Welcome to AP Chemistry What is AP Chemistry
































- Slides: 47

Welcome to AP Chemistry

What is AP Chemistry? • It is several things • Equivalent of 1 year college inorganic chemistry class • A class that will prepare you for a test – May 17 • Hard work • A wonderful way to start the day • Now on to the details

Rules and Procedures • You know the basic rules but here a few that are specific for this class • No food drink or gum • LATE WORK • If you forget to bring in your homework, I will accept it ONE day late with a parent's signature, for full credit. • I will not accepted it any later.

Rules and Procedures • MAKE-UP WORK • It is your responsibility to make up all the work you missed. You have the same number of days that you were absent to turn in the missing work. • Pick up any missing work, and notes before or after class. • If you miss a test or quiz, it must be made up outside class.

Rules and Procedures • TARDIES • You will be allowed one ”free" tardy per semester. • The second and every subsequent tardy will result in a detention. • Repeated tardies will result in parent contacts, and referrals, AND MAY RESULT IN BEING DROPPED FROM THE CLASS.

Rules and Procedures • PASSES • Since every minute of class time is valuable, hall passes will be given only on an emergency basis, with a limit of one per semester, except under special circumstances.

Rules and Procedures • LAB- Because of the importance of safety in the lab, violation of laboratory safety rules and procedures may result in loss of lab privileges.

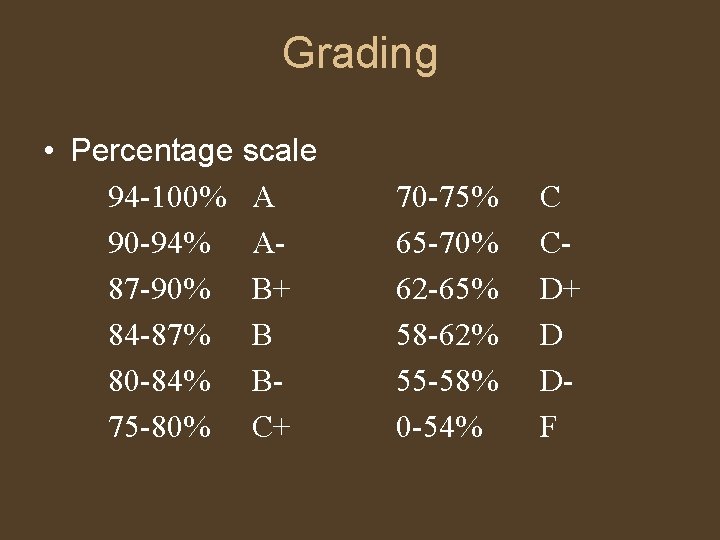
Grading • Percentage scale 94 -100% A 90 -94% A 87 -90% B+ 84 -87% B 80 -84% B 75 -80% C+ 70 -75% 65 -70% 62 -65% 58 -62% 55 -58% 0 -54% C CD+ D DF

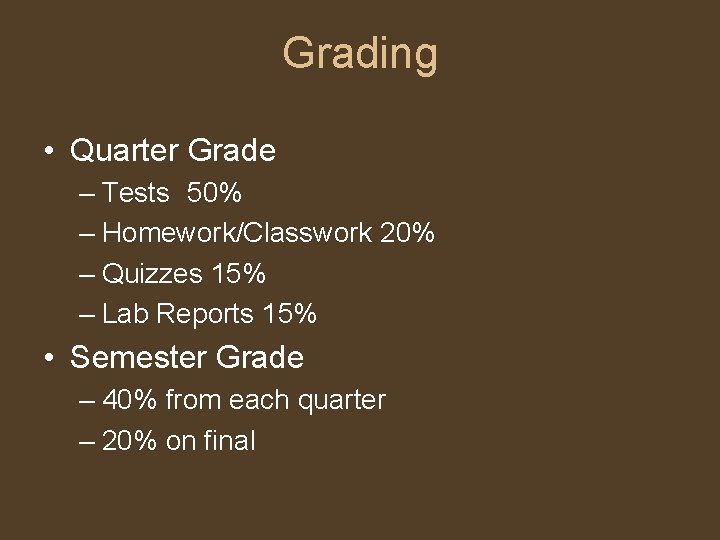
Grading • Quarter Grade – Tests 50% – Homework/Classwork 20% – Quizzes 15% – Lab Reports 15% • Semester Grade – 40% from each quarter – 20% on final

Extra Credit!! • Assignments will be provided approximately mid- quarter. • They may be turned in any time until the due date, (during the last week of the quarter) • Extra credit may be used to raise the quarter grade by up to one letter grade. • Extra credit is meant to be extra, so it will not be accepted if more than 10% of the other assignments are not turned in.

What you need for class • • Paper Pencil or pen, Calculator- scientific Book? – Not unless I let you know • Lab Notebook

Internet Ready • http: //mrgreen. tierranet. com • My email is tvgreen@aol. com

Why First Period? • College chemistry labs take more than 56 minutes, • To do those labs we will have to come early • I will give you notice of when

Any questions? • Lets get started

Significant figures • Meaningful digits in a MEASUREMENT • Exact numbers are counted, have unlimited significant figures • If it is measured or estimated, it has sig figs. • If not it is exact. • All numbers except zero are significant. • Some zeros are, some aren’t

Which zeroes count? • • • In between other sig figs does Before the first number doesn’t After the last number counts iff it is after the decimal point is written in 3200 2 sig figs • 3200. 4 sig figs

Doing the math • Multiplication and division, same number of sig figs in answer as the least in the problem • Addition and subtraction, same number of decimal places in answer as least in problem.

More Preliminaries Scientific Method Metric System Uncertainty

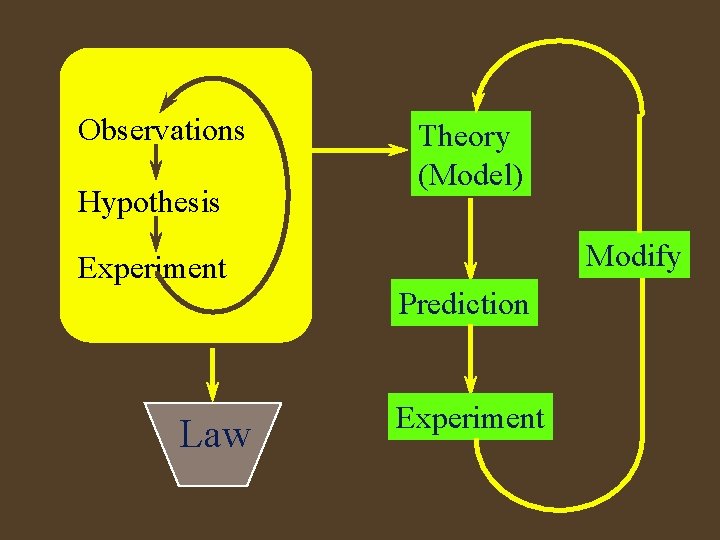
Scientific method. • A way of solving problems • Observation- what is seen or measured • Hypothesis- educated guess of why things behave the way they do. (possible explanation) • Experiment- designed to test hypothesis • leads to new observations, • and the cycle goes on

Scientific method. • After many cycles, a broad, generalizable explanation is developed for why things behave the way they do • Theory • Also regular patterns of how things behave the same in different systems emerges • Laws are summaries of observations

Scientific method. • Theories have predictive value. • The true test of a theory is if it can predict new behaviors. • If the prediction is wrong, theory must be changed. • Theory- why • Law - how

Observations Hypothesis Theory (Model) Modify Experiment Prediction Law Experiment

Metric System • • Every measurement has two parts Number Scale (unit) SI system (le Systeme International) based on the metric system • Prefix + base unit • Prefix tells you the power of 10 to multiply by - decimal system -easy conversions

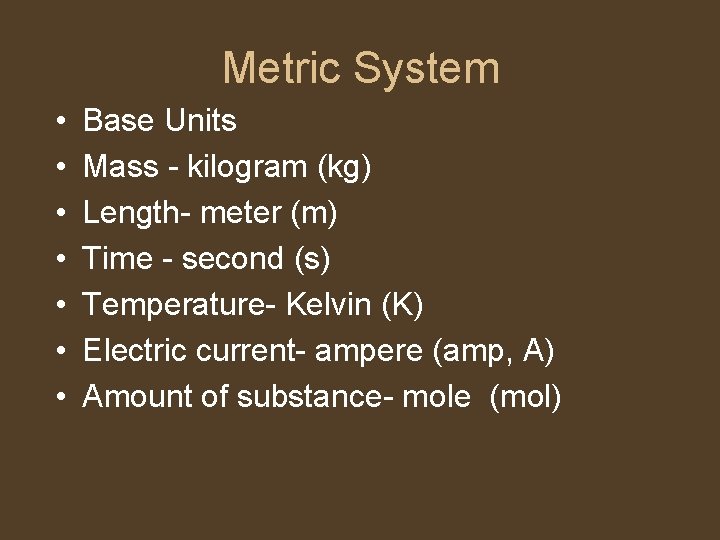
Metric System • • Base Units Mass - kilogram (kg) Length- meter (m) Time - second (s) Temperature- Kelvin (K) Electric current- ampere (amp, A) Amount of substance- mole (mol)

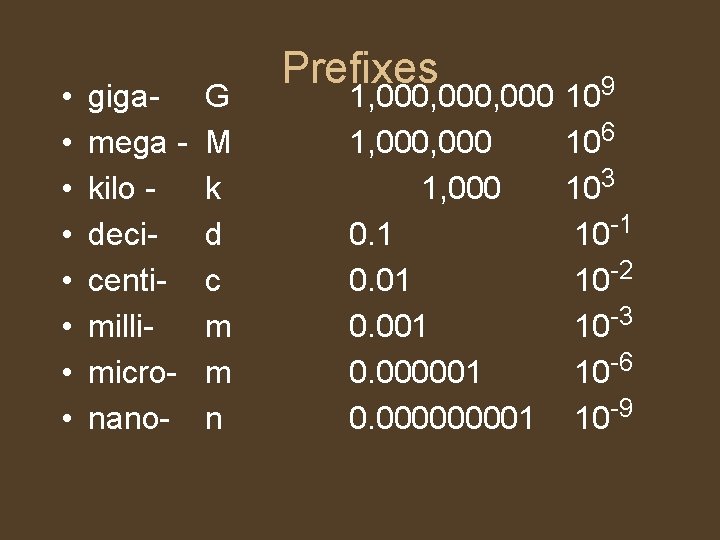
• • gigamega kilo decicentimillimicronano- G M k d c m m n Prefixes 1, 000, 000 1, 000 0. 1 0. 001 0. 000000001 109 106 103 10 -1 10 -2 10 -3 10 -6 10 -9

Deriving the Liter 3 • Liter is defined as the volume of 1 dm 3 • gram is the mass of 1 cm

Mass and Weight • Mass is measure of resistance to change in motion • Weight is force of gravity. • Sometimes used interchangeably • Mass can’t change, weight can

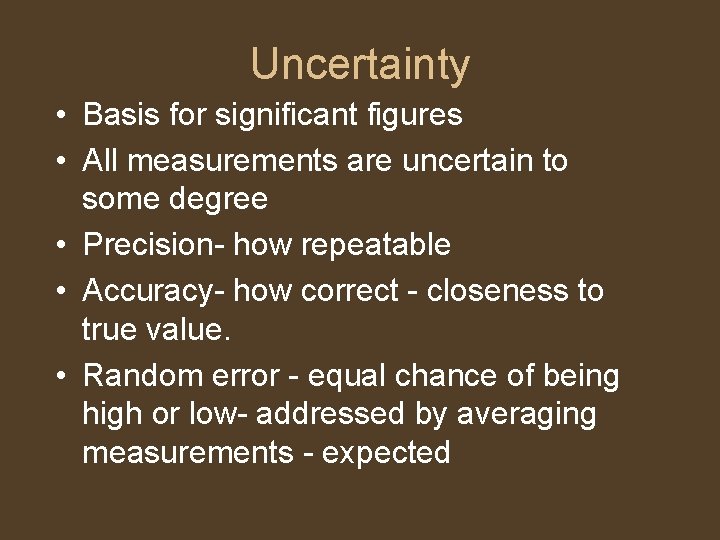
Uncertainty • Basis for significant figures • All measurements are uncertain to some degree • Precision- how repeatable • Accuracy- how correct - closeness to true value. • Random error - equal chance of being high or low- addressed by averaging measurements - expected

Uncertainty • Systematic error- same direction each time • Want to avoid this • Better precision implies better accuracy • you can have precision without accuracy • You can’t have accuracy without precision

Dimensional Analysis Using the units to solve problems

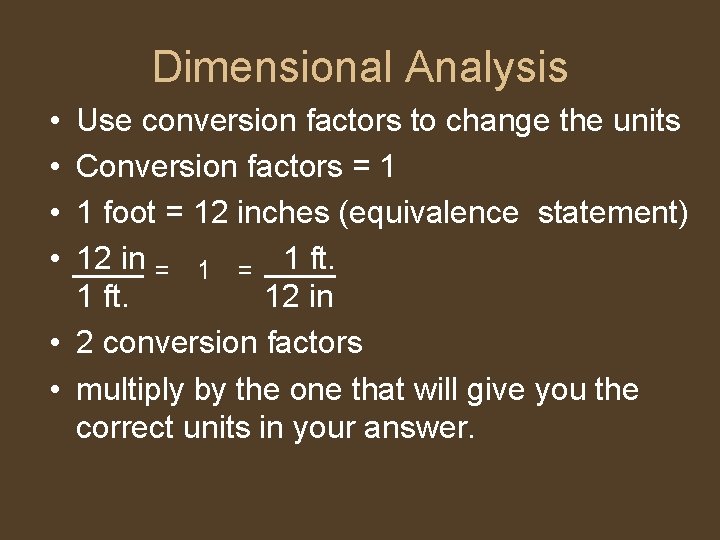
Dimensional Analysis • • Use conversion factors to change the units Conversion factors = 1 1 foot = 12 inches (equivalence statement) 12 in = 1 ft. 12 in • 2 conversion factors • multiply by the one that will give you the correct units in your answer.

Examples • • 11 yards = 2 rod 40 rods = 1 furlong 8 furlongs = 1 mile The Kentucky Derby race is 1. 25 miles. How long is the race in rods, furlongs, meters, and kilometers? • A marathon race is 26 miles, 385 yards. What is this distance in rods, furlongs, meters, and kilometers?

Examples • Science fiction often uses nautical analogies to describe space travel. If the starship U. S. S. Enterprise is traveling at warp factor 1. 71, what is its speed in knots? • Warp 1. 71 = 5. 00 times the speed of light • speed of light = 3. 00 x 108 m/s • 1 knot = 2000 yd/h exactly

Examples • Apothecaries (druggists) use the following set of measures in the English system: • 20 grains ap = 1 scruple (exact) • 3 scruples = 1 dram ap (exact) • 8 dram ap = 1 oz. ap (exact) • 1 dram ap = 3. 888 g • 1 oz. ap = ? oz. troy • What is the mass of 1 scruple in grams?

Examples • The speed of light is 3. 00 x 108 m/s. How far will a beam of light travel in 1. 00 ns?

Temperature and Density

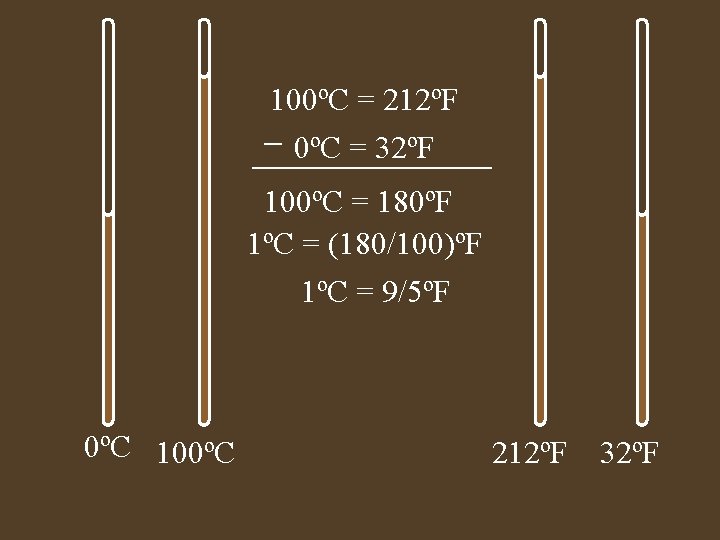
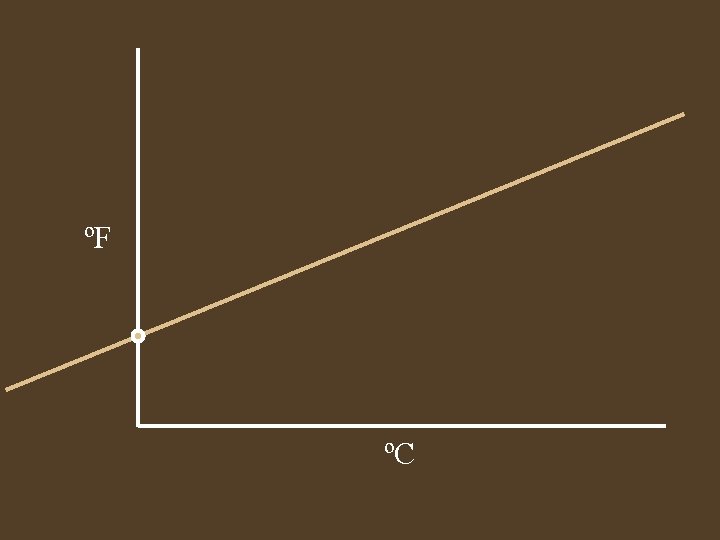
Temperature • A measure of the average kinetic energy • Different temperature scales, all are talking about the same height of mercury. • Derive a equation for converting ºF toºC

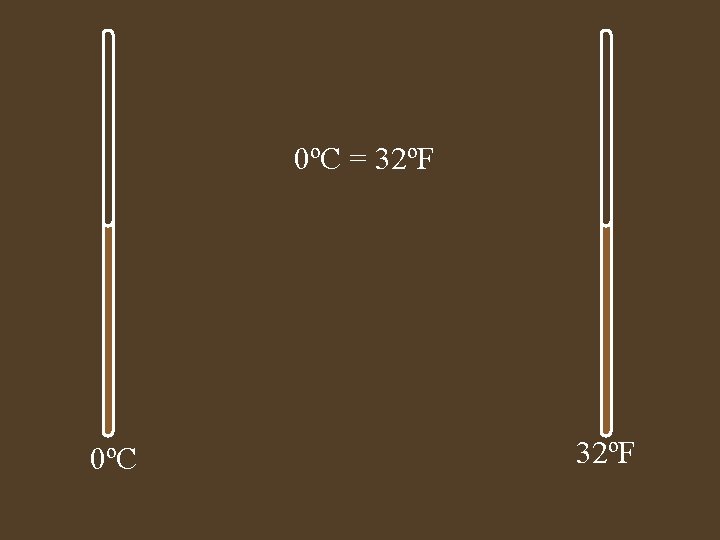
0ºC = 32ºF 0ºC 32ºF

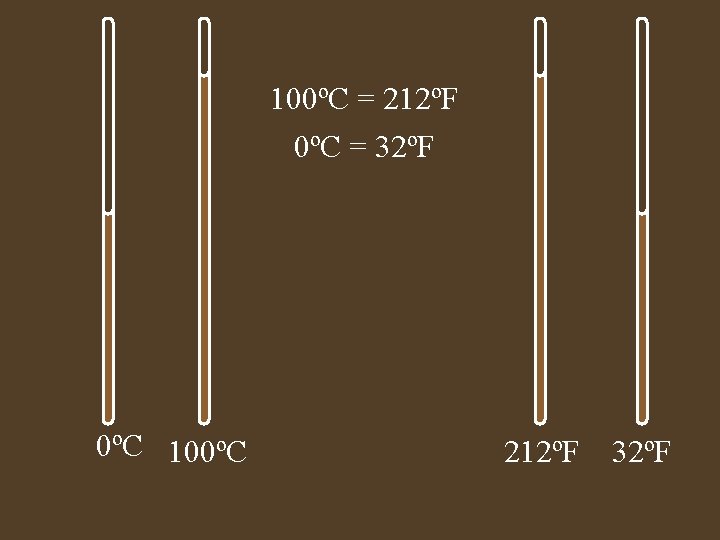
100ºC = 212ºF 0ºC = 32ºF 0ºC 100ºC 212ºF 32ºF

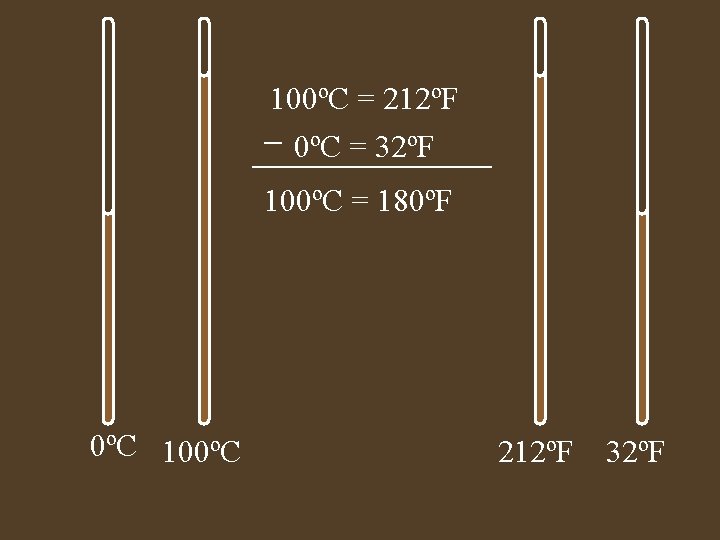
100ºC = 212ºF 0ºC = 32ºF 100ºC = 180ºF 0ºC 100ºC 212ºF 32ºF

100ºC = 212ºF 0ºC = 32ºF 100ºC = 180ºF 1ºC = (180/100)ºF 1ºC = 9/5ºF 0ºC 100ºC 212ºF 32ºF


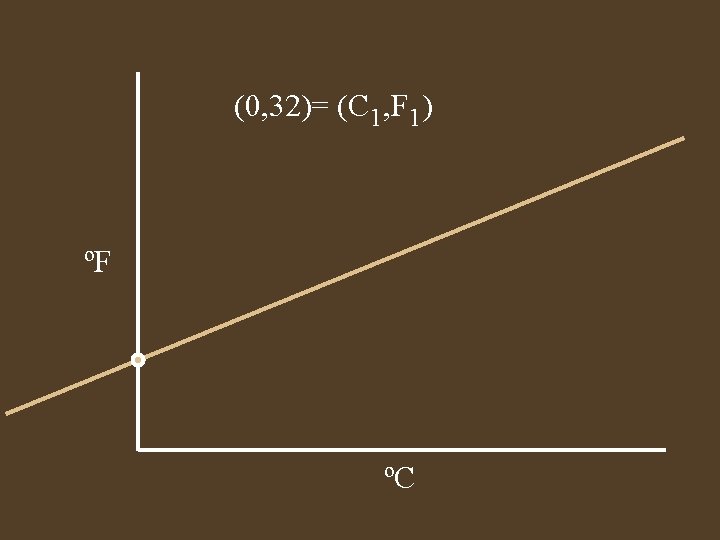
(0, 32)= (C 1, F 1) ºF ºC

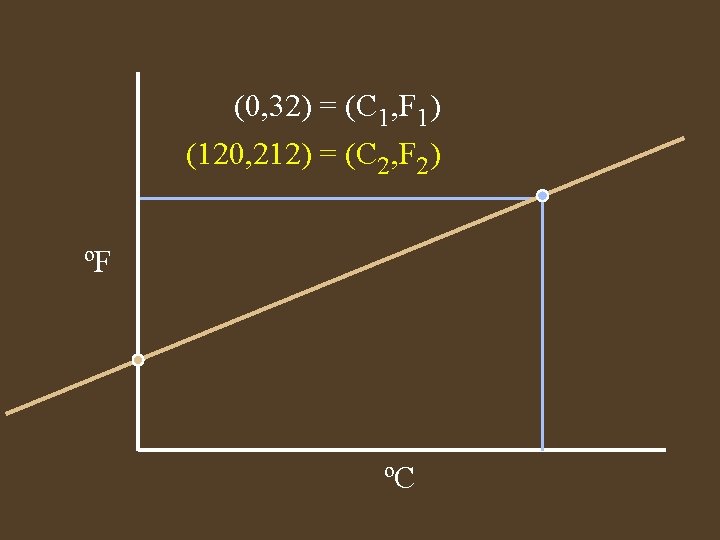
(0, 32) = (C 1, F 1) (120, 212) = (C 2, F 2) ºF ºC

Density • • • Ratio of mass to volume D = m/V Useful for identifying a compound Useful for predicting weight An intrinsic property- does not depend on what the material is

Density Problem • An empty container weighs 121. 3 g. Filled with carbon tetrachloride (density 1. 53 g/cm 3 ) the container weighs 283. 2 g. What is the volume of the container?

Density Problem • A 55. 0 gal drum weighs 75. 0 lbs. when empty. What will the total mass be when filled with ethanol? density 0. 789 g/cm 3 gal = 3. 78 L 454 g 1 1 lb =
 Wise men three clever are we
Wise men three clever are we Welcome to chemistry class
Welcome to chemistry class Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Welcome to quarter 3
Welcome to quarter 3 Welcome to edmodo
Welcome to edmodo Welcome back i hope you enjoyed your vacation
Welcome back i hope you enjoyed your vacation Welcome prayer service
Welcome prayer service Welcome to the netherlands a tiny country that only extends
Welcome to the netherlands a tiny country that only extends Welcome glad you're here
Welcome glad you're here Krb_ap_err_modified
Krb_ap_err_modified Welcome speech for chairman
Welcome speech for chairman Welcome to today's class
Welcome to today's class Welcome to our class
Welcome to our class Welcome to week 3
Welcome to week 3 Welcome to curriculum night
Welcome to curriculum night Assaignment
Assaignment Welcome dear parents
Welcome dear parents Chorus pro suivre le traitement d'une facture
Chorus pro suivre le traitement d'une facture Well welcome to the class everybody
Well welcome to the class everybody Dear guests welcome
Dear guests welcome Welcome to our class
Welcome to our class Jeopardy game builder
Jeopardy game builder Welcome to spanish class
Welcome to spanish class Welcome to primary 2
Welcome to primary 2 Welcome to super hero high
Welcome to super hero high Welcome and housekeeping
Welcome and housekeeping Welcome to 2nd semester
Welcome to 2nd semester Chapter 1 welcome to the industry
Chapter 1 welcome to the industry Class 5 english unit 14
Class 5 english unit 14 Welcome to russia meme
Welcome to russia meme Us cellular welcome kit
Us cellular welcome kit Welcome program
Welcome program Welcome to week 3
Welcome to week 3 How to self validate
How to self validate Agenda welcome and introductions
Agenda welcome and introductions Oh shut up question tag
Oh shut up question tag Welcome to my webpage
Welcome to my webpage Welcome the stranger bible
Welcome the stranger bible Welcome to your senior year of high school
Welcome to your senior year of high school Good morning, class
Good morning, class Welcome to our english lesson
Welcome to our english lesson Welcome to all participants
Welcome to all participants Welcome my dear students
Welcome my dear students We will start soon
We will start soon Unit 1 welcome back
Unit 1 welcome back Welcome canada amount for international students
Welcome canada amount for international students Wellcome
Wellcome