene �Apatite �Staurolite

Mineral Associations �Minerals will often form in specific environments and be associated with specific minerals. Sometimes a mineral is only associated with a certain suite of minerals. Hence an assemblage of minerals is invariably diagnostic of the conditions under which particular rocks formed than the individual mineral. �An important aid in mineral identification is the knowledge of characteristic and widespread mineral associations, which helps by the presence of some easily recognizable minerals first to presuppose and then identify other unknown minerals characteristic of a given association.

Mineral Associations Cont. �Mineral associations formed in the interior of the crust (endogenic genesis) differ greatly from those originated at the crust surface (exogenic genesis). �Among the endogenic mineral associations one can distinguish magmatic, pegmatic, pueumatolytic and hydrothermal ones. �Mineral associations of exogenic origin are formed by chemogenic, organic and mechanical processes.


Uses of Minerals �Minerals are useful in many industries in the past and today. �Minerals that are used for gems are usually hard. �Artists use certain minerals to carve because of their softness. Talc, serpentine, jade, and malachite are soft enough to carve and produce beautiful smooth figures. �The colours of some of the minerals also make them excellent choices for ornamental uses. �Silicon, used in the computer industry is obtained from quartz which is composed of silicon and oxygen. There are many other minerals that are useful to our society; roads we ride or drive on and the buildings we live learn and work in all contain minerals from iron, tin, nickel, tin, uranium etc.

EXAMPLES OF MINERALS

EXAMPLES OF MINERALS

GOLD
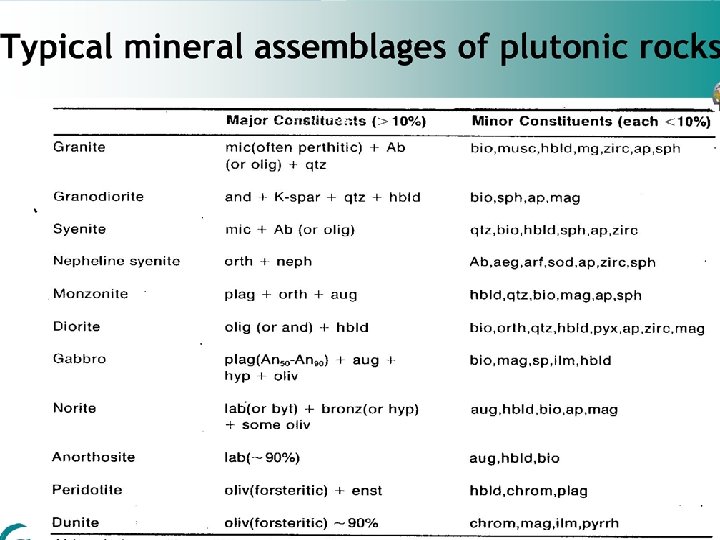
 Igneous sedimentary and metamorphic rocks
Igneous sedimentary and metamorphic rocks What is this
What is this Interference figures in optical mineralogy
Interference figures in optical mineralogy Biaxial indicatrix
Biaxial indicatrix Types of extinction angle
Types of extinction angle Mineralogy lab
Mineralogy lab Flash figure optical mineralogy
Flash figure optical mineralogy Mineralogy
Mineralogy Pseudomorphism in minerals
Pseudomorphism in minerals Optical mineralogy
Optical mineralogy Chapter 3 standardized test practice answers
Chapter 3 standardized test practice answers Chapter 3 standardized test practice answers
Chapter 3 standardized test practice answers Latino rock
Latino rock Rock cycle
Rock cycle Compaction and cementation
Compaction and cementation Climbing rock climbing
Climbing rock climbing Rocks cycle
Rocks cycle Mining frequent patterns associations and correlations
Mining frequent patterns associations and correlations Associations and correlations in data mining
Associations and correlations in data mining Savings and loan associations
Savings and loan associations Savings and loan associations
Savings and loan associations Savings and loan associations
Savings and loan associations Lichen are association of
Lichen are association of Mining frequent patterns associations and correlations
Mining frequent patterns associations and correlations Lichens are symbiotic associations of fungi and ________.
Lichens are symbiotic associations of fungi and ________. Secondary brand elements
Secondary brand elements Brand secondary associations
Brand secondary associations Co occurrence matrix example
Co occurrence matrix example Animal texture
Animal texture Partial failure
Partial failure Gypsum occurrence
Gypsum occurrence Occurrence verbs
Occurrence verbs Occurrence of silicon
Occurrence of silicon Aggregate exceedance probability
Aggregate exceedance probability Cardinality and connectivity
Cardinality and connectivity Examples of irony in an occurrence at owl creek bridge
Examples of irony in an occurrence at owl creek bridge An occurrence at owl creek bridge pov
An occurrence at owl creek bridge pov An occurrence at owl creek bridge tone
An occurrence at owl creek bridge tone An occurrence at owl creek bridge climax
An occurrence at owl creek bridge climax It is an occurrence of harmony
It is an occurrence of harmony Occurrence sampling
Occurrence sampling The recurring aspects of designs are called design
The recurring aspects of designs are called design Occurrence of zinc
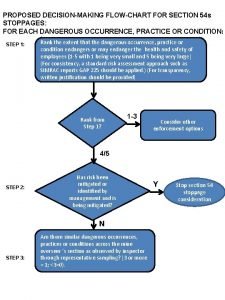
Occurrence of zinc Dangerous occurrence
Dangerous occurrence Come to occurrence
Come to occurrence An occurrence at owl creek bridge comprehension questions
An occurrence at owl creek bridge comprehension questions Minerals sources functions and deficiency chart
Minerals sources functions and deficiency chart Occipital brow presentation
Occipital brow presentation Fundal height transverse lie
Fundal height transverse lie Loose associations thought process
Loose associations thought process Loose thought process
Loose thought process Nigrostriatal pathway
Nigrostriatal pathway Loose associations psychology
Loose associations psychology Negative explanatory style
Negative explanatory style Grandiose thinking
Grandiose thinking Loose associations
Loose associations Loose associations
Loose associations Example of loose associations in schizophrenia
Example of loose associations in schizophrenia Loose thought process
Loose thought process Leveraging brand equity
Leveraging brand equity Maison des associations bastia
Maison des associations bastia Loosening of associations
Loosening of associations