Rocks Minerals Geology at its finest Minerals Minerals





















- Slides: 30

Rocks & Minerals Geology at its finest

Minerals • Minerals can be defined by the 5 properties that they must possess. – 1) Inorganic – a mineral must not be alive or formed from once living material. Coal would NOT be a mineral because it was once alive (organic) – 2) Occurs naturally in the earth. Steel and cement would not be minerals because they are man made. – 3) Always a solid. – 4) Has a definite chemical composition. The same atoms in the same ratios. – 5) The atoms form a repeated pattern called a crystal

Minerals (Cont. ) • There are more than 2000 minerals. • Minerals can be made of a single element. – Ex. Gold, Copper, Iron, Sulphur. • Minerals may be a compound. – Ex. Halite (salt)

Mineral Formation • Most minerals form from cooling magma or lava. • Some minerals form from mineral particles dissolved in a liquid that evaporates. – Calcite forms this way. • The rate of mineral formation determines the size of the crystal in the mineral – When a mineral forms quickly the mineral crystal will be small. – When a mineral forms slowly the mineral crystal will be large.

Exceptions to the 5 criteria rules of minerals • Diamonds. – Diamonds ARE considered minerals although they form from coal which is organic and not considered a mineral. • Obsidian – Obsidian is considered a mineral even though it cools so quickly that crystals don’t form at all.

Geodes • A geode is a rock with a hallow interior lined with mineral crystals. – Most geodes contain quartz crystals.

Composition of Minerals • The 2 most common elements that make up minerals are silicon and oxygen. • The next 6 most common elements are aluminum, calcium, iron, magnesium, potassium, sodium. • Even though these 8 are the most common elements in minerals, only 100 are made of combinations of these elements and fewer than 20 are found everywhere. – Many minerals are rare.

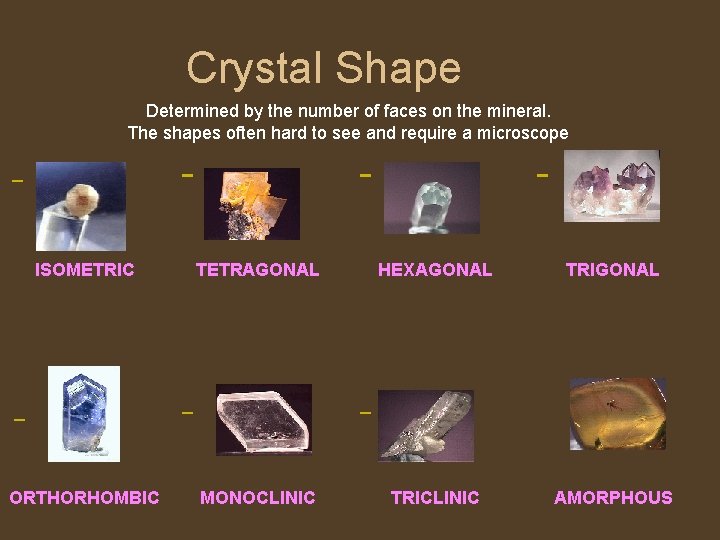
Identifying Minerals • There are 8 ways to help determine what minerals are. – – – – Color Luster Hardness Streak Density (specific gravity) Crystal Shape How it breaks (Cleavage or Fracture) – Special Properties

Color • Obvious, but not always definitive. • Sulfur is (almost) always yellow, and there a few others, but not many minerals have a fixed color. • Small amounts of impurities can drastically change a mineral's color. • Many minerals share the same color. (Black is common) Sulfur

Luster • • The quantity and quality of light reflected from the surface. (How shiny it is). Most are relatively obvious, but some minerals can exhibit a range of lusters (ex. Hematite). – Metallic: looks like a metal. Metallic minerals are commonly shiny and opaque – Non-metallic: doesn't look like a metal. There are many subtle differences in the non-metallic lusters, but most are relatively dull, and are often transparent to translucent on thin edges. (May be called pearly, glassy, earthy, even brilliant) Galena Asbestos

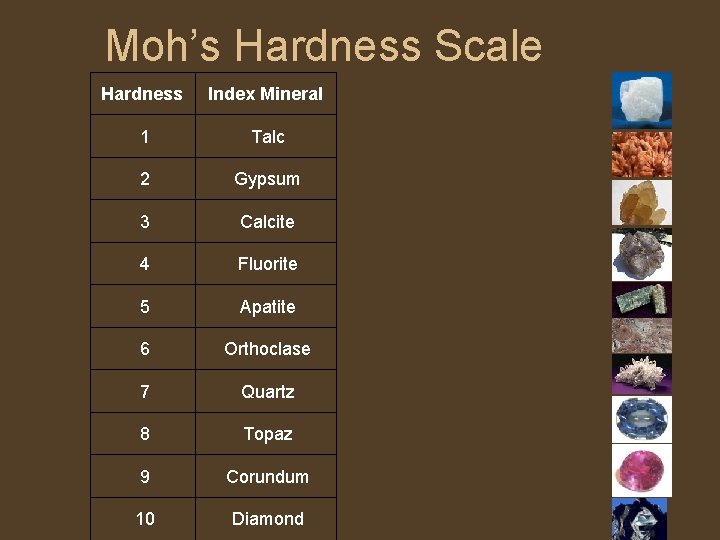
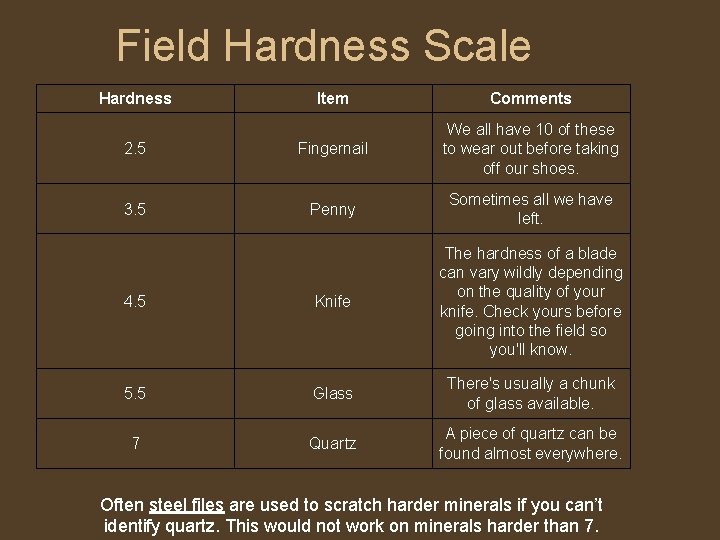
Hardness • • • Hardness is the resistance of a mineral to scratching. It does NOT refer to how easily the mineral is broken. Hardness is a measure of the bonding strength between atoms. If these bonds are strong, the mineral is not easily scratched. Minerals with weaker bonds are more easily scratched. Pencil "lead" is softer than paper, so it writes. Hardness in minerals can vary due to impurities, but is usually definitive. We determine the relative hardness of minerals using a scale devised by mineralogist Friedrich Mohs. The scale assigns hardness to ten common index minerals, and is based on the ability of one mineral to scratch another. Hardness may be relatively determined by the field hardness scale (1 -7)

Moh’s Hardness Scale Hardness Index Mineral 1 Talc 2 Gypsum 3 Calcite 4 Fluorite 5 Apatite 6 Orthoclase 7 Quartz 8 Topaz 9 Corundum 10 Diamond

Field Hardness Scale Hardness Item Comments 2. 5 Fingernail We all have 10 of these to wear out before taking off our shoes. 3. 5 Penny Sometimes all we have left. 4. 5 Knife The hardness of a blade can vary wildly depending on the quality of your knife. Check yours before going into the field so you'll know. 5. 5 Glass There's usually a chunk of glass available. 7 Quartz A piece of quartz can be found almost everywhere. Often steel files are used to scratch harder minerals if you can’t identify quartz. This would not work on minerals harder than 7.

Streak • The color of the powdered mineral. • The test is usually performed by scraping the mineral across a piece of unglazed porcelain. • Streak can be definitive. Good examples include hematite (always red-brown no matter what form it's in) and chromite (distinguished from the hundreds of other black minerals by its chocolate-brown streak). • Some minerals don’t streak or streak is clear. Some have the same color streak.

Density (Specific Gravity) • Defined as "the weight of a specific volume of a mineral divided by the weight of an equal volume of water (at 4°C. )" • Since water is always 1. 0, it's the same number as density without any units (they cancel). • This is almost impossible to measure in the field, but a rough approximation and be determined.

Crystal Shape Determined by the number of faces on the mineral. The shapes often hard to see and require a microscope ISOMETRIC TETRAGONAL HEXAGONAL TRIGONAL ORTHORHOMBIC MONOCLINIC TRICLINIC AMORPHOUS

How the mineral breaks • How a mineral breaks is determined by its internal structure, and is therefore very important (and nearly always diagnostic). Unfortunately, it can also be the hardest to determine (sorry). There are two (2) major subdivisions: fracture and cleavage. – Fracture: The mineral just breaks, leaving an uneven surface. (jagged) Most are irregular but there are some special cases (ex: the conchoidal fractures common to quartz and glass) – Cleavage: The mineral splits along closely spaces parallel planes, leaving a mirror surface which will flash at you if rotated in the light. Smooth and even.

Special Properties • • • Effervescence (the Fizz test): Minerals containing calcium carbonate (Ca. CO 3) will generally react when exposed to weak acid (usually hydrochloric acid (HCl), but even vinegar will work). Carbon dioxide (CO 2) is released and the mineral or rock literally "fizzes. " Magnetism: Magnetite is naturally magnetic. Don't put a chunk near your computer! Taste: Some minerals have a distinctive taste. Notable examples include Halite (rock salt), and Chalcanthite (a copper sulfate - be careful with this one!!). I don't generally recommend the taste test. Smell: Some minerals have a distinctive odor. Sulfur is a good example. Fluorescence: comes from mineral fluorite. It glows under a UV light.

Uses of minerals • Minerals are used for many everyday uses. • They are often found in ores (rock deposits where economically useful minerals are mined for profit) • They must be mined and have impurities removed. • Sometimes impurities are removed by the process of smelting. (metals) • Can mine both metals and non metals.

Metals • Many useful minerals are metals. – Shiny – Good conductors – Malleability (the ability to be hammered into thin sheets without breaking) – Ductility (the ability to be drawn into wires without breaking) • Ex Gold, silver, copper, iron, lead, aluminum, ect.

Non Metals • NOT good conductors, malleable, ductile or shiny. – Ex. Sulfur, asbestos, and halite • Includes Gems (rare, durable and beautiful minerals) – Precious stones – rarest and most valuable gems, • Ex Diamonds, emeralds, rubies and sapphires – Semiprecious stones- not as rare or valuable • Ex. Amethyst, garnet, opal, turquoise, zircons.

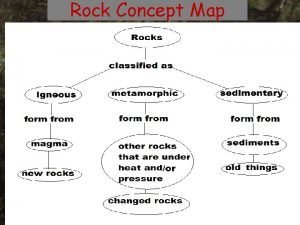
Rocks • Solids made of one or more minerals. • “All minerals are rocks, but not all rocks are minerals” • The study of rocks is called Petrology – The word petrology comes from the apostle Peter. Matthew 16: 18

Types of Rocks • There are 3 main types of rock. – Igneous – Sedimentary – Metamorphic

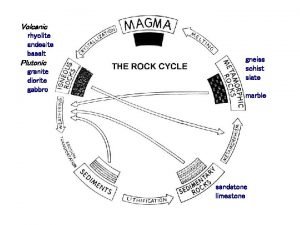
Igneous Rocks • Igneous rocks are called fire rocks and are formed either underground from magma or above ground from lava. • Most minerals are also igneous rocks.

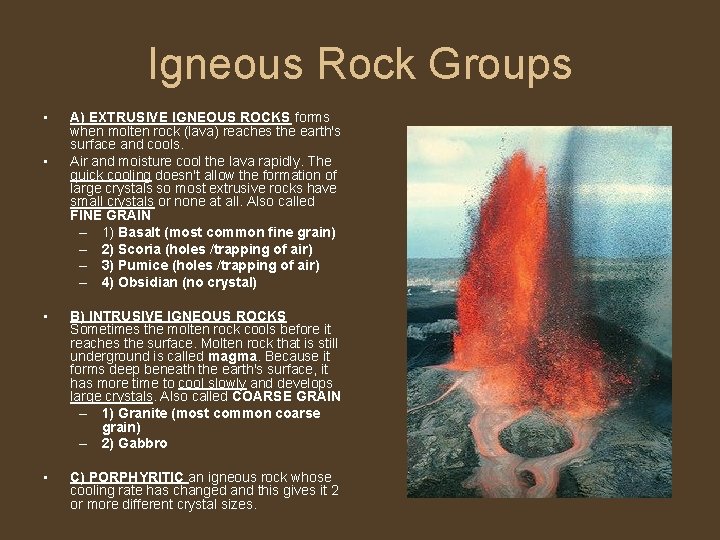
Igneous Rock Groups • • A) EXTRUSIVE IGNEOUS ROCKS forms when molten rock (lava) reaches the earth's surface and cools. Air and moisture cool the lava rapidly. The quick cooling doesn't allow the formation of large crystals so most extrusive rocks have small crystals or none at all. Also called FINE GRAIN – 1) Basalt (most common fine grain) – 2) Scoria (holes /trapping of air) – 3) Pumice (holes /trapping of air) – 4) Obsidian (no crystal) • B) INTRUSIVE IGNEOUS ROCKS Sometimes the molten rock cools before it reaches the surface. Molten rock that is still underground is called magma. Because it forms deep beneath the earth's surface, it has more time to cool slowly and develops large crystals. Also called COARSE GRAIN – 1) Granite (most common coarse grain) – 2) Gabbro • C) PORPHYRITIC an igneous rock whose cooling rate has changed and this gives it 2 or more different crystal sizes.

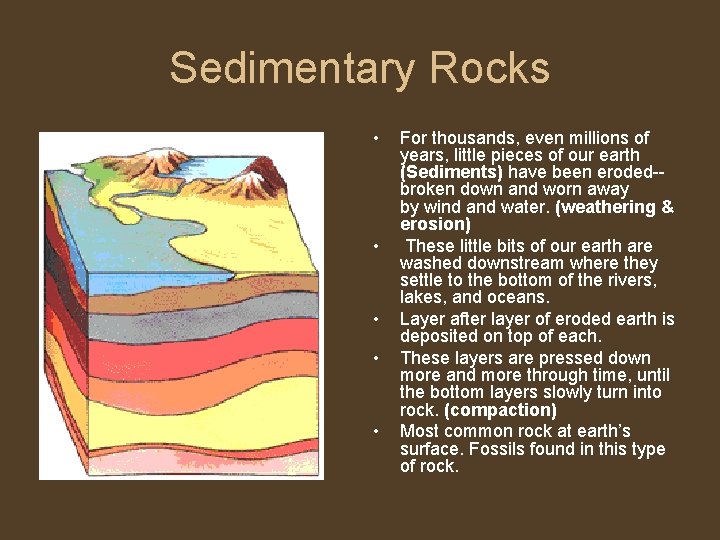
Sedimentary Rocks • • • For thousands, even millions of years, little pieces of our earth (Sediments) have been eroded-broken down and worn away by wind and water. (weathering & erosion) These little bits of our earth are washed downstream where they settle to the bottom of the rivers, lakes, and oceans. Layer after layer of eroded earth is deposited on top of each. These layers are pressed down more and more through time, until the bottom layers slowly turn into rock. (compaction) Most common rock at earth’s surface. Fossils found in this type of rock.

Sedimentary Rock Groups • • • A) CLASTIC or DETRITAL classified by size of sediment (mud, sand, or gravel) – 1)Gravel sized • a) Conglomerate (round) • b) Breccia (angular) – 2) Sand sized • a) sandstone – 3) Mud sized • a) shale • b) siltstone B) ORGANIC classified by forming directly or indirectly from once living material. – 1) Limestone – 2)Chalk – 3)Coal C) CHEMICAL formed by chemical means such as water evaporation and sediments cementing together. – 1) rock salt – 2) gypsum – 3) limestone Breccia, sandstone, shale Organic (fossiliferous) limestone and coal Rock salt and chemical limestone

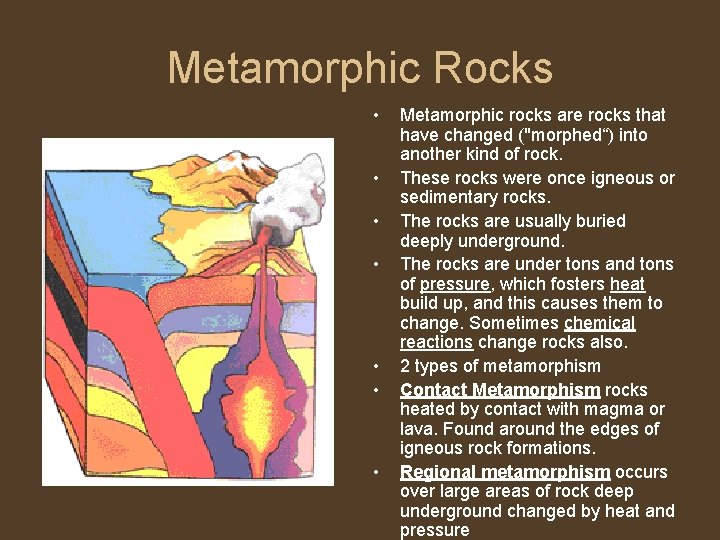
Metamorphic Rocks • • Metamorphic rocks are rocks that have changed ("morphed“) into another kind of rock. These rocks were once igneous or sedimentary rocks. The rocks are usually buried deeply underground. The rocks are under tons and tons of pressure, which fosters heat build up, and this causes them to change. Sometimes chemical reactions change rocks also. 2 types of metamorphism Contact Metamorphism rocks heated by contact with magma or lava. Found around the edges of igneous rock formations. Regional metamorphism occurs over large areas of rock deep underground changed by heat and pressure

Metamorphic Rock Groups • 2 groups of metamorphic rocks. • A) FOLIATED TEXTURE mineral crystals arranged in parallel layers. Due to different minerals having different densities they often separate into alternating light and dark bands. – 1) Slate (used to be shale) – 2) Schist (used to be granite, basalt or slate – 3)Gneiss (used to be granite) • B) UNFOLIATED TEXTURE no bands and do not break in layers. – 1) Quartzite (used to be sandstone) – 2) Marble (used to be limestone)

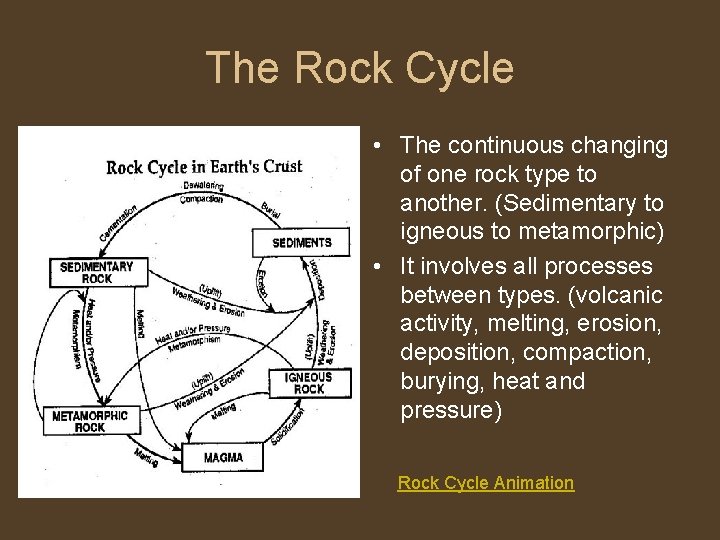
The Rock Cycle • The continuous changing of one rock type to another. (Sedimentary to igneous to metamorphic) • It involves all processes between types. (volcanic activity, melting, erosion, deposition, compaction, burying, heat and pressure) Rock Cycle Animation
 Rock cycle
Rock cycle Igneous rocks metamorphic rocks and sedimentary rocks
Igneous rocks metamorphic rocks and sedimentary rocks Https://geology.com/rocks/
Https://geology.com/rocks/ Come live with me poem
Come live with me poem Types of soil
Types of soil The finest grained soils are richest in
The finest grained soils are richest in The holidays finest
The holidays finest The passionate shepherd to his love analysis
The passionate shepherd to his love analysis The holidays finest
The holidays finest Canterbury tales the skipper
Canterbury tales the skipper Topfeeds
Topfeeds Rock type
Rock type Quartzite rock cycle
Quartzite rock cycle 3 types of rocks concept map
3 types of rocks concept map Rocks are aggregates of minerals
Rocks are aggregates of minerals Poem about minerals and rocks 3 stanza
Poem about minerals and rocks 3 stanza The rock cycle song
The rock cycle song What kind of rock is this
What kind of rock is this Difference between minerals and rocks
Difference between minerals and rocks Rock cycle
Rock cycle Extrusive rock
Extrusive rock Volcanic rocks and plutonic rocks
Volcanic rocks and plutonic rocks When a train increases its velocity, its momentum
When a train increases its velocity, its momentum Its not easy but its worth it
Its not easy but its worth it Sunny rainy cloudy windy stormy
Sunny rainy cloudy windy stormy If its a square it's a sonnet summary
If its a square it's a sonnet summary The emigree annotations
The emigree annotations Its halloween its halloween the moon is full and bright
Its halloween its halloween the moon is full and bright What is geochemistry in geology
What is geochemistry in geology Earth science vs geology
Earth science vs geology Geology
Geology