Introduction to the World of Chemistry By Dr

Introduction to the World of Chemistry By, Dr. Sophia K. Phillip Assistant Professor in Chemistry Christian College, Chengannur

Welcome to the World of Chemistry

Chemistry our life and our future

v. Celebrated the 100 th anniversary of the founding of the International Association of Chemical Societies (precursor to IUPAC).

objectives Increase the public appreciation of chemistry in meeting world needs (ck X {´ s¯ ¡p dn¨v tem. I P X ¡pÅ Adnhv hfÀ¯n Cu imkv{X im J sb ¡p dn¨v a. Xn ¸p m ¡p I).

v Increase interest of young people in chemistry (Ip «n Ifnepw bph P § fnepw ck X{´ Xm ]cyw hÀ²n ¸n ¡p I). Generate enthusiasm for the creative future of chemistry (ck X {´ ¯nsâ kÀKm ßI (Creative) amb `mhn sb ¸än P § fn Bth i ap m ¡p I).

v. Celebrate the role of women in chemistry and major historical events in chemistry, including the centenaries of Mme. Curie’s Nobel Prize

Marie Curie and Pierre Curie Madame Curie was awarded Nobel Prize two times. Her husband Pierre Curie, daughter Irene Curie& her husband Frederic Joliot Curie also received Nobel Prizes. Their family was blessed with Nobel Prize several times!!!! They dedicated their lives for the welfare of human Kind.

She introduced the Magic metal radium to the world


Let me dedicate this lecture to the memory of Madame Curie whose life is an inspiration to all of us

Chemistry is an experimental, creative, central science
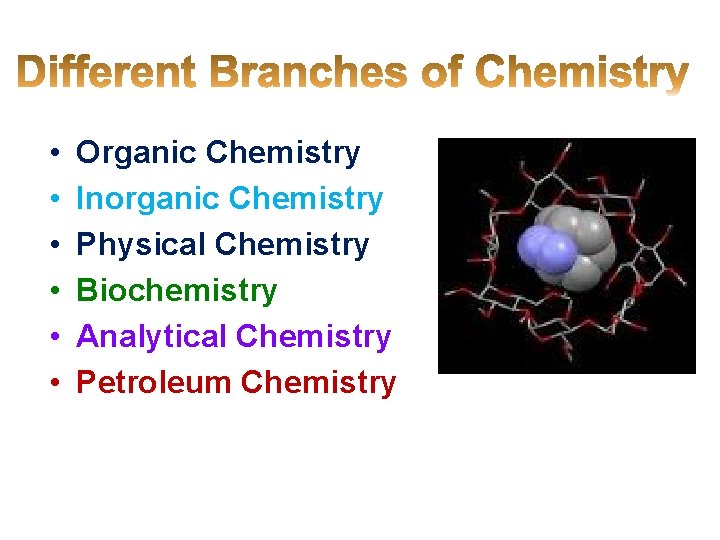
• • • Organic Chemistry Inorganic Chemistry Physical Chemistry Biochemistry Analytical Chemistry Petroleum Chemistry
• Dye Chemistry • • Industrial Chemistry Environmental Chemistry Green Chemistry Polymer Chemistry Radiation Chemistry Photochemistry Electrochemistry
![DWÀs¶gp t¶ ¡p ¶Xp ap. X Dd §p ¶ Xp hsc D] tbm Kn DWÀs¶gp t¶ ¡p ¶Xp ap. X Dd §p ¶ Xp hsc D] tbm Kn](http://slidetodoc.com/presentation_image/e52ae6e4448f0b0967072b2a7b0c7594/image-15.jpg)
DWÀs¶gp t¶ ¡p ¶Xp ap. X Dd §p ¶ Xp hsc D] tbm Kn ¡p¶ hkv. Xp ¡ fn te Xn se ¦nepw ck X {´ ¯nsâ ss. I ]Xn bm ¯ Xpt m? {_jv, t]Ìv, _¡ äv, Sm¸v, a. Kv, ¥mkv, tkm¸v, Pew, `£ Ww, hkv{Xw, ]u. UÀ, s]À^yqw. . cmk amen y §Ä, hnj ¸p I, Io. S m in n IÄ, cmk h kv. Xp ¡Ä, kvt^m. SI hkv. Xp ¡Ä, cmkm bp [ §Ä, ¹mÌn Iv FÃmw ck X {´ ¯nsâ kw`m h IÄ Xs¶.

BUILDING MATERIALS MEDICINES CLOTHING POLYMERS FERTILIZERS PAINTS GRAINS COSMETICS Means, anything & everything in the universe… EXPLOSIVES INSECTICIDES PRESERVATIVES ALL COSMIC OBJECTS FUELS INSULATORS NANO MATERIALS CONDUCTORS SEMICONDUCTORS METALS AND ALLOYS HIGH TEPT. SUPERCODUCTORS

Chemistry has given us so much!! We can expect more in Future

United Nations Millennium Goals Goal 1: Eradicate extreme poverty and hunger Goal 2: Achieve universal primary education Goal 3: Promote gender equality and empower women Goal 4: Reduce child mortality Goal 5: Improve maternal health Goal 6: Combat HIV/AIDS, malaria and other diseases Goal 7: Ensure environmental sustainability Goal 8: Develop a Global Partnership for Development CHEMISTRY is vital to achieving these goals!

A Brief History of Chemistry It is said that science began with the Greeks. • Chemistry A branch of science that has been around for a long time. Date back to the prehistoric times. Split into four general chronological categories. prehistoric times - beginning of the Christian era (black magic) • beginning of the Christian era - end of 17 th century (alchemy), • end of 17 th century - mid 19 th century (traditional chemistry) and • mid 19 th century - present (modern chemistry) • •

• Who was the first chemist? • The first known chemist was a woman. • A Mesopotamian cuneiform tablet from the second millenium B. C. describes Tapputi, a perfumer and palace overseer who distilled the essences of flowers and other aromatic materials, filtered them, added water and returned them to the still several times until she got just what she wanted. • This is also the first known reference to the process of distillation and the first recorded still. Cuneiform tablet

Prehistoric Times - Beginning of the Christian Era Black Magic v 1700 BC v King Hammurabi's reign over Babylon v Known metals such as gold, silver, mercury, lead, tin, iron and copper were matched with the brightest heavenly bodies: Sun, Moon, Mercury, Saturn, Jupiter, Mars and Venus respectively. These symbols are used today by astronomers as the symbols for planets.
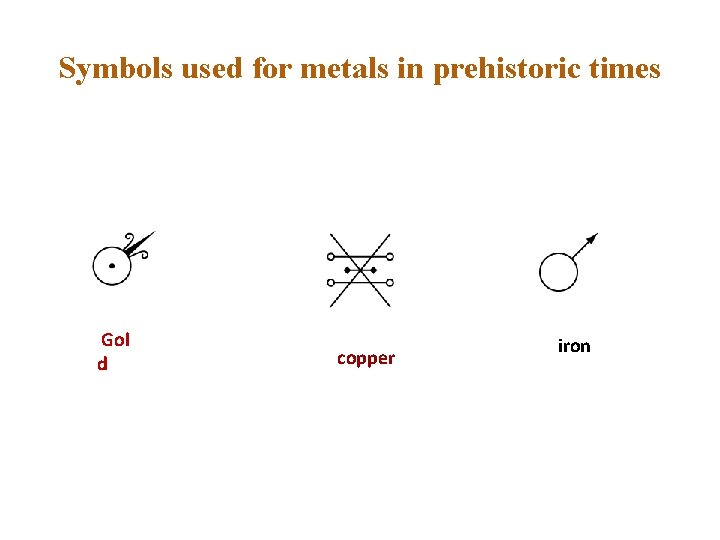
Symbols used for metals in prehistoric times Gol d copper iron
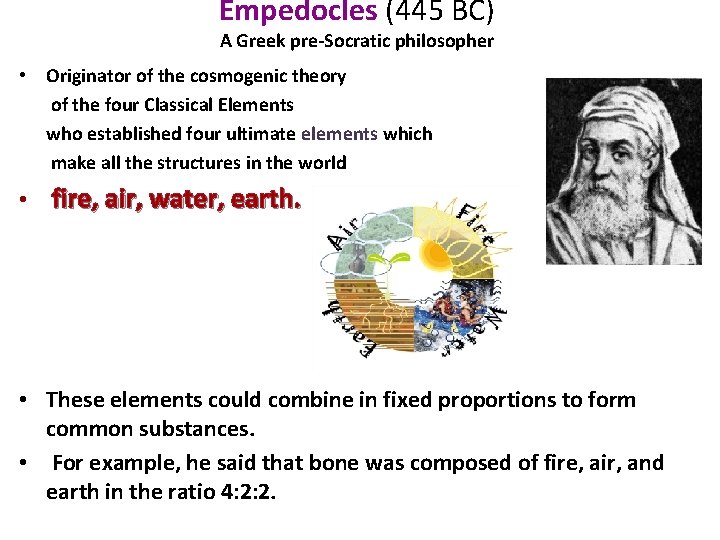
Empedocles (445 BC) A Greek pre-Socratic philosopher • Originator of the cosmogenic theory of the four Classical Elements who established four ultimate elements which make all the structures in the world • fire, air, water, earth. • These elements could combine in fixed proportions to form common substances. • For example, he said that bone was composed of fire, air, and earth in the ratio 4: 2: 2.

430 BC Democritus of ancient Greece Developed an early atomic theory. Elements were composed of tiny indivisible corpuscles called atoms (atomom is Greek for indivisible) that moved in empty space. • Atoms varied in shape and size and combined in various ways to produce different substances. • For example, substances that have a sour taste are composed of angular, small, thin atoms • substances that taste sweet are round, moderatesized atoms. • •

Prehistoric Times - Beginning of the Christian Era (Black Magic) Aristotle of ancient Greece (384 -323 BC) • Developed the idea of properties of the elements, saying different types of matter depend on a specific balance of the qualities of hot, cold, wet, and dry. • He reasoned that • there must be a way to change one element into another by modifying its balance. The idea really caught on, especially that of changing cheap metals to gold, and persisted for nearly 2, 000 years.

Proto-Chemistry • Alchemy is considered a prechemistry, or protochemistry. • Through alchemy we have gotten herbal remedies, alloys, and most importantly a desire to manipulate the world around us! • The alchemists learned how to use metallic compounds and plant-derived materials to treat diseases.

Alchemist Elixir of life • Their main goal was the transmutation of ordinary metals into gold. • They also wanted to find a chemical concoction that would enable people to live longer and cure all ailments. This elixir of life never happened either.

13 th Century (1200's) - 15 th Century (1400's) • Failure of the Gold Business • Although Pope John XXII issued an edict against gold-making, the gold business continued. • Despite the alchemists' efforts, transmutation of cheap metals to gold never happened within this time period.

Beginning of the Christian Era -End of 17 th Century (Alchemy ) • 300 BC -300 AD • The Advent of the Alchemists • Influenced greatly by Aristotle's ideas, alchemists attempted to transmute cheap metals to gold. • The substance used for this conversion was called the Philosopher’s stone. Salt, sulphur, and mercury were some of the main ingredients they used.

End of 17 th Century Death of Alchemy The disproving of Aristotle's four-elements theory and the publishing of the book, The Skeptical Chymist (by Robert Boyle), combined to destroy this early form of chemistry.
Robert Boyle 1627 -91 The first modern chemist One of the founders of modern chemistry, and one of the pioneers of modern experimental scientific method. He is best known for Boyle's law. Among his works, The Sceptical Chymist is seen as a cornerstone book in the field of chemistry.

The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes Title of Robert Boyle's masterpiece of scientific literature • Published in London in 1661. • In the form of a dialogue, the Sceptical Chymist presented Boyle's hypothesis • That matter consisted of atoms and clusters of atoms in motion • Every phenomenon was the result of collisions of particles in motion. Title page

End of 17 th Century - Mid 19 th Century (Traditional Chemistry) 1700's Phlogiston Theory Johann J. Beecher (1635 -1682) He believed in a substance called phlogiston. When a substance is burned, phlogiston was supposedly added from the air to the flame of the burning object. • In some substances, a product is formed. • • •

Air is not an elementary substance Joseph Priestley, 1733 -1804 • In 1774 • Priestley found that "air is not an elementary substance, but a composition, " or mixture, of gases. • Some 2, 500 years ago, the ancient Greeks identified • air — along with earth, fire and water — as one of the four elemental components of creation. • The idea persisted until the late 18 th century. • It was changed by Joseph Priestley.

Discovery of Oxygen Priestley heated mercuric oxide (calx of mercury) by focussing sun’s rays and he named the gas obtained as "dephlogisticated air" which was actually oxygen • He invented carbonated water and the rubber eraser, identified a dozen key chemical compounds • Wrote one of the first comprehensive treatises on electricity. • But the world recalls Priestley best as the man who discovered oxygen, the active ingredient in our planet's atmosphere. • In the process, he helped dethrone an idea that dominated science for 23 uninterrupted centuries:

• It was Antoine Lavoisier who disproved the Phlogiston Theory. • He renamed the "dephlogisticated air" oxygen when he realized that the oxygen was the part of air that combines with substances as they burn. • Because of Lavoisier's work, Lavoisier is now called the "Father of Modern Chemistry". • Lavoisier demonstrated the role of oxygen in the rusting of metal, as well as oxygen's role in animal and plant respiration.

Lavoisier's Laboratory, Paris

John Dalton (1766 – 1844), an English chemist Best Known for his • pioneering work in the development of modern atomic theory • research into colour blindness. • other works include Law of Multiple Proportions, Dalton's Law of Partial Pressures, Daltonism • 1803 • John Dalton publishes his Atomic Theory which states that all • matter is composed of atoms, which are small and indivisible. (Examination of his preserved eyeball in 1995 demonstrated that Dalton actually had a less common kind of colour blindness, deuteroanopia, in which medium wavelength sensitive cones are missing)

Five main points of Dalton's atomic theory • Elements are made of extremely small particles called atoms • Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. • Atoms cannot be subdivided, created, or destroyed. • Atoms of different elements combine in simple wholenumber ratios to form chemical compounds. • In chemical reactions, atoms are combined, separated, or rearranged. • Dalton's theory quickly became theoretical foundation in chemistry.
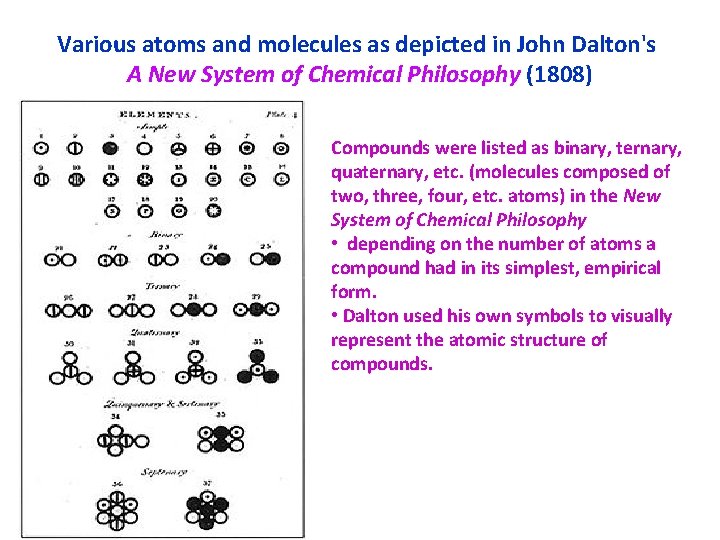
Various atoms and molecules as depicted in John Dalton's A New System of Chemical Philosophy (1808) Compounds were listed as binary, ternary, quaternary, etc. (molecules composed of two, three, four, etc. atoms) in the New System of Chemical Philosophy • depending on the number of atoms a compound had in its simplest, empirical form. • Dalton used his own symbols to visually represent the atomic structure of compounds.

THE GREATNESS OF ANCIENT INDIA’S DEVELOPMENTS VEDIC CHEMISTRY • Anu and Parmanu It was Kanada who first propounded that • the Parmanu (atom) was an indestrutible particle of matter. • According to him the material universe is made up of Kana. • When matter is divided and sudivided, we reach a stage beyond which no division is possible, this undivisible element of matter is Parmanu. • Kanada explained that this indivisible, indestructible parmanu cannot be sensed through any human organ.

• There are different types of Parmanu for the • five Pancha Mahabhootas, Earth, water, fire, air and ether. • Each Parmanu has a peculiar property which depends, on the substance to which it belongs. • In this context Kanada seems to arrived at conclusions which were surpassed only many centuries after him.
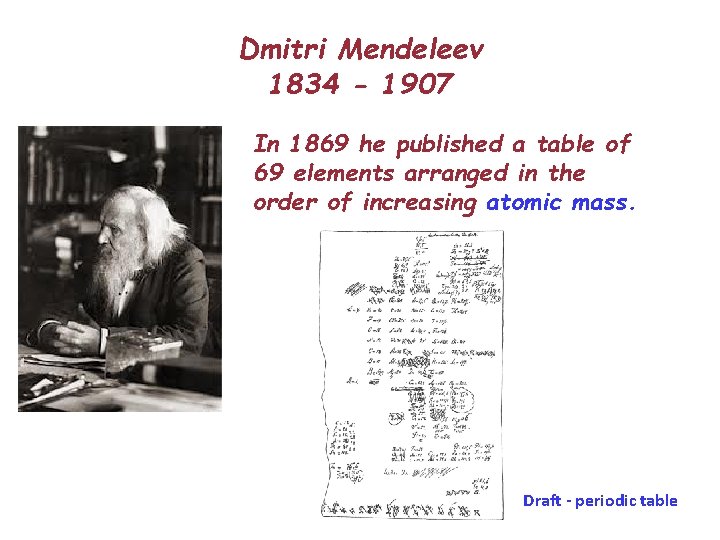
Dmitri Mendeleev 1834 - 1907 In 1869 he published a table of 69 elements arranged in the order of increasing atomic mass. Draft - periodic table

Mid 19 th Century -Present (Modern Chemistry or 20 th Century Chemistry) • 1854 Heinrich Geissler creates the first vacuum tube. • 1879 Cathode Rays William Crookes Made headway in modern atomic theory when he used the vacuum tube made by Heinrich Geissler to discover cathode rays. Crookes created a glass vacuum tube which had a zinc sulphide coating on the inside of one end, a metal cathode imbedded on the other end a metal anode in the shape of a cross in the middle of the tube. When electricity was run through the apparatus, an image of the cross appeared and the zinc sulphide glowed. Crookes hypothesized that there must have been rays coming from the cathode which caused the zinc sulphide to fluoresce and the cross to create a shadow and these rays were called cathode rays.

William Crookes 1832 -1919 Cathode-Ray Tube

Discharge Tube
cathode rays • For some decades whether cathode rays are waves or particles remained a matter of considerable controversy, with the German physicists strongly for waves and the English physicists strongly for particles. • One way to test between these two options was to see if these cathode rays were deflected by a magnet. • Light was not deflected by a magnet. • Both Julius Plucker (1801 to 1868) and Crookes performed this test • found that a magnet did deflect cathode rays. • Thus, these objects had to be particles • But what were they? Magnets affect metal objects, • so these cathode ray particles could be metal, or maybe they had a charge. If they were charged particles, then an electric field would deflect them. • Unfortunately, neither Plucker or Crooke were able to detect this effect.

The Proton 1885 • Eugene Goldstein (1850 – 1930) • Discovered positive particles by using a tube filled with • • hydrogen gas (similar to Thomson's tube). Tubes with a perforated cathode also emit a glow at the cathode end. In addition to the already-known cathode rays, there is another ray that travels in the opposite direction. The positive particle had a charge equal and opposite to the electron. It was named the proton.
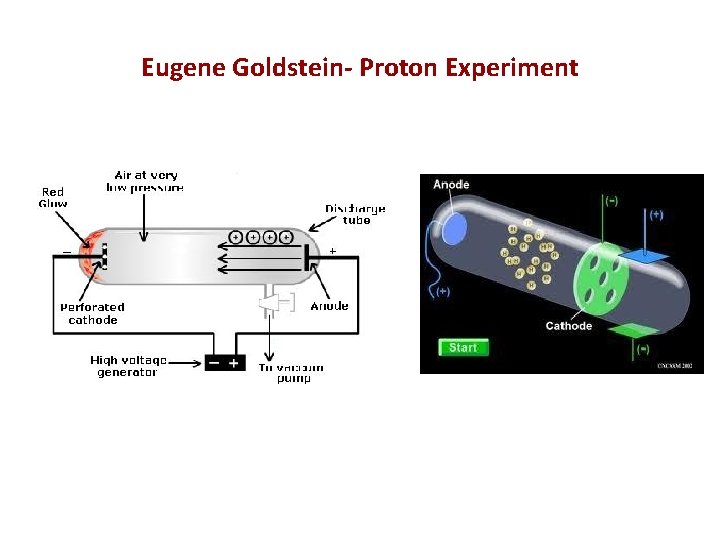
Eugene Goldstein- Proton Experiment
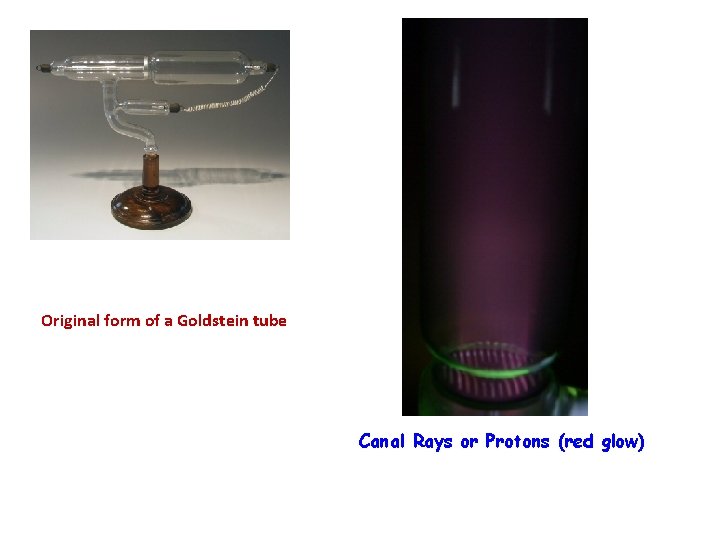
Original form of a Goldstein tube Canal Rays or Protons (red glow)

X-rays 1895 • Wilhelm Roentgen (1845 -1923) • Accidentally discovered x-rays while researching the glow produced by cathode rays. • Roentgen performed his research on cathode rays within a dark room • a thin aluminium window had been added to permit the cathode rays to exit the tube but a cardboard covering was added to protect the aluminium from damage by the strong electrostatic field that is necessary to produce the cathode rays • He noticed that a bottle of barium platinocyanide was glowing on a shelf. • He discovered that the rays that were causing the fluorescence could also pass through glass, cardboard and walls. • The rays were called x-rays.

WILHELM ROENTGEN 1845 -1923 • 1901 • Roentgen was awarded the very first Nobel Prize in Physics "in recognition of the extraordinary services he has rendered by the discovery of the remarkable rays subsequently named after him". • He donated the monetary reward from his Nobel Prize to his university. • Refused to take out patents related to his discovery, as he wanted mankind as a whole to benefit from practical applications of the same. • He did not even want the rays to be named after him
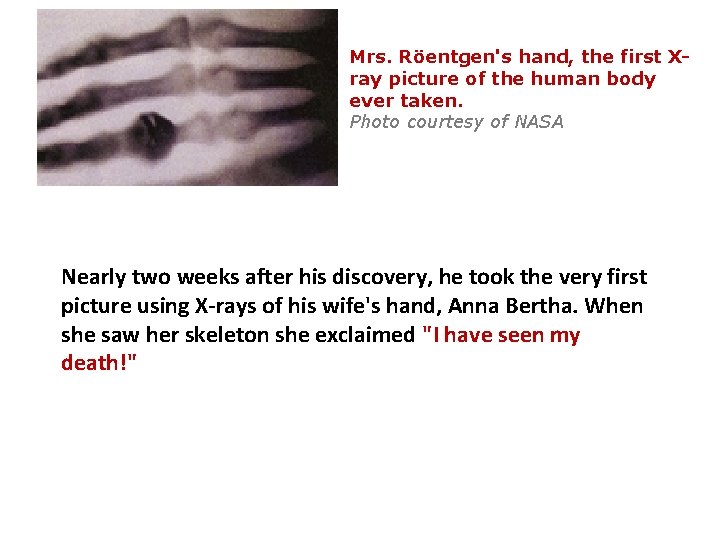
Mrs. Röentgen's hand, the first Xray picture of the human body ever taken. Photo courtesy of NASA Nearly two weeks after his discovery, he took the very first picture using X-rays of his wife's hand, Anna Bertha. When she saw her skeleton she exclaimed "I have seen my death!"

Radioactivity 1896 • While investigating phosphorescence in uranium salts, Becquerel accidentally discovered radioactivity. • Investigating the work of Roentgen, Becquerel wrapped a fluorescent substance, potassium uranyl sulphate, in photographic plates and black material in preparation for an experiment requiring bright sunlight. • However, prior to actually performing the experiment, Becquerel found that the photographic plates were fully exposed. This discovery led Becquerel to investigate the spontaneous emission of nuclear radiation. • For his discovery of spontaneous radioactivity Becquerel was awarded half of the Nobel Prize for Physics in 1903, the other half being given to Pierre and Marie Curie for their study of the Becquerel radiation.
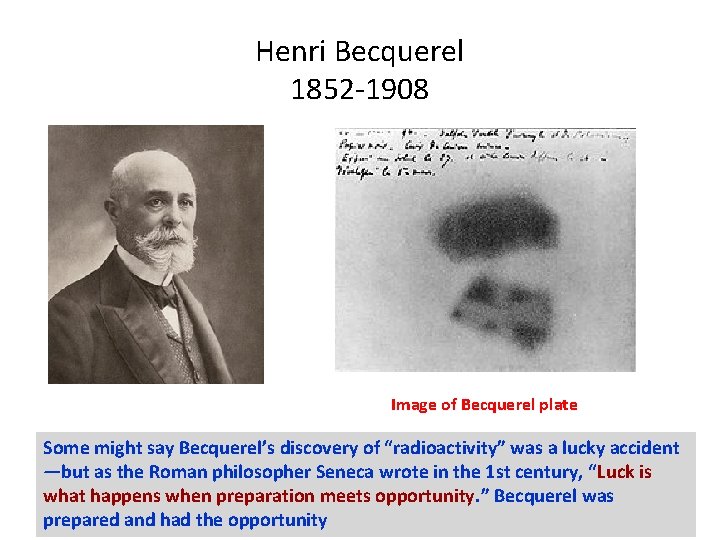
Henri Becquerel 1852 -1908 Image of Becquerel plate Some might say Becquerel’s discovery of “radioactivity” was a lucky accident —but as the Roman philosopher Seneca wrote in the 1 st century, “Luck is what happens when preparation meets opportunity. ” Becquerel was prepared and had the opportunity
1897 The Electron and Its Properties Radioactive Elements • J. J. Thomson placed the Crookes' tube within a magnetic field as well as an electric field. • He found that the cathode rays were negatively charged • Each charge had a mass ratio of 1. 759 x 10 -8 coulombs per gram. • He concluded that all atoms have this negative charge (through more experiments) • He renamed the cathode rays electrons. • Thomson received the 1906 Nobel Prize in physics.
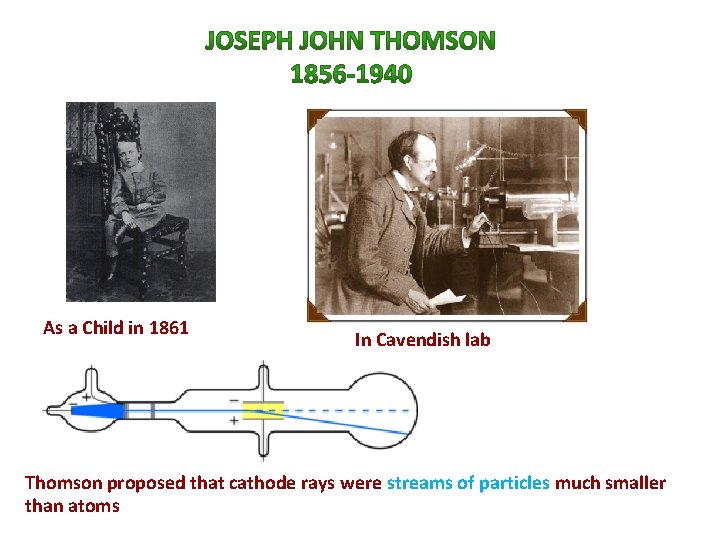
As a Child in 1861 In Cavendish lab Thomson proposed that cathode rays were streams of particles much smaller than atoms

• Thomson’s discovery meant that the atom was divisible! There was an assumption that all matter is neutral (equal amount of positive and negative). Therefore, if atoms contained negatively charged particles (the electrons) then there must be positive charges in the atom too! Thomson came up with his own “Atomic Theory”.

Thomson’s Plum-Pudding Model The positive charge is evenly spread out while the negative charge is in bits – like chocolate chips in cookies
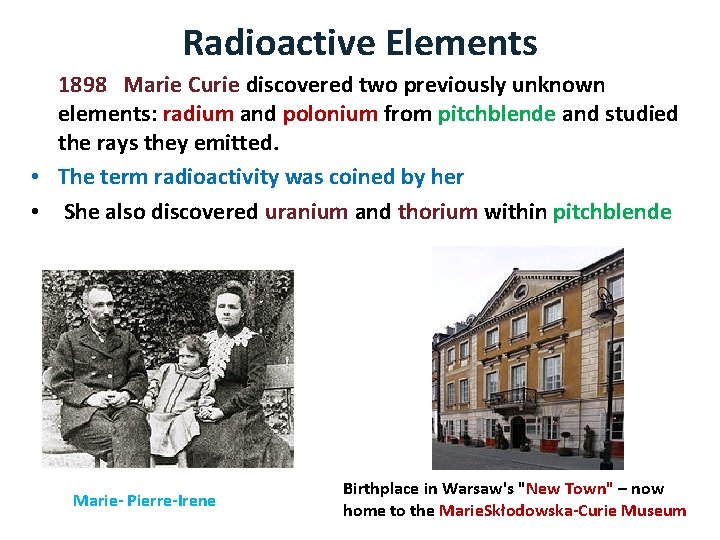
Radioactive Elements 1898 Marie Curie discovered two previously unknown elements: radium and polonium from pitchblende and studied the rays they emitted. • The term radioactivity was coined by her • She also discovered uranium and thorium within pitchblende Marie- Pierre-Irene Birthplace in Warsaw's "New Town" – now home to the Marie. Skłodowska-Curie Museum

Marie Skłodowska-Curie (1867 – 1934) She was the first woman to win a Nobel Prize, the only woman to win in two fields, and the only person to win in multiple sciences. She was also the first female professor at the University of Paris • Her co-discovery with her husband Pierre Curie of the radioactive elements radium and polonium represents one of the best known stories in modern science for which they were recognized in 1903 with the Nobel Prize in Physics. • In 1911, Marie Curie was honored with a second Nobel prize, this time in chemistry, to honour her for successfully isolating pure radium • •

Mass of the Electron 1909 • Robert Millikan • discovered the charge of an electron by introducing charged oil droplets into an electrically charged field. • The charge of the electron was found to be • 1. 602 x 10 -19 coulombs. • Using Thomson's mass ratio, Millikan found the mass of one electron to be 9. 11 x 10 -28 grams. • Millikan received the 1932 Nobel Prize in Physics for this discovery.
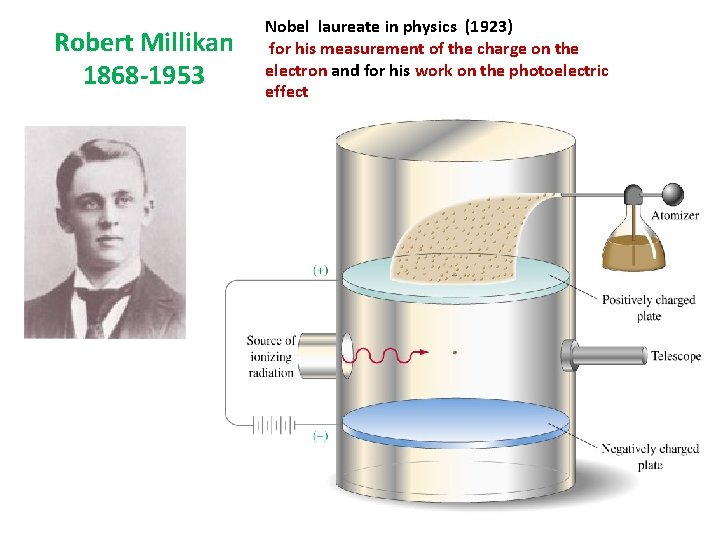
Robert Millikan 1868 -1953 Nobel laureate in physics (1923) for his measurement of the charge on the electron and for his work on the photoelectric effect
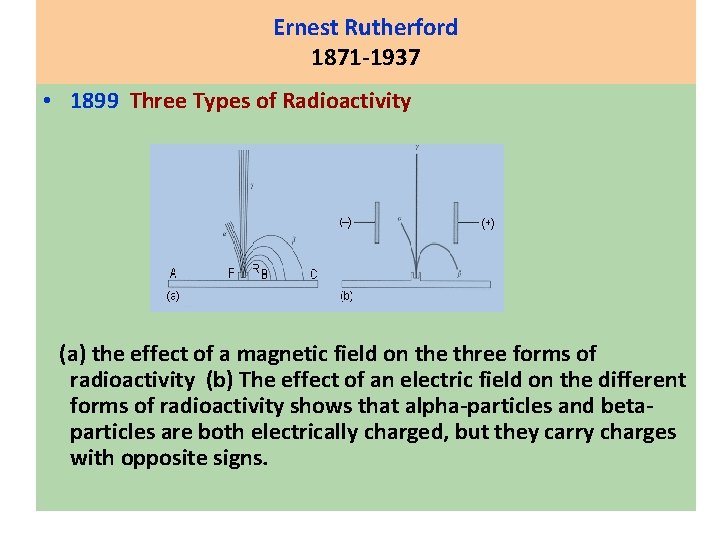
Ernest Rutherford 1871 -1937 • 1899 Three Types of Radioactivity (a) the effect of a magnetic field on the three forms of radioactivity (b) The effect of an electric field on the different forms of radioactivity shows that alpha-particles and betaparticles are both electrically charged, but they carry charges with opposite signs.

Ernest Rutherford 1871 -1937 Nobel prize in chemistry in 1908 At Mc. Gill University
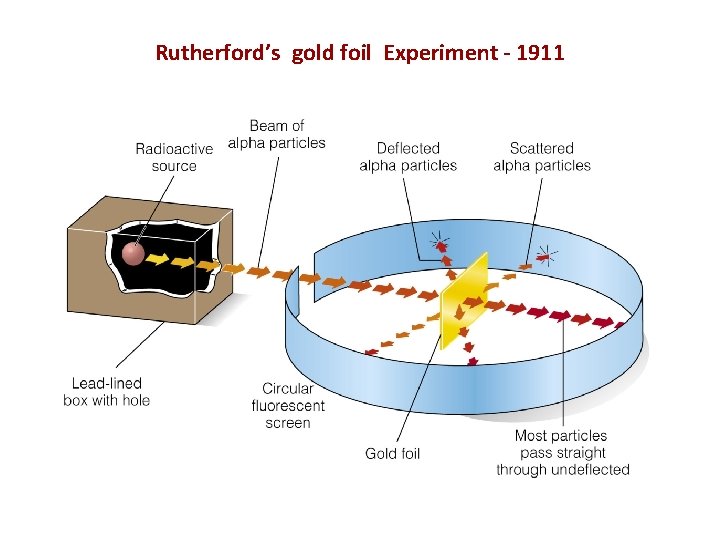
Rutherford’s gold foil Experiment - 1911
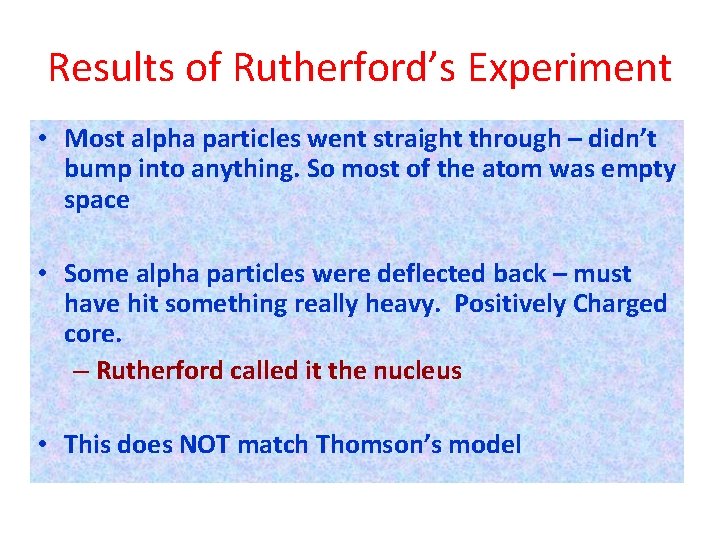
Results of Rutherford’s Experiment • Most alpha particles went straight through – didn’t bump into anything. So most of the atom was empty space • Some alpha particles were deflected back – must have hit something really heavy. Positively Charged core. – Rutherford called it the nucleus • This does NOT match Thomson’s model
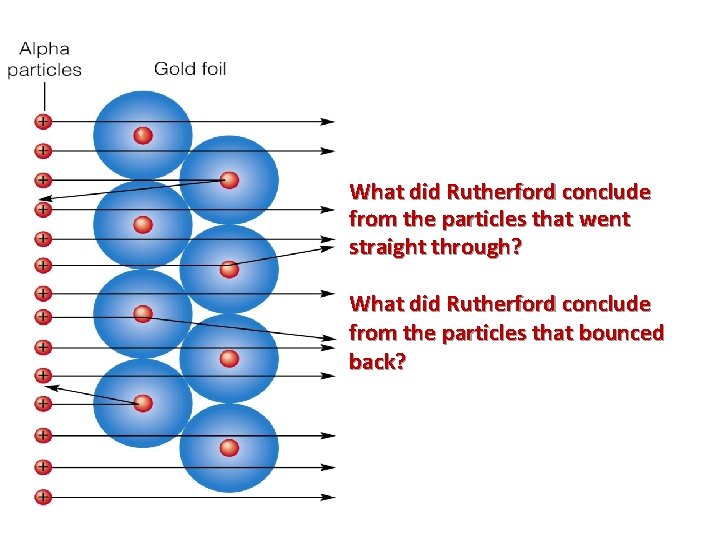
What did Rutherford conclude from the particles that went straight through? What did Rutherford conclude from the particles that bounced back?
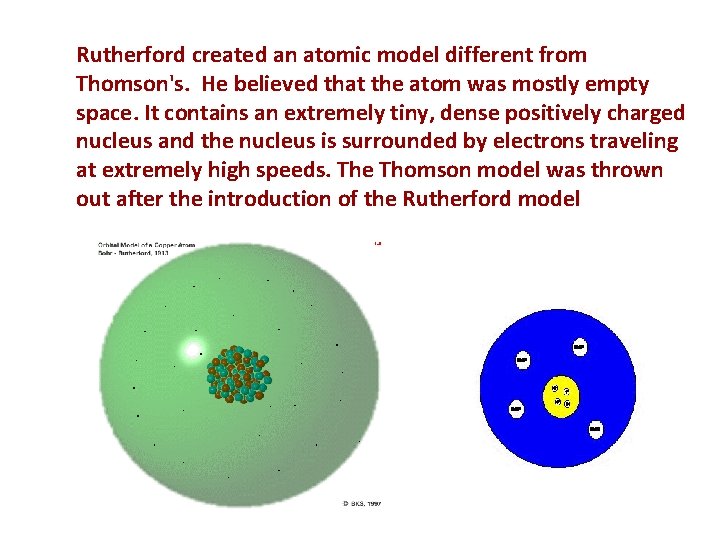
Rutherford created an atomic model different from Thomson's. He believed that the atom was mostly empty space. It contains an extremely tiny, dense positively charged nucleus and the nucleus is surrounded by electrons traveling at extremely high speeds. The Thomson model was thrown out after the introduction of the Rutherford model

Henry Moseley 1887 -1915 • In 1913, Moseley observed and measured the X-ray spectra of various chemical elements (mostly metals) • Discovered a systematic mathematical relationship between the wavelengths of the X-rays produced and the atomic numbers of the metals that were used as the targets in Xray tubes. This has become known as Moseley's law.
Henry Moseley When World War I broke out in Western Europe, Moseley left his research work at the University of Oxford behind to volunteer for the Royal Engineers of the British Army Moseley was assigned to the force of British Empire as a telecommunications officer. Moseley was shot and killed during the Battle of Gallipoli on 10 August 1915, at the age of 27. Moseley could have been awarded the Nobel Prize in Physics in 1916, had he not died in the service of the British Army.
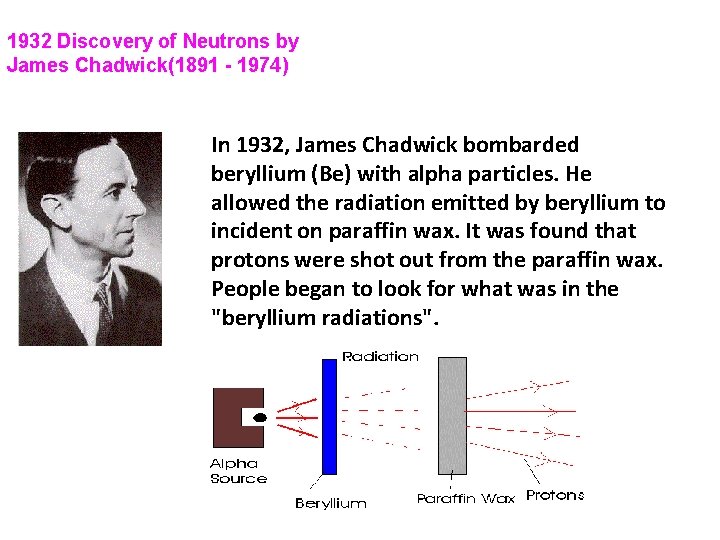
1932 Discovery of Neutrons by James Chadwick(1891 - 1974) In 1932, James Chadwick bombarded beryllium (Be) with alpha particles. He allowed the radiation emitted by beryllium to incident on paraffin wax. It was found that protons were shot out from the paraffin wax. People began to look for what was in the "beryllium radiations".
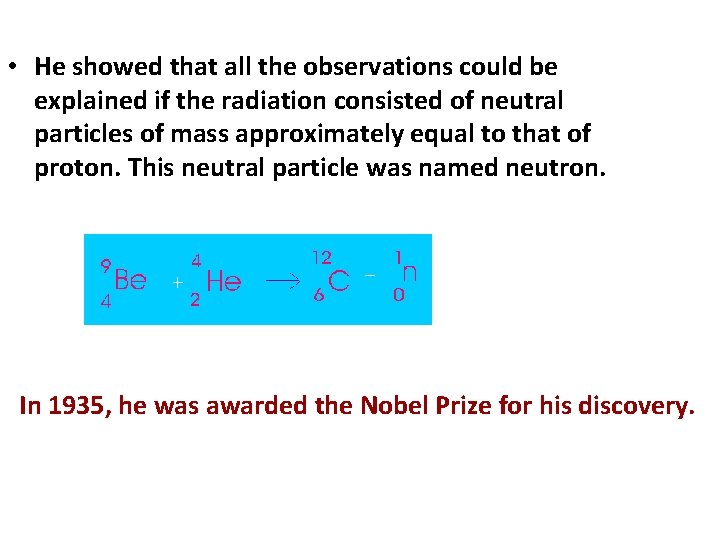
• He showed that all the observations could be explained if the radiation consisted of neutral particles of mass approximately equal to that of proton. This neutral particle was named neutron. In 1935, he was awarded the Nobel Prize for his discovery.
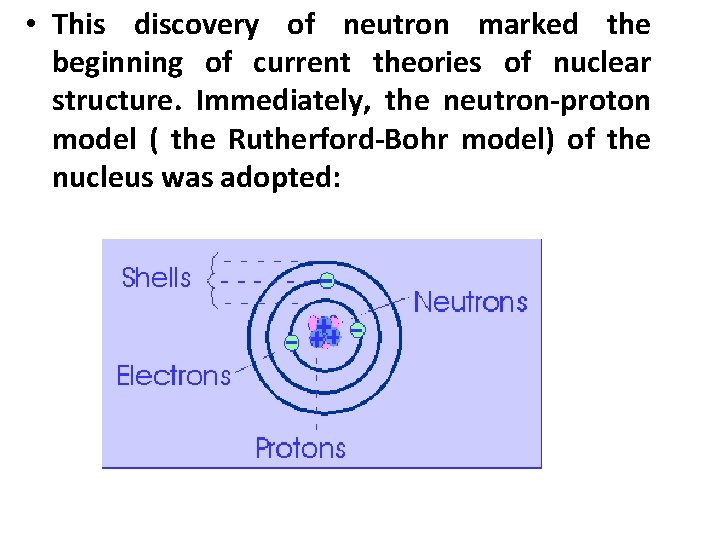
• This discovery of neutron marked the beginning of current theories of nuclear structure. Immediately, the neutron-proton model ( the Rutherford-Bohr model) of the nucleus was adopted:

The nuclear bomb • Neutrons are very penetrating because they are uncharged. • This makes them very useful to nuclear physicists, as they can be fired into the nucleus without being repelled like the proton. • A neutron can even be made to stop inside a nucleus, transforming elements into more massive types. • This understanding of the neutron allowed scientists to develop nuclear power, and nuclear weapons during the Second World War. • Chadwick helped in theory behind the first nuclear bombs, and used a particle accelerator in Liverpool

Enrico Fermi (1901 -1954) Nuclear fission • Enrico Fermi and his colleagues, during the early 1930's, • studied in detail theory of neutrons; • They bombarded most of the elements in the periodic table with them. • They slowed down the neutrons, and among other things, • produced a strange new product when bombarding uranium with neutrons • which later was recognized to be a splitting of the uranium atoms. • Nuclear fission occurred when Fermi bombarded uranium with neutrons. • He received the 1938 Nobel Prize in physics.

• At that time it was recognized that nuclear fission (the splitting of the atom) had taken place in Fermi's and other similar experiments. • Scientists felt that this principle might be applied to construct an "atomic bomb". • With World War II raging in Europe, the ability to produce such a bomb was of the greatest importance in the balance of power in the world. • Enrico Fermi moved to the University of Chicago to be in charge of the first major step in making feasible the building of the atomic bomb. • Fermi’s momentous accomplishments caused him to be recognized as one of the great scientists of the 20 th century.
Artificial Radioactivity • 1934 • Irene Curie and Frederic Joliot-Curie • Realized the alchemist's dream of turning one element into another. • They created radioactive nitrogen from boron and then radioactive isotopes of phosphorus from aluminium and silicon from magnesium. • They created artificial radioactive elements by bombarding the alpha particles (helium nuclei, He 2+) on various light elements. • These isotopes rapidly became important tools in biomedical research and in the treatment of cancer and related diseases. • They were given the 1935 Nobel Prize.

Irene Curie and Frederic Joliot-Curie

Manhattan Project • 1940’s • The Manhattan Project was a research and development program by the United States with the United Kingdom and Canada that produced • the first atomic bomb during World War II • Albert Einstein and Enrico Fermi both warned the United States about Germany's extensive research on atomic fission reaction. • Below the football field at the University of Chicago, the United States developed the very first working nuclear fission reactor.

• By the summer of 1945, weapon development and design were sufficiently far advanced so that an actual field test of a nuclear explosive could be scheduled. By this time the original $6, 000 authorized for the Manhattan Project had grown to $2 billion. • The first atomic bomb was exploded at 5: 30 AM on July 16, 1945, at a site on the Alamogordo air base, New Mexico. It was detonated on top of a steel tower surrounded by scientific equipment, with remote monitoring taking place in bunkers occupied by scientists and a few dignitaries 10, 000 yards (9 km) away. • The explosion came as an intense light flash, a sudden wave of heat, and later a tremendous roar as the shock wave passed and echoed in the valley. A ball of fire rose rapidly, followed by a mushroom cloud extending to 40, 000 feet (12, 200 metres). • The bomb generated an explosive power equivalent to 15, 000 to 20, 000 tons of TNT; the tower was completely vaporized and the surrounding desert surface fused to glass for a radius of 800 yards (730 metres). • The following month, two other atomic bombs produced by the project, the first using uranium-235 and the second using plutonium, were dropped on Hiroshima and Nagasaki , Japan.

The Trinity test
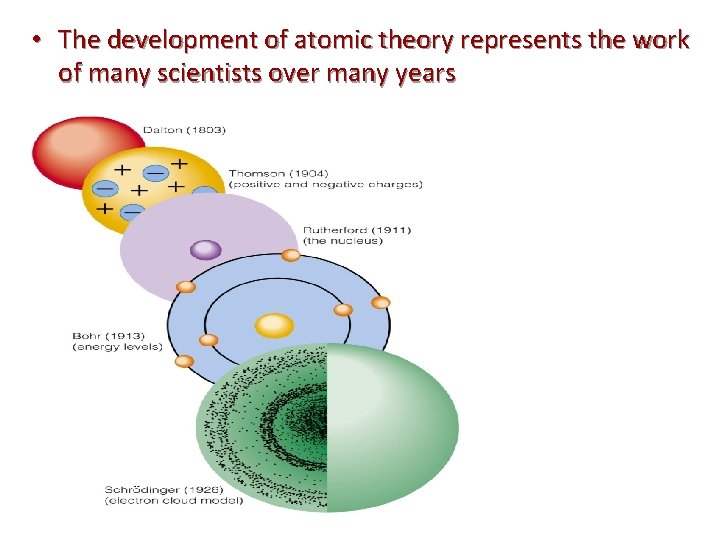
• The development of atomic theory represents the work of many scientists over many years

Educational Opportunities • • • At the postgraduate level Indian Institutes of Technology at Chennai, Delhi, Kanpur and Mumbai offer Chemistry. Postgraduate diplomas of one-year duration in related subjects are offered by some universities, e. g. , Analytical Chemistry – SNDT Women’s University (Mumbai), University of Mumbai; Industrial Chemistry – University of Pune, Sardar patel University (Gujarat), Textile Chemistry-Jai Narain Vyas University (Jodhpur), MS University of Baroda. Pre-doctoral and doctoral courses are available in most of the universities which offer M. Sc (Chemistry) course. Indian Institute of Science (Bangalore) has an integrated Ph. D programme in Chemical Science. Candidates possessing B. Sc degree with chemistry or biochemistry as one of the subjects at the B. Sc level and mathematics or chemistry or physics at the 10+2 level are eligible for the course. A number of research institutions including those under the Council of Scientific and Industrial Research (CSIR), such as, Indian Institute of Chemical Technology (Hyderabad), National Chemical Laboratory (Pune), Central Leather Research Institute (Chennai) are recognized university centres for pursuing doctoral research. Candidates possessing valid UGC-CSIR NET scores can take advantage of this facility as Junior Research Fellows • Useful links in Educational Opportunities http: //www. insplore. in

Career Opportunities • Chemistry considered as the "mother" science offers a wide range of career opportunities in such diverse areas as teaching, research, chemical and related industries, consultancy and entrepreneurship. Entering the teaching profession needs valid UGC-CSIR NET score or State Level Eligibility Test (SLET) score. As stated earlier, UGC-CSIR NET score is also a passport to research career although those who do not need such financial supports can also pursue research work. Persons with industrial experience can either opt for consultancy, advising others on how to set up chemical industries or can themselves enter into the venture.

Jobs in Chemistry • Web Resources • Useful links www. india. com/jobs/india/chemistry • www. jobsinchemistry. com/

Thank You
- Slides: 87