You Light up my Life Lab Activity Chem

You Light up my Life! Lab Activity

Chem. Catalyst If you were to drop a spoonful of salt, Na. Cl, into a glass of water, what would happen? If you were to drop a gold ring into a glass of water, what would happen? What do you think is different about the atoms of these two substances? Why wouldn’t the individual gold atoms come apart?

The Big Question What patterns do we see in the properties of substances?

At the end of this lab… You will be able to: Predict whether Mg. SO 4(aq), epsom salts, will conduct electricity.
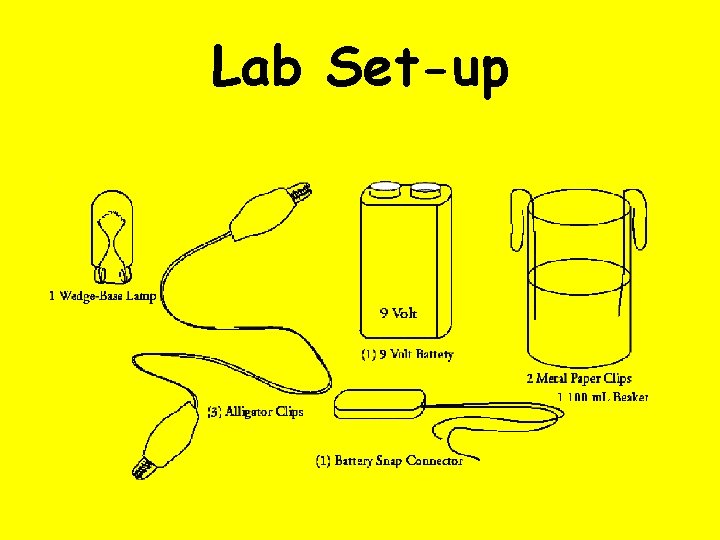
Lab Set-up

Activity Purpose: This lesson allows you to collect evidence regarding some of the properties of substances, and look for patterns.

Reminders… • • • Safety first! (goggles, sleeves, chemicals etc. ) Pay attention to what you are doing! Practice high levels of Safety! If a light burns out or something spills, let me know! Practice high levels of Safety! Don’t play with the equipment! Practice high levels of Safety! Work together and efficiently! Practice high levels of Safety!
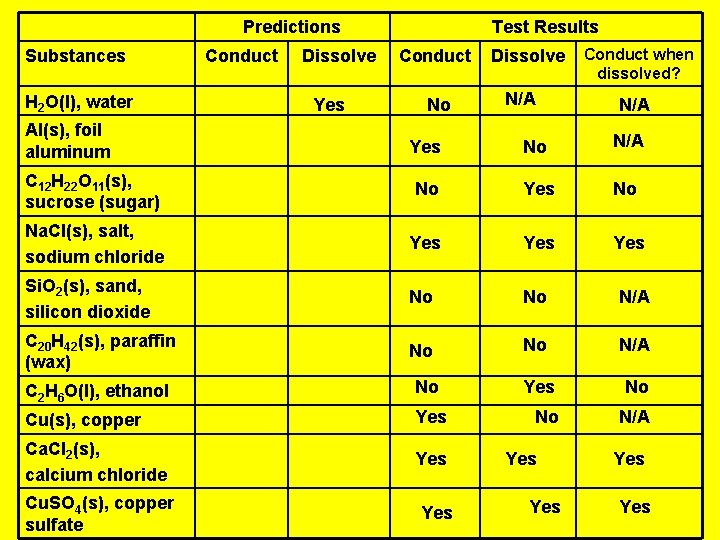
Predictions Substances H 2 O(l), water Al(s), foil aluminum Conduct Dissolve Yes Test Results Conduct No Dissolve N/A Conduct when dissolved? N/A Yes No N/A C 12 H 22 O 11(s), sucrose (sugar) No Yes No Na. Cl(s), salt, sodium chloride Yes Yes Si. O 2(s), sand, silicon dioxide No No N/A C 20 H 42(s), paraffin (wax) No No N/A Yes No No N/A C 2 H 6 O(l), ethanol No Cu(s), copper Yes Ca. Cl 2(s), calcium chloride Yes Cu. SO 4(s), copper sulfate Yes Yes Yes

1. Substances that don’t Dissolve in water, BUT conduct electricity • Copper • Aluminum foil • Both metals and solids!
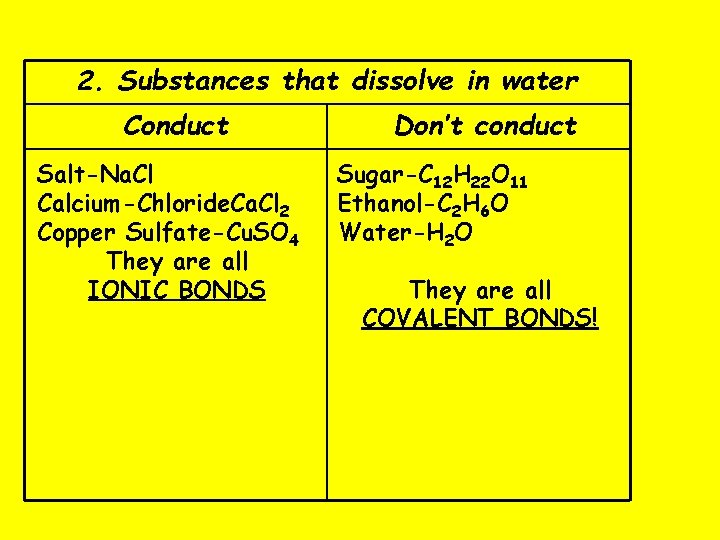
2. Substances that dissolve in water Conduct Salt-Na. Cl Calcium-Chloride. Ca. Cl 2 Copper Sulfate-Cu. SO 4 They are all IONIC BONDS Don’t conduct Sugar-C 12 H 22 O 11 Ethanol-C 2 H 6 O Water-H 2 O They are all COVALENT BONDS!

3. What do the substances that conduct electricity once dissolved have in common? They are all made up of ionic bonds. Ionic bonds are made up of a metal and a non-metal. Metals are good conductors of heat and electricity.

4. What do the substances that Don’t conduct electricity once dissolved have in common? They are all made up of covalent bonds. Covalent bonds are made up of all nonmetals. Non-Metals are NOT good conductors of heat and electricity.

5. & 6. Write a generalizing statement about the substances that DO and Don’t light up the bulb. Hint: • Think about bonds • What the bonds are made up of • Any properties they may have The substances that do not conduct electricity when dissolved in water are held together by covalent bonds (2 non-metals), and they are not good conductors. The substances that do conduct electricity when dissolved in water are held together by ionic bonds (metal and non-metals), and they are good conductors. .
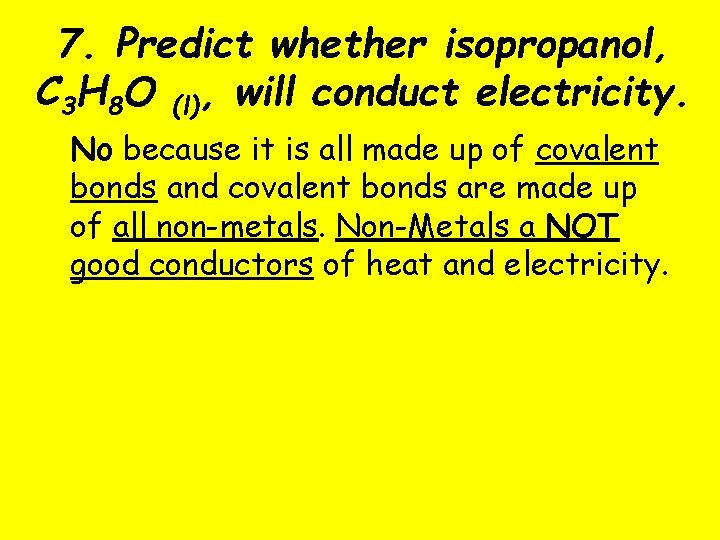
7. Predict whether isopropanol, C 3 H 8 O (l), will conduct electricity. No because it is all made up of covalent bonds and covalent bonds are made up of all non-metals. Non-Metals a NOT good conductors of heat and electricity.

Making Sense If it is dangerous to take a bath with a blow dryer, what must also be true about the water in the bathtub? The tap water will have a charge because it has ions floating in it. Pure (distilled) water has no ions in it so it is not conductive!
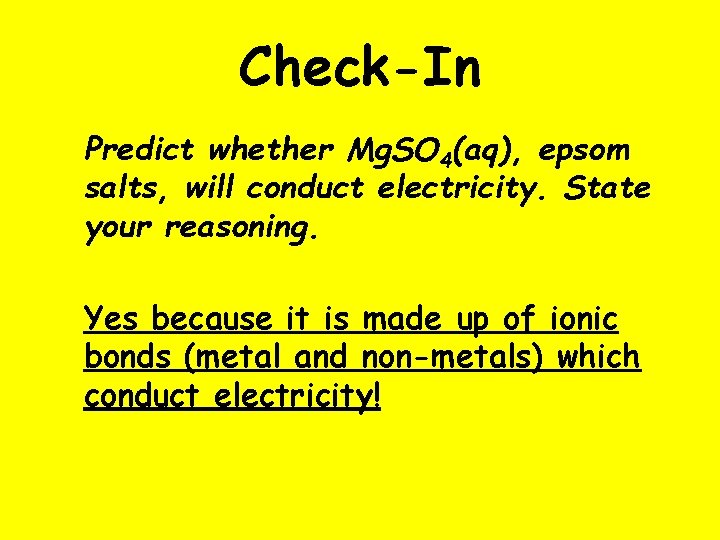
Check-In Predict whether Mg. SO 4(aq), epsom salts, will conduct electricity. State your reasoning. Yes because it is made up of ionic bonds (metal and non-metals) which conduct electricity!
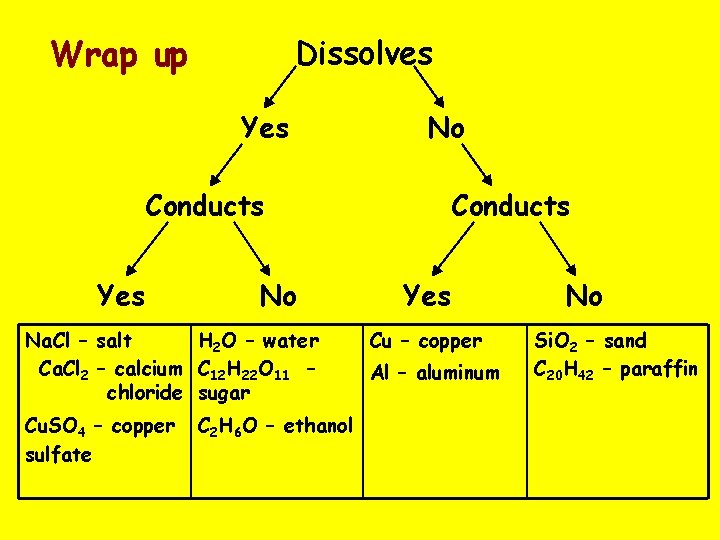
Wrap up Dissolves Yes No Conducts Yes No Na. Cl – salt H 2 O – water Ca. Cl 2 – calcium C 12 H 22 O 11 – chloride sugar Cu. SO 4 – copper sulfate C 2 H 6 O – ethanol Conducts Yes Cu – copper Al – aluminum No Si. O 2 – sand C 20 H 42 – paraffin
- Slides: 17