Minerals Humans cannot survive without minerals 16 minerals


















- Slides: 31

Minerals

Humans cannot survive without minerals • 16 minerals needed for humans to survive • . 03% of what we eat but we would not survive without the minerals • Sodium, potassium, calcium, magnesium, copper, phosphorous


Minerals make-up many practical parts of our lives

Glass is made from 6 minerals • • Silica Limestone Magnesium Boric acid Soda Aluminum 40 billion glass containers/year in USA • 35 % are recycled

Gold in California • Discovered in the American River, 1848 • Gold Rush- 1849 • Population of SF- 575 males, 177 females, 60 children- March, 1848 • 100, 000 - December 1849 • Chinese, Welsh, German, English, Mexican, Spanish and French • Diversity of California

Salt • Early people collected salt before they understood how important the mineral is for survival • Mediterranean-salt cakes were used as money • Greeks traded salt for slaves • England flourished when fuel for boiling brine changed from wood to coal

Minerals are mined for our use Magmatic copper, magnetite, uranium

What is a mineral? • Naturally occurring • Inorganic • Crystalline structure Halite, salt, sodium chloride

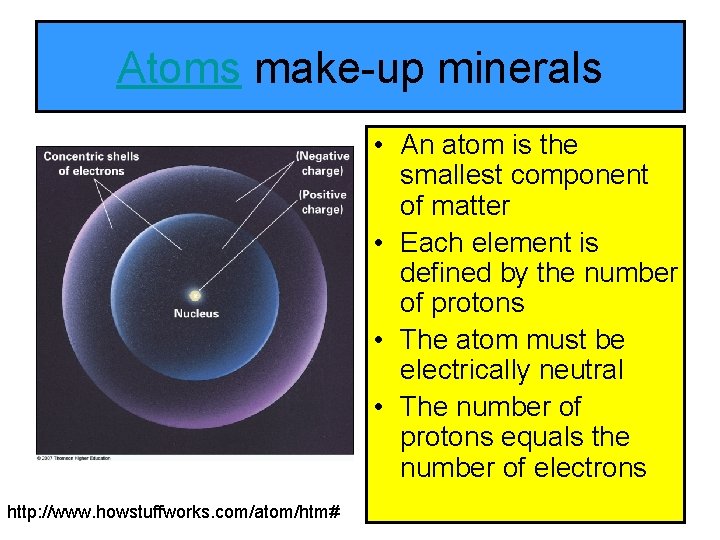
Atoms make-up minerals • An atom is the smallest component of matter • Each element is defined by the number of protons • The atom must be electrically neutral • The number of protons equals the number of electrons http: //www. howstuffworks. com/atom/htm#

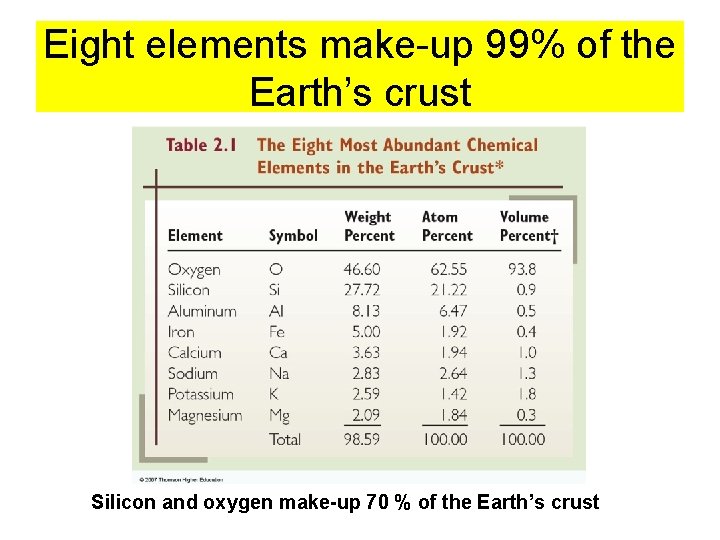
Eight elements make-up 99% of the Earth’s crust Silicon and oxygen make-up 70 % of the Earth’s crust

Why do atoms combine to form minerals? • Write the following questions and answer in your notes: • Look at the salt with the hand lens • What is the color? • What is the shape of the crystals? • Does the shape match the halite crystals? • What is a distinguishing characteristic of salt?

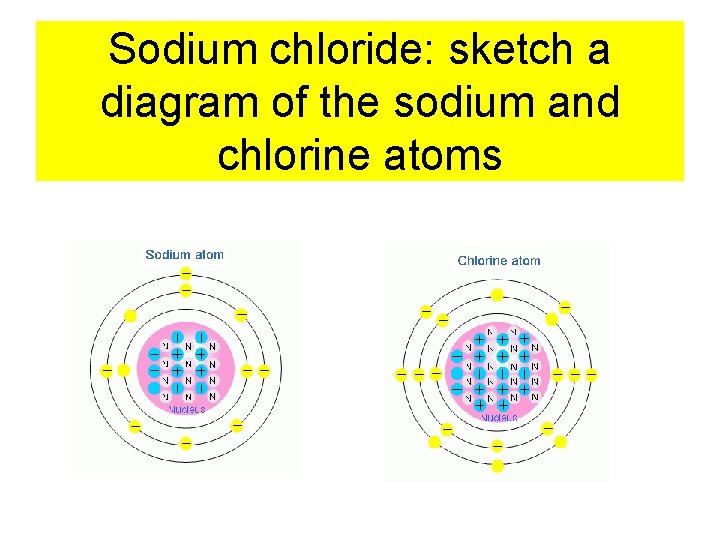
Salt is also named Sodium Chloride • Look at the periodic table: • Write the chemical notation, atomic number and atomic weight for sodium and chlorine • Now sketch an atom with the nucleus containing the correct number of protons and neutrons • Sketch the electrons on the “rings” around the nucleus

Sodium chloride: sketch a diagram of the sodium and chlorine atoms

Why do sodium and chlorine combine to form salt? • Explain why sodium and chlorine combine to form salt

The configuration of electrons determines if an atom will respond with another atom The sodium atom has one electron on its outer ring. The Chlorine atom has 7 electrons on its outer ring. The two atoms share electrons forming an ionic bond.

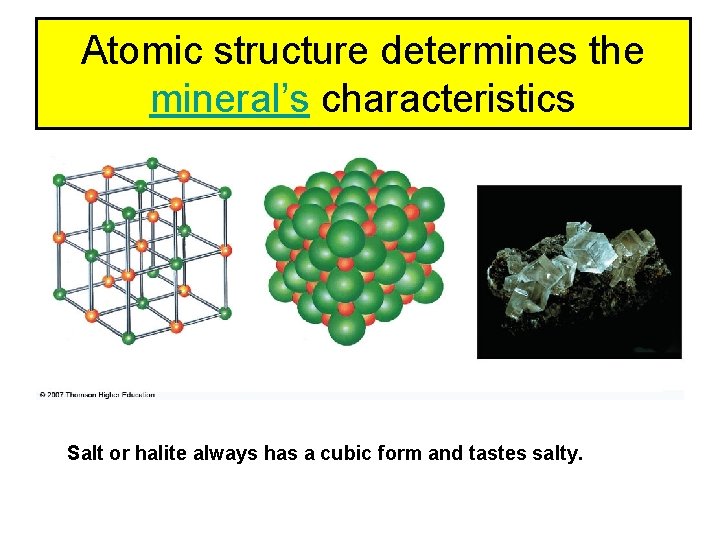
Atomic structure determines the mineral’s characteristics Salt or halite always has a cubic form and tastes salty.

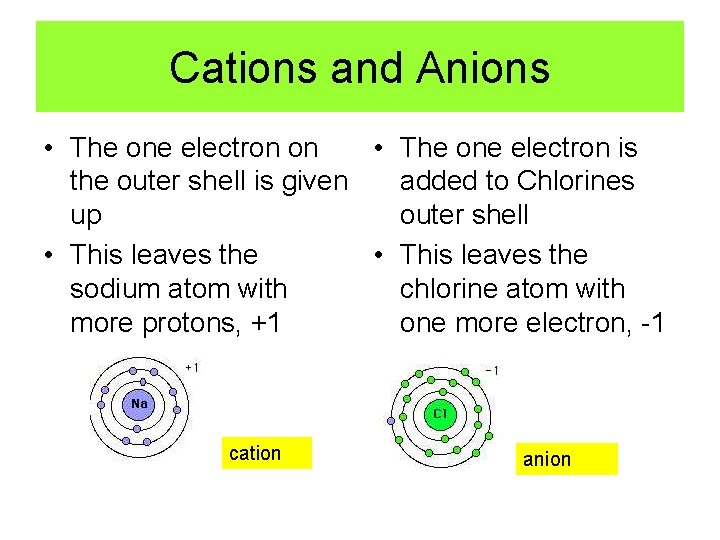
Cations and Anions • The one electron on • The one electron is the outer shell is given added to Chlorines up outer shell • This leaves the sodium atom with chlorine atom with more protons, +1 one more electron, -1 cation anion

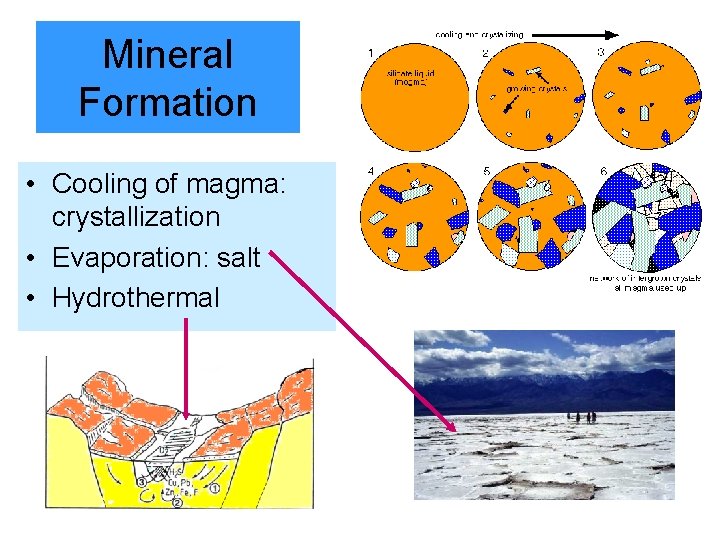
Mineral Formation • Cooling of magma: crystallization • Evaporation: salt • Hydrothermal

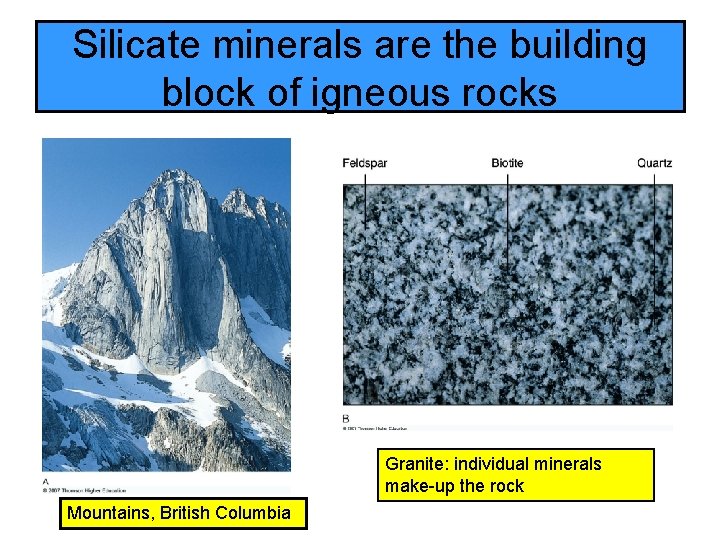
Silicate minerals are the building block of igneous rocks Granite: individual minerals make-up the rock Mountains, British Columbia

Minerals are the building block of rocks Quartz Biotitie Feldspar crystal Hornblende

Minerals can be identified by physical properties • Crystal habit • Cleavage • Fracture Quartz has a conchoidal fracture Equant garnet: same dimension in all directions Mica has a single, perfect cleavage

Color malachite apatite sulfur

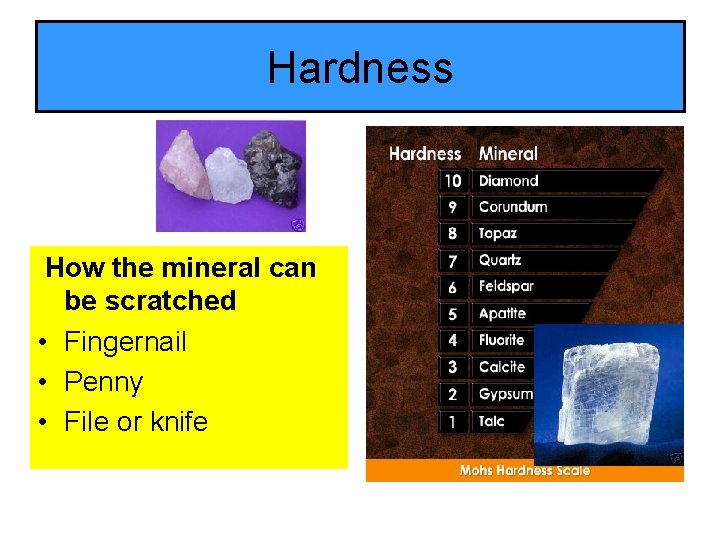
Hardness How the mineral can be scratched • Fingernail • Penny • File or knife

Streak • Minerals leave a distinct residue on a porcelain plate

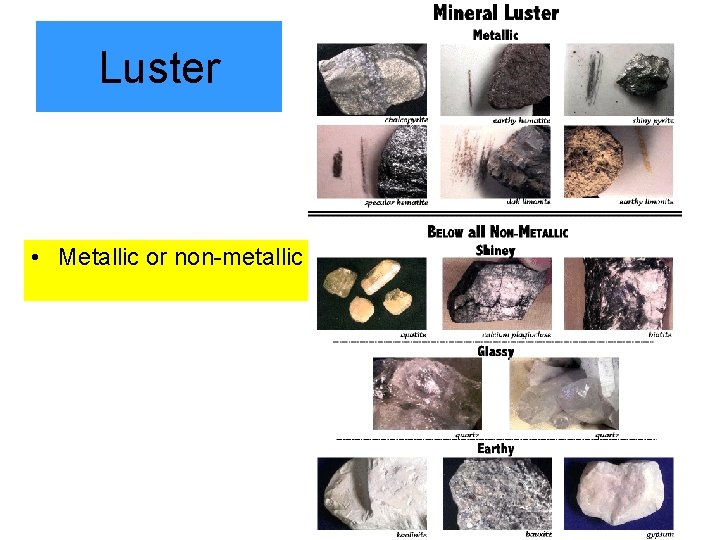
Luster • Metallic or non-metallic

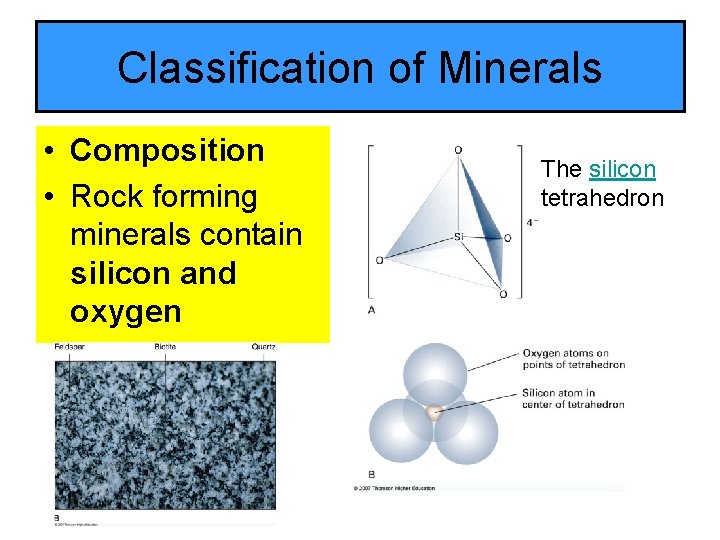
Classification of Minerals • Composition • Rock forming minerals contain silicon and oxygen The silicon tetrahedron

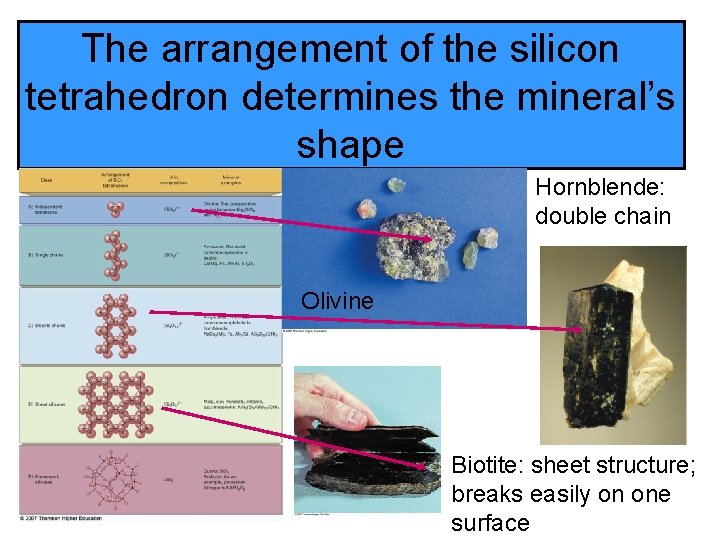
The arrangement of the silicon tetrahedron determines the mineral’s shape Hornblende: double chain Olivine Biotite: sheet structure; breaks easily on one surface

Mineral Classification: based on dominant element Sulfides: contains the element sulfur Pyrite: fool’s gold; Fe. S 2 Galena: Pb. S; important ore of lead

Mineral Classification: based on dominant element • Carbonates: contains calcium carbonate; Ca. CO 3 Calcite: Ca. Co 3 Dolomite: Ca. Mg(CO 3)2

Summary • Mineral definition • How do minerals form? • Read and understand information associated with the periodic table of the elements • Be able to sketch a diagram of an atom: hydrogen, helium, carbon, sodium, chlorine • How are minerals classified: rock forming minerals? Silicates, sulfides, carbonates • Name and describe the physical characteristics to distinguish minerals.