MINERALS MINERALS v Inorganic compounds v Minerals do

















































- Slides: 73

MINERALS

MINERALS v Inorganic compounds v Minerals do not give energy v Important roles in many biological activities v Normal growth and homeostatic balance v Mediation of metabolic reactions in the skeleton, tissues, body fluids, digestive juices, etc

v. Excessive intake of certain minerals can also disrupt homeostatic balance and lead to toxic effects. v. For example, excess sodium intake is associated with high blood pressure and excessive iron ……. liver damage.

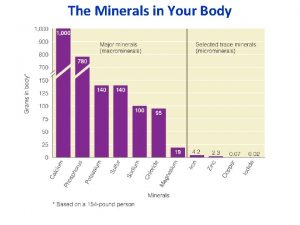
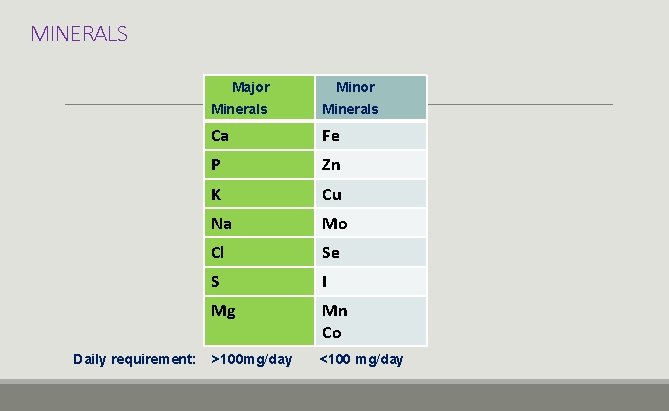
MINERALS Major Daily requirement: Minor Minerals Ca Fe P Zn K Cu Na Mo Cl Se S I Mg Mn Co >100 mg/day <100 mg/day

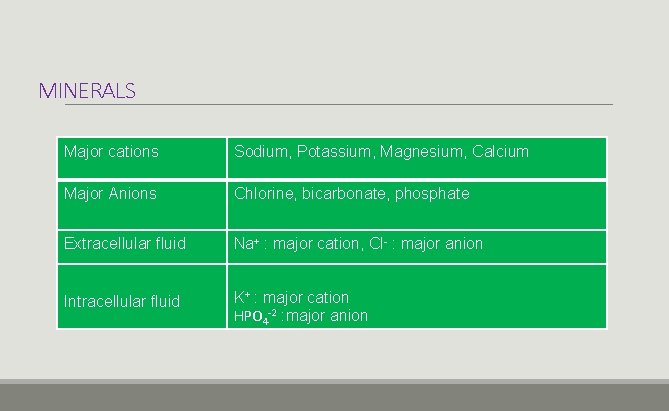
MINERALS Major cations Sodium, Potassium, Magnesium, Calcium Major Anions Chlorine, bicarbonate, phosphate Extracellular fluid Na+ : major cation, Cl- : major anion Intracellular fluid K+ : major cation HPO 4 -2 : major anion

Calcium (Ca) v v v v Bone mineralization Muscle contraction Nerve transmission Blood coagulation Secretion Enzyme reactions Hormon and neurotranmitter secretion

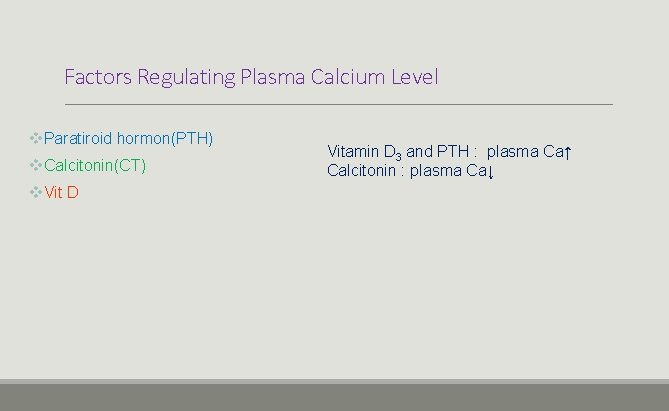
Factors Regulating Plasma Calcium Level v. Paratiroid hormon(PTH) v. Calcitonin(CT) v. Vit D Vitamin D 3 and PTH : plasma Ca↑ Calcitonin : plasma Ca↓

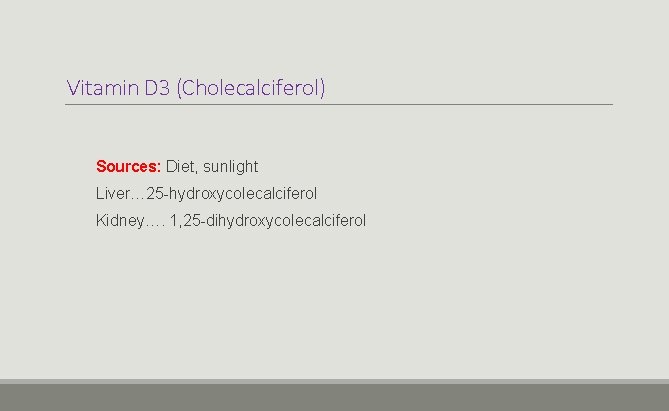
Vitamin D 3 (Cholecalciferol) Sources: Diet, sunlight Liver… 25 -hydroxycolecalciferol Kidney…. 1, 25 -dihydroxycolecalciferol

Calcium v. Total calcium: 1 -1. 5 kg v%99 bone (Hidroxyapetite) v%1 blood, ECF, very little cytosol, soft tissues

Extracellular calcium levels; are 10 000 times higher than intracellular levels higher and both are under very strict control. (Ca gradient) Extracellular calcium; is important for excitation-contraction relationship in muscle tissues, synaptic transmission in the nervous system, coagulation and the secretion of hormones. Intracelular calcium; is an important second messenger for cell division, motility, membrane permeability and regulation of secretion

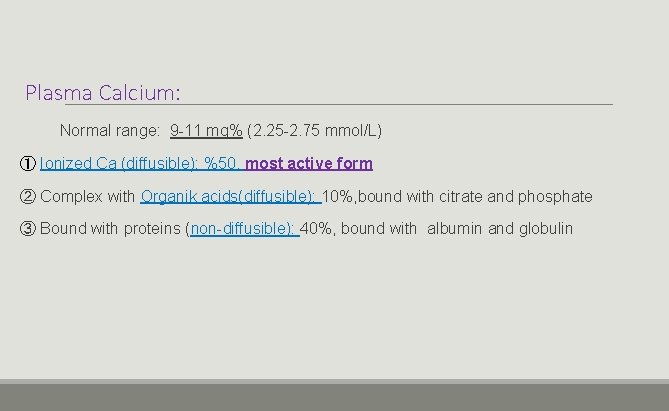
Plasma Calcium: Normal range: 9 -11 mg% (2. 25 -2. 75 mmol/L) ① Ionized Ca (diffusible): %50, most active form ② Complex with Organik acids(diffusible): 10%, bound with citrate and phosphate ③ Bound with proteins (non-diffusible): 40%, bound with albumin and globulin

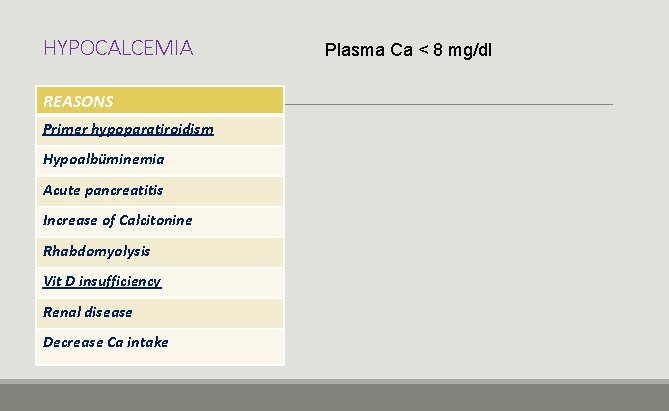
HYPOCALCEMIA REASONS Primer hypoparatiroidism Hypoalbüminemia Acute pancreatitis Increase of Calcitonine Rhabdomyolysis Vit D insufficiency Renal disease Decrease Ca intake Plasma Ca < 8 mg/dl

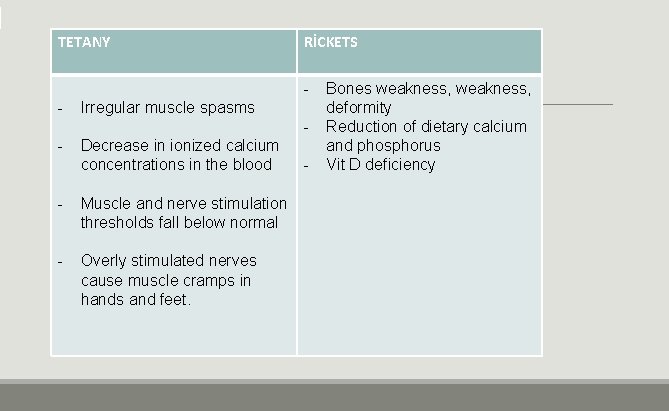
TETANY RİCKETS - Irregular muscle spasms - Decrease in ionized calcium concentrations in the blood - Muscle and nerve stimulation thresholds fall below normal - Overly stimulated nerves cause muscle cramps in hands and feet. - Bones weakness, deformity Reduction of dietary calcium and phosphorus Vit D deficiency

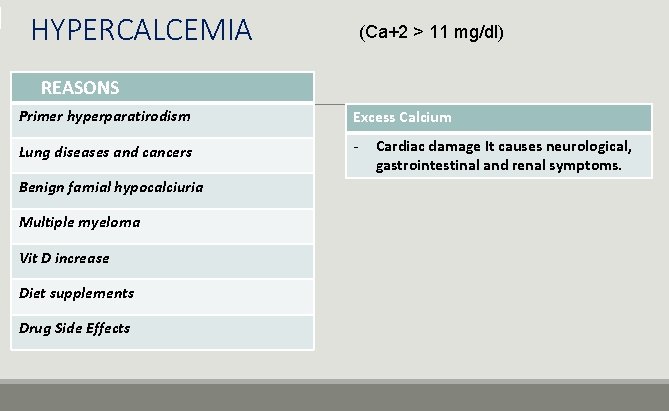
HYPERCALCEMIA (Ca+2 > 11 mg/dl) REASONS Primer hyperparatirodism Excess Calcium Lung diseases and cancers - Benign famial hypocalciuria Multiple myeloma Vit D increase Diet supplements Drug Side Effects Cardiac damage It causes neurological, gastrointestinal and renal symptoms.

Factors affecting Ca absorption 1. Vit. D 2. Parathyrioid hormone (PTH) 3. Acidity (p. H düşüklüğü) 4. Lactose 5. Lysine and arginine 6. Estrogen

Factors inhibiting Ca absorption 1. 2. 3. 4. 5. 6. Phytates and oxalate The high content of dietary phosphate results in the formation of insoluble Ca phosphate and prevent Ca uptake. (Ca : P ---1: 1 2: 1) Free fatty acids Alkaline conditions High content of dietary fiber interferes with Ca absorption. Low estrogen levels (postmenopausal women)

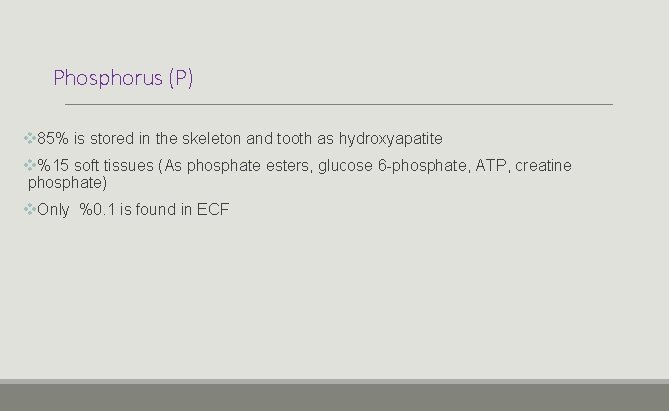
Phosphorus (P) v 85% is stored in the skeleton and tooth as hydroxyapatite v%15 soft tissues (As phosphate esters, glucose 6 -phosphate, ATP, creatine phosphate) v. Only %0. 1 is found in ECF

Phosphorus is found in plasma in two ways: Inorganic Phosphorus: (%30) (PO 4) %55; Ionic, %10‐ 15; bound to proteins, %35; complex with Na , Ca ve Mg Organic phosphour: (%70) In the cell; cytosol, cell membrane (phospholipid),

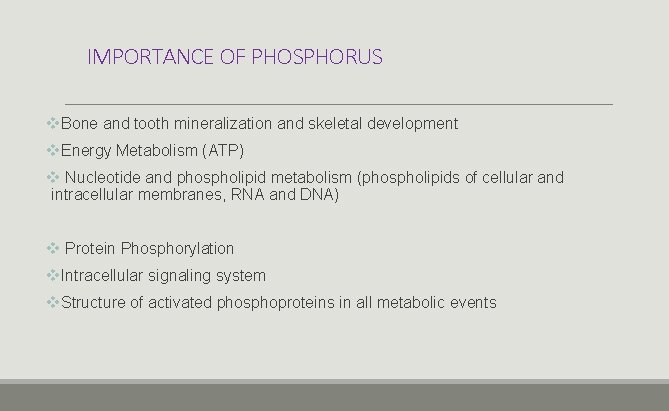
IMPORTANCE OF PHOSPHORUS v. Bone and tooth mineralization and skeletal development v. Energy Metabolism (ATP) v Nucleotide and phospholipid metabolism (phospholipids of cellular and intracellular membranes, RNA and DNA) v Protein Phosphorylation v. Intracellular signaling system v. Structure of activated phosphoproteins in all metabolic events

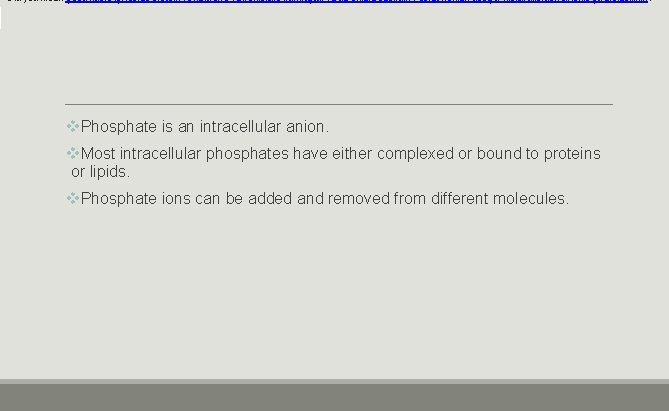
Did you mean Çoğu hücre içi fosfat, protein ve lipitlerle ya kompleks kurmuştur ya da onlara bağlıdır. Fosfat iyonları değişik moleküllerden eklenip çıkartılabilinir. ? v. Phosphate is an intracellular anion. v. Most intracellular phosphates have either complexed or bound to proteins or lipids. v. Phosphate ions can be added and removed from different molecules.

Paratyhroid hormone: v v v The major regulator of phosphate absorbtion First regulator of renal phosphate absorbtion It decreases renal phosphate absorbtion (proximal tubes) It increases phosphate excreation via urine It enhances the uptake of phosphate from the intestine and bones into the blood. Calcitriol (Vit D); v Increases calcium and phosphate absorbtion from intestine v The absorption of phosphate is not as dependent on vitamin D as is that of calcium.

ØInsulin, increases renal phosphate reabsorbtion ØCa: P ratio 1: 2 -2: 1; → Ca and P absorbtion is optimum

Hypophosphatemia v. Phosphate < 2, 5 mg/dl v. Reduction in intestinal absorbtion v. Increased excretion of urine v. Physical effects are rare. v. In uncontrolled diabetes occurs acutely v. Mild hypophosphatemia occurs after kidney transplantation v. Long-term alcohol use and chronic malnutrition cause reduction of body phosphate levels.

v. Reductions in serum phosphorus levels are seen in rickets and hyperparathyroidism. v. Phosphourus Deficiency; cause osteomalacia in adults and rickets in children. v. Absorption of phosphate in intestinal diseases such as Crohn's disease, ulcerative colitis is decreased v Chronic diarrhea can cause moderate hypophophatemia.

v. The release of insulin after meals is the main factor in the entry of phosphate into the cell. v. When hypophosphatemia is exacerbated, patients may experience muscle weakness. v. Chronic hypophosphatemia (in patients with genetically modified phosphate deficiency) may be seen as a finding of curvature in the legs. Bone pain, muscle weakness, skeletal deformities are seen.

HYPERPHOSPHATEMIA v. Increase in phosphate intake or increase in the release of cellular phosphate v. Reduction of renal phosphate excretion v. It is seen in chronic nephritis and hypoparathyroidism. v. D vit intoxication

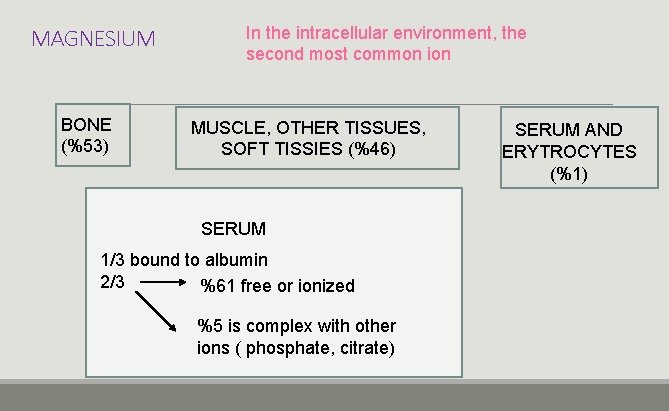
MAGNESIUM BONE (%53) In the intracellular environment, the second most common ion MUSCLE, OTHER TISSUES, SOFT TISSIES (%46) SERUM 1/3 bound to albumin 2/3 %61 free or ionized %5 is complex with other ions ( phosphate, citrate) SERUM AND ERYTROCYTES (%1)

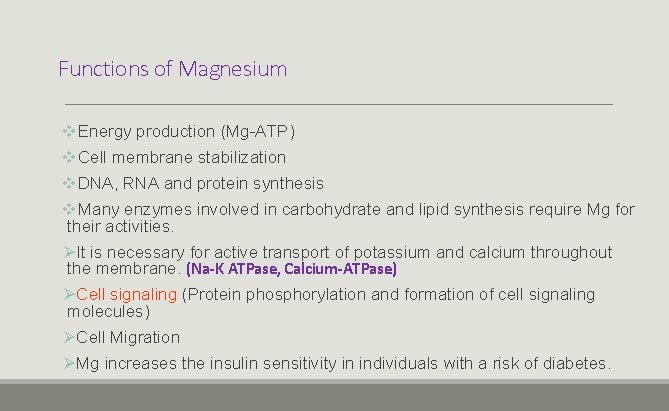
Functions of Magnesium v. Energy production (Mg-ATP) v. Cell membrane stabilization v. DNA, RNA and protein synthesis v. Many enzymes involved in carbohydrate and lipid synthesis require Mg for their activities. ØIt is necessary for active transport of potassium and calcium throughout the membrane. (Na-K ATPase, Calcium-ATPase) ØCell signaling (Protein phosphorylation and formation of cell signaling molecules) ØCell Migration ØMg increases the insulin sensitivity in individuals with a risk of diabetes.

Hypomagnesemia v. Many Mg deficiency can affect Vit D and calcium homeostasis. v. Magnesium deficiency is related with cardiovascular diseases, osteoporosis, metabolic diseases (associated with hypertension and diabetes) v. Clinical results: Weakness, fatigue, muscle cramps, tetany, arrhythmia are seen.

Hypermagnesemia: v. It is less common than hypomagnesemia. v Decreased excretion (Chronic or acute renal damage, hypothyroidism, hypoaldosteronism) v. Increased intake (Use of drugs containing Mg)

IRON(Fe) v. Essential element v. Its biological significance is due to its ability to bind to oxygen and to play a role in electron transfer reactions.

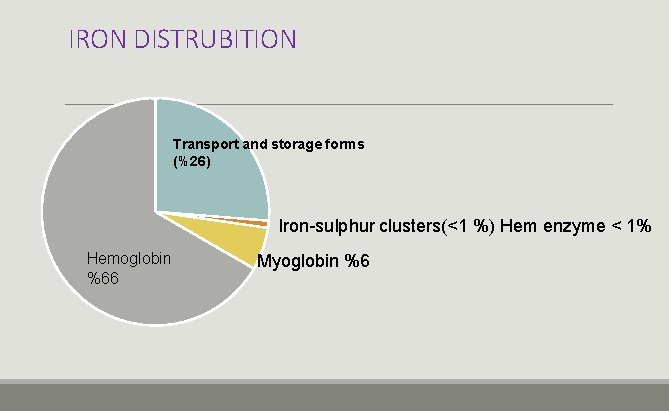
IRON DISTRUBITION Transport and storage forms (%26) Iron-sulphur clusters(<1 %) Hem enzyme < 1% Hemoglobin %66 Myoglobin %6

v. Iron deficiency is a common problem v. Iron overload…. . damages the heart, liver and endocrine organs v. Ferrous iron ↑ free radical formation v. Factors affecting dietary iron absorption and bioavailability are strictly controlled throughout the body.

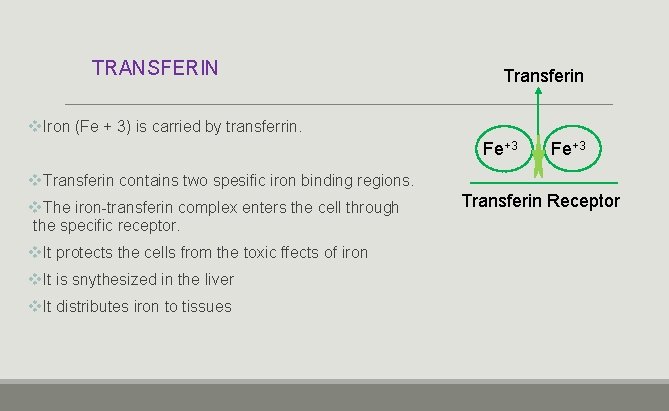
TRANSFERIN Transferin v. Iron (Fe + 3) is carried by transferrin. Fe+3 v. Transferin contains two spesific iron binding regions. v. The iron-transferin complex enters the cell through the specific receptor. v. It protects the cells from the toxic ffects of iron v. It is snythesized in the liver v. It distributes iron to tissues Transferin Receptor

v It is found in the form of Hb in the structure of erythrocytes in the blood and plasma in the structure of the transferin v It is transported in the form of transferrin, stored in the form of ferritin or hemosiderin. v About 35 mg of iron undergoes turnover on a daily basis. v Only about 1 mg of iron is lost by skin epithelial cells, GI and urinary canals, and a small amount of erythrocyte is lost with urine and stool. v Women lose 20 -40 mg of iron with the menstrual cycle

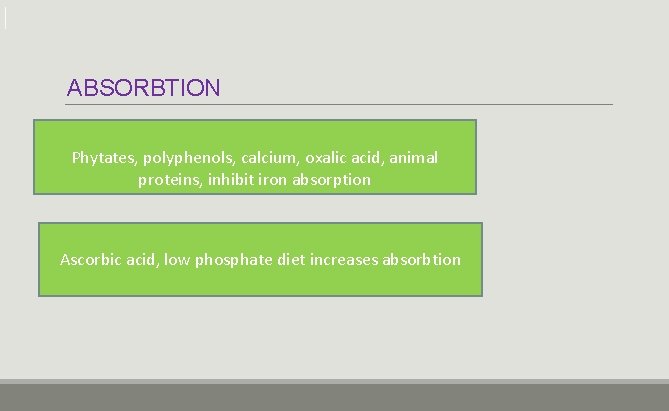
ABSORBTION Phytates, polyphenols, calcium, oxalic acid, animal proteins, inhibit iron absorption Ascorbic acid, low phosphate diet increases absorbtion

FUNCTIONS OF IRON v. It is found in the structure of many biologically important molecules. v. Hemoglobin is the primary protein found in red blood cells, It is also an iron-containing molecule v. Myoglobin is responsible for the transport and short-term storage of oxygen in muscle cells, It provides oxygen support to working muscles. v. Cytochrome C is the mobile component of ETS. They are conjugated proteins. v. They contain both the group containing the porphyrin ring and the iron atom. v. Cytochromes are enzymes that contain both and play important roles in mitochondrial electron transport. v Cytochrome p 450 enzymes metabolize toxic compounds in the liver (drugs, endogenous metabolism products such as fatty acids, steroids, vitamins A, K, bilurubin)

FUNCTIONS OF IRON v. It is found in the structure of Fe-S proteins. (Succinate dehydrogenase, isocitrate dehydrogenase, NADH dehydrogenase) v. Takes part in DNA replication and repair (DNA polymerases and DNA helicases) v. Non-heme enzymes that require iron as a cofactor: (phenylalanine, lysine hydroxylase, ribonucleotide reductase) v. Proteins responsible for iron transport and storage: (Nonheme proteins) (Ferritin, transferrin, haptoglobin, hemopexin, lactoferrin). v. Catalase and some peroxidases

Disorders of Iron Metabolism Iron deficiency v Iron deficiency mainly leads to anemia, fatigue, a decrease in working capacity and a decrease in learning ability, especially in children. Iron Excess v Hemosiderosis or haemochromotosis occurs in excess iron. v There is an increase in iron depots, Fe absorption is very high v Complications include joint inflammation (arthritis), diabetes, liver cirrhosis, heart rhythm irregularities and failure, increased skin pigmentation ( tanning).

COPPER (Cu) v. Third trace element after iron and zinc v. The human body contains about 100 mg of copper. v. It is found in muscles, liver, bone marrow, brain, kidney, heart and hair. v 0. 9 mg / day (for adult women and men) (Recommended Dietary Allowance) v Transition element v It can be found as Cu +2, Cu +1 v It is found in vegetables, legumes, cereals, animal products.

COPPER (Cu) v 10% of the dietary copper is absorbed. v Copper is mainly excreted in bile. v. Normal serum copper levels are 25 -50 mg / dl.

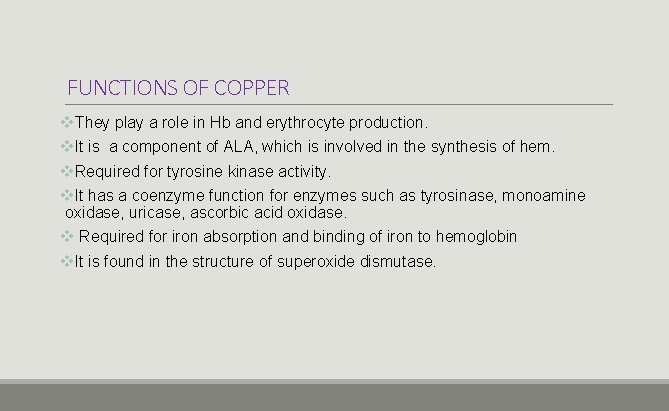
FUNCTIONS OF COPPER v. They play a role in Hb and erythrocyte production. v. It is a component of ALA, which is involved in the synthesis of hem. v. Required for tyrosine kinase activity. v. It has a coenzyme function for enzymes such as tyrosinase, monoamine oxidase, uricase, ascorbic acid oxidase. v Required for iron absorption and binding of iron to hemoglobin v. It is found in the structure of superoxide dismutase.

FUNCTIONS OF COPPER Energy Production: Copper-dependent enzyme, cytoxrome c oxidase plays a role in energy production. Connective tissue formation: Lysyl oxidase; cross-link formation in collagen and elastin, preservation of connective tissue integrity in blood vessels and heart Iron metabolism: Multi-copper oxidase enzymes (MCO) (ferroxidases) oxidize ferrous (Fe + 2) iron to ferric (Fe + 3) iron. Fe + 3 can be linked to transferine MCO family; Contains ceruloplasmin, hephaestin.

Copper Absorption v. Effect of p. H and digestion v. Sodium v. The effect of phytate on copper absorption is not as common as zinc or calcium. v. Copper can precipitate with phytates in the presence of excess calcium v. Dietary fructose enhances the effects of copper deficiency.

Ceruloplasmin v v It is the major copper transport protein. It increases in active liver diseases and tissue damage It decreases in Wilson's disease. It facilitates iron metabolism through copper-dependent ferroxidase activity. v Hephaestin is another copper-dependent ferroxidase and is expressed in the duedonal mucosa and facilitates the transfer of ferric ion transport to the transfer of the basolateral surface.

v. Decreased ceruloplasmin and hephaestin activities cause impaired iron absorption from the small intestine v. Systemic transport is disrupted by transfrin v. Insufficient iron incorporation occurs in protoporphyrin. v. Hem synthesis weakens

v. In Cu deficiency, growth stop, hair loss, milk reduction, walking disorders are seen. v. Copper deficiency restricts copper-dependent enzymatic activity. v. It causes hypopigmentation, osteoporosis, anemia, neutropenia, myelopathy and peripheral neuropathy in the hair and skin.

Copper Metabolism Disorders: Wilson disease v 1/50. 000 v. Wilson disease gen ATP 7 B mutation v. ATP 7 B protein is a copper-transporting P type ATP ase v. Autosomal ressesive disease of copper transport v. Ceruloplasmin drop in the blood and copper accumulation v. Copper excretion with bile decreases

Wilson Disease Ø Accumulation of copper in the liver…. Hepatocellular degeneration Ø Accumulation of brain in basal ganglia ……. Leticular degeneration Ø Accumulation around the cornea…. . Kayser-Kleischer ring

Menkes kinky hair syndrome v. Copper transport disease v. X dependent ressesive disease v. Mutations in genes encoding ATP 7 A v. ATP 7 A provides the release of copper out of the cell from the intracellular environment v. Characteristic findings: Kinky hair, growth retardation, nervous system disorders, reduced growth, hypothermia, brain degeneration, hair discoloration, plasma Cu levels decrease, copper accumulates inside the cell

ZINC v Zinc is the second most abundant trace element necessary for living things. v It is found as Zn+2

ZINC v Major sources of Zinc include cereals, beans, meat, and shellfish. v. More than 300 enzymes in the human body are zinc-dependent. (Carboxypeptidase, carbonic anhydrase, alkaline phosphatase, lactate dehydrogenase, alcohol dehydrogenase) v. Compared to adults; babies, children, adolescents, pregnant and lactating women have increased requirements for zinc, and therefore the risk of zinc depletion is higher.

v Epidermal, gastrointestinal, central nervous, immune, skeletal and reproductive systems are the organs most affected clinically by zinc deficiency. v Many dietary factors affect absorption. v. Phytate, calcium, copper and iron ↓ v. Small peptids and amino acids ↑

Excreation v Loss of zinc through the gastrointestinal tract accounts for about half of all zinc removed from the body. v. A significant amount of zinc is secreted from bile and intestinal secretions, but most are reabsorbed. v. Other ways of excreting zinc include urine and surface losses (skin, hair, sweat).

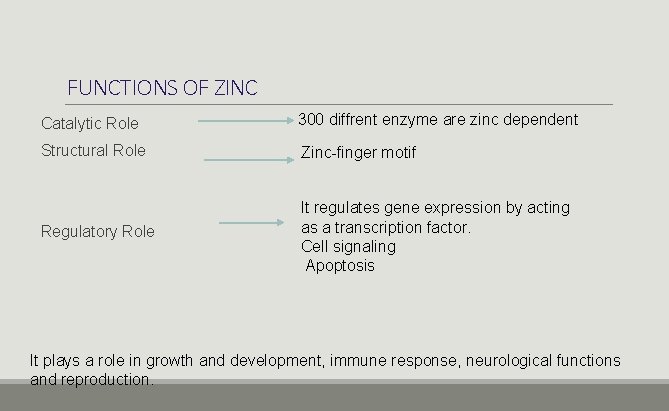
FUNCTIONS OF ZINC Catalytic Role 300 diffrent enzyme are zinc dependent Structural Role Zinc-finger motif Regulatory Role It regulates gene expression by acting as a transcription factor. Cell signaling Apoptosis It plays a role in growth and development, immune response, neurological functions and reproduction.

Zinc- Finger Motif Ø It plays a role in the interactions between proteins and nucleic acids for replication, transcription and translation and also it is central to the regulation of these processes. Ø Zinc plays an important role in the structure of proteins and cell membranes. Ø A finger-like structure known as a zinc finger motif stabilizes the structure of a number of proteins

Zinc Deficiency v. Reduction in wound healing v. Skin lesions v. Disruption of spermatogenesis v. Dermatitis v. Loss of appetite v. Disruption of immune functions v. Hair loss v. Diarrhea

SODIUM (Na) v. Sodium is the most abundant cation in extracellular fluid v. It determines the osmolality, plasma volume and acid base balance of plasma v. It has a great effect on the osmotic pressure of body fluids. v. Normal plasma osmolality of about 295 nmol / L v. Caused by 270 mmol / L sodium and related anions

Regulation v Plasma sodium concentration mainly depends on water intake and excretion and to a lesser extent on renal sodium regulation. The three events are of primary importance. 1. Water intake 2. Water excretion 3. Blood volume status affects sodium excretion.

Regulation v Kidneys are capable of removing or protecting sodium in large quantities depending on the sodium content of ECF and blood volume.

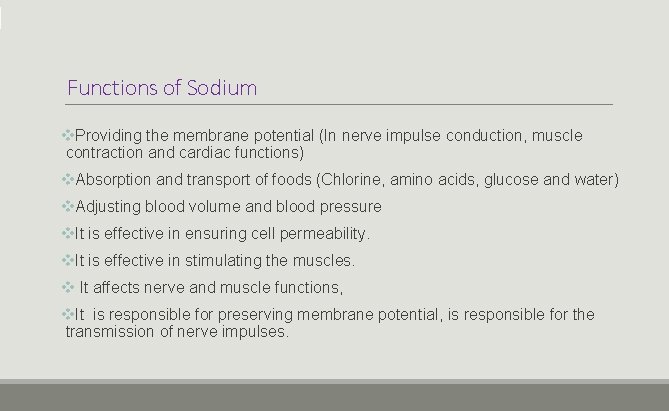
Functions of Sodium v. Providing the membrane potential (In nerve impulse conduction, muscle contraction and cardiac functions) v. Absorption and transport of foods (Chlorine, amino acids, glucose and water) v. Adjusting blood volume and blood pressure v. It is effective in ensuring cell permeability. v. It is effective in stimulating the muscles. v It affects nerve and muscle functions, v. It is responsible for preserving membrane potential, is responsible for the transmission of nerve impulses.

Functions of Sodium v It is involved in the absorption of monosaccharides, amino acids, pyrimidines and bile salts. v Changes in osmotic pressure largely depend on sodium concentrations. v Its metabolism is regulated by aldosterone.

Functions of Sodium v Sodium is rapidly absorbed in the form of sodium ions and is added to the circulation. v Its excretion is mainly from the kidneys in the form of sodium chloride or sodium phosphate. Reasonable losses are lost with sweating. v. This loss varies in proportion to the humidity of the air.

Hypernatremia v. It is an increase in sodium levels in the blood. v. It occurs in Cushing disease. ( ACTH production increases). v. Dietary intake of excess sodium causes hypertension

Hypernatremia v. It is an increase in sodium levels in the blood. v. It is seen in Cushings Disease. ACTH production increases, adrenal glands are stimulated v. Dietary intake of excess sodium causes hypertension. v. Excessive water loss, less water intake and excess sodium intake

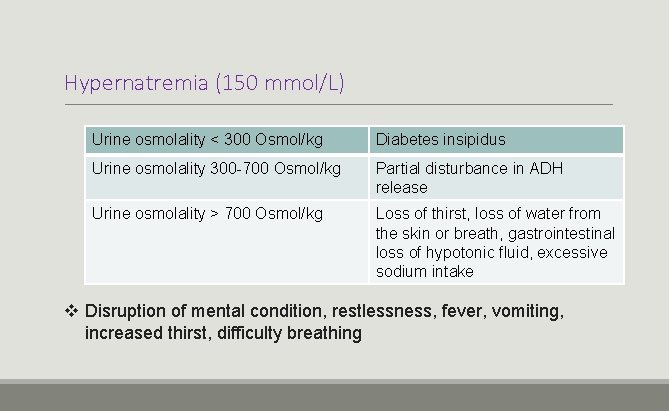
Hypernatremia (150 mmol/L) Urine osmolality < 300 Osmol/kg Diabetes insipidus Urine osmolality 300 -700 Osmol/kg Partial disturbance in ADH release Urine osmolality > 700 Osmol/kg Loss of thirst, loss of water from the skin or breath, gastrointestinal loss of hypotonic fluid, excessive sodium intake v Disruption of mental condition, restlessness, fever, vomiting, increased thirst, difficulty breathing

Hyponatremia v Low sodium levels in serum v. It is common in the elderly people v. Acute hyponatremia… Headache, nausea, vomiting, muscle cramps are seen. v. Rapidly progressive hyponatremia may include brain edema coma and brain damage. v. It occurs in Addison's disease. (cortisol and aldosterone hormone decreased in the blood

Potassium (K) v. Major cation of intracellular fluid. v. It plays role in acid-base balance. v. Regulation of osmotic pressure v. Nerve impulse transmission v. Muscle contraction v. Providing membrane potential v. Cofactor of enzymes (Sodium-potassium ATPase, pyruvate kinase)

Many functions of sodium and potassium are in coordination.

Hyperkalemia v. Serum potassium levels are increased. v. It occurs in Addison's disease (Fatigue, loss of appetite, weight loss, nausea, vomiting, dizziness, darkening of the skin, muscle and joint pain, such as signs and symptoms are observed. ) v. Excess salt demand, Sodium level decline, potassium increase

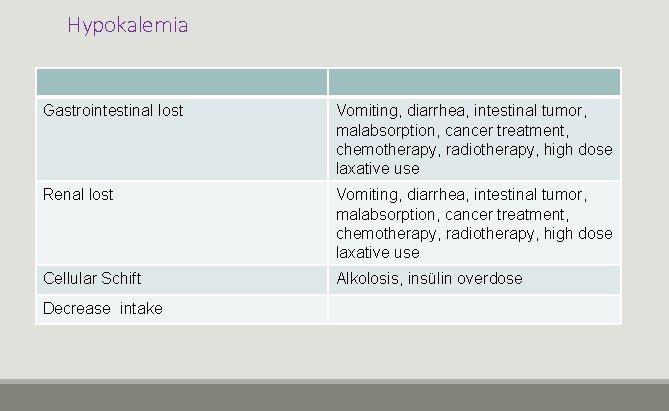
Hypokalemia Gastrointestinal lost Vomiting, diarrhea, intestinal tumor, malabsorption, cancer treatment, chemotherapy, radiotherapy, high dose laxative use Renal lost Vomiting, diarrhea, intestinal tumor, malabsorption, cancer treatment, chemotherapy, radiotherapy, high dose laxative use Cellular Schift Alkolosis, insülin overdose Decrease intake

Chloride (Cl) v. Cl is the major anion in the extracellular fluid. v. It is responsible for providing osmotic pressure of extracellular fluid. v. It is a major anion of gastric fluid. v. Participates in the production of gastric acid. v. It is excreted via urine and gaita.

REFERENCES 1. Electrolytes Joani E. Polancic, Chapter 14 pages 297 -300 2. Hiponatremi; Güncel Tanı ve Tedavisi Hyponatremia; Current Diagnosis and Treatment, Gürsel YILDIZ Mansur KAYATAŞ Ferhan Candan, Turk Neph Dial Transpl 2011; 20 (2): 115 -131 3. Copper absorption and bioavailability, Raul A Wapnir. Am J Clin Nutr 1998; 67: 1054 S-60 S. 4. R. Hossain, D. D. Grier, Copper Deficiency, Journal of Science and Medicine 5. Calcium Metabolism in Health and Disease, Munro Peacock, CJASN January 2010, 5 (Supplement 1) S 23 -S 30; DOI: https: //doi. org/10. 2215/CJN. 05910809, Clinical Journal of the American Society of Nephrology. Vol. 5, Issue Supplement 11 Jan 2010 6. Fong J 1, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. 2012 Feb; 58(2): 158 -62 7. R Swaminathan, Magnesium Metabolism and its Disorders, Clin Biochem Rev. 2003 May; 24(2): 47– 66. 8. Murray J Favus, David A. Bushinsky, Jacob, Chapter 13. Regulation of Calcium, Magnesium, and Phosğhate Metabolism, American Society for Bone and Mineral Research, 2006. 9. Review on iron and its importance for human health, Nazanin Abbaspour, Richard Hurrel, Roya Kelishadi, J Res Med Sci. 2014 Feb; 19(2): 164– 174 10. Transfus Med Hemother. 2014 Jun; 41(3): 213– 221, Physiology of Iron Metabolism, Sophie Waldvogel. Abramowski, a Gérard Waeber, b Christoph Gassner, c Andreas Buser, d Beat M. Frey, c Bernard Favrat, e and Jean-Daniel Tissota, * 11. Şule Mine BAKANAY ÖZTÜRK, Iron Metabolism, Turkiye Klinikleri J Hematol-Special Topics. 2017; 10(3): 161 -6 12. Biochemistry, Iron Absorption, Thomas Ems, Martin R. Huecker 13. Maret W 1. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013 Jan 1; 4(1): 82 -91. 14. Pfeiffer CC, Braverman ER. Zinc, the brain and behavior. Biol Psychiatry. 1982 Apr; 17(4): 513 -32. 15. Nazanin Roohani, Richard Hurrell, Roya Kelishadi, Rainer Schulin, Zinc and its importance for human health: An integrative review, J Res Med Sci. 2013 Feb; 18(2): 144– 157. 16. Taiho Kambe, Tokuji Tsuji, Ayako Hashimoto, Naoya Itsumura, The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism, 2015, https: //doi. org/10. 1152/physrev. 00035. 2014
 Difference between organic and inorganic
Difference between organic and inorganic Organic vs inorganic compounds
Organic vs inorganic compounds Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Importance of inorganic chemistry in pharmacy
Importance of inorganic chemistry in pharmacy Importance of organic compounds
Importance of organic compounds What is inorganic matter
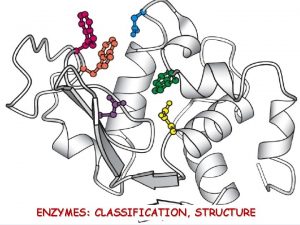
What is inorganic matter Enzim
Enzim Iron deficiency anemia smear
Iron deficiency anemia smear Ionic vs covalent venn diagram
Ionic vs covalent venn diagram Inorganic non metallic materials examples
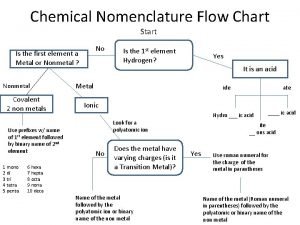
Inorganic non metallic materials examples Nomenclature flow chart chemistry
Nomenclature flow chart chemistry Inorganic gases
Inorganic gases What is smear layer
What is smear layer Organic matrix of bone
Organic matrix of bone Conclusion of anemia ppt
Conclusion of anemia ppt Fajans rule
Fajans rule Inorganic vs organic
Inorganic vs organic Inorganic growth disadvantages
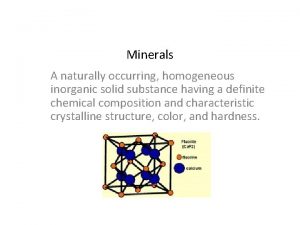
Inorganic growth disadvantages Inorganic mineral definition
Inorganic mineral definition Difference between cold and vanishing cream
Difference between cold and vanishing cream The pros and cons of pesticides
The pros and cons of pesticides Inorganic nomenclature flow chart
Inorganic nomenclature flow chart Inorganic plants
Inorganic plants Gravimetric methods of analysis
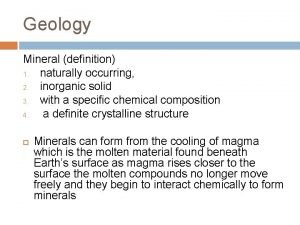
Gravimetric methods of analysis Inorganic geology definition
Inorganic geology definition Inorganic nomenclature worksheet
Inorganic nomenclature worksheet Veins are often formed from hot water solutions
Veins are often formed from hot water solutions What is enzymes
What is enzymes Protonic and non protonic solvents
Protonic and non protonic solvents Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Homogeneous inorganic substances
Homogeneous inorganic substances Unsaturated hydrocarbon
Unsaturated hydrocarbon Organic and inorganic cofactors
Organic and inorganic cofactors Naturally occurring inorganic solid material
Naturally occurring inorganic solid material Organic and inorganic nutrients
Organic and inorganic nutrients Organic vs inorganic chemistry
Organic vs inorganic chemistry Coprecipitation errors
Coprecipitation errors Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Organic and inorganic cofactors
Organic and inorganic cofactors What is inorganic pollution
What is inorganic pollution Inorganic nomenclature
Inorganic nomenclature Inert pair effect
Inert pair effect Silicones and phosphazenes
Silicones and phosphazenes Inorganic content of calculus
Inorganic content of calculus Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme Organic vs inorganic
Organic vs inorganic Asam oksalat rumus kimia
Asam oksalat rumus kimia Organic vs inorganic
Organic vs inorganic Organic growth vs inorganic growth
Organic growth vs inorganic growth Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Induced fit model
Induced fit model Organic vs inorganic
Organic vs inorganic Naming binary ionic compounds
Naming binary ionic compounds What compounds do cells need?
What compounds do cells need? Ternary ionic compounds
Ternary ionic compounds Ionic charge of magnesium
Ionic charge of magnesium What is the classification of organic compounds
What is the classification of organic compounds Tannin
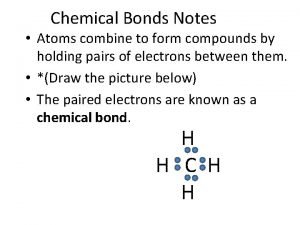
Tannin Atoms combine to form compounds
Atoms combine to form compounds Chemical formula vs molecular formula
Chemical formula vs molecular formula Classification of heterocyclic compounds
Classification of heterocyclic compounds Alkane alkene alkyne
Alkane alkene alkyne Lithium and nitrogen formula
Lithium and nitrogen formula All compounds are molecules
All compounds are molecules Verbal compounds examples
Verbal compounds examples Covelant compounds
Covelant compounds How are ionic compounds held together
How are ionic compounds held together Is titanium multivalent
Is titanium multivalent Molecule vs compound
Molecule vs compound Is o2ionic or covalent
Is o2ionic or covalent Unscramble loyal
Unscramble loyal Two types of compounds
Two types of compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Binary molecular compounds are made of two
Binary molecular compounds are made of two