Welcome Welcome to Welcome to Dr Chus Welcome





- Slides: 22

Welcome

Welcome to

Welcome to Dr. Chu’s

Welcome to Dr. Chu’s Vodcast

Today’s

Today’s topic is …

Gases Part B: Gas Laws Dr. Chu River Dell Regional High School

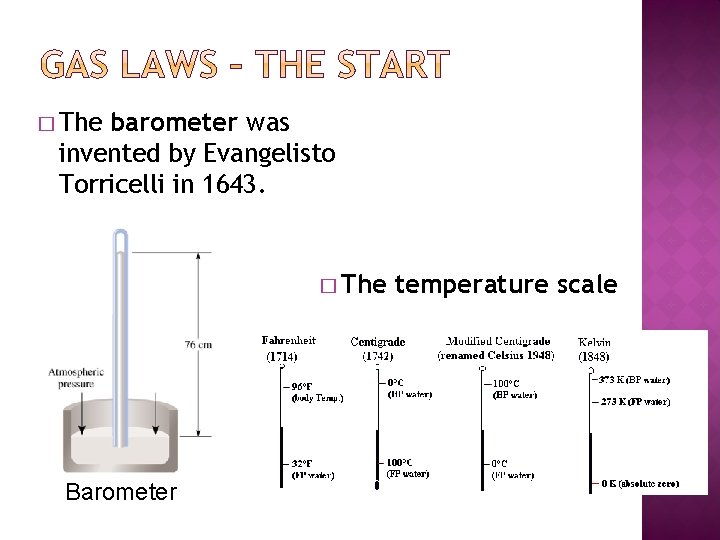
� The barometer was invented by Evangelisto Torricelli in 1643. � The Barometer temperature scale

Websites: • http: //phet. colorado. edu/en/simulation/gasproperties • http: //www. mhhe. com/physsci/chemistry/ess entialchemistry/flash/gasesv 6. swf

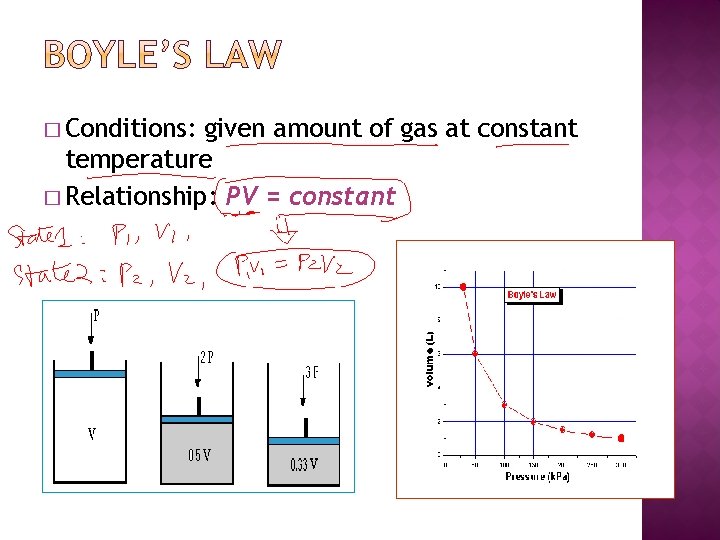
� Conditions: given amount of gas at constant temperature � Relationship: PV = constant

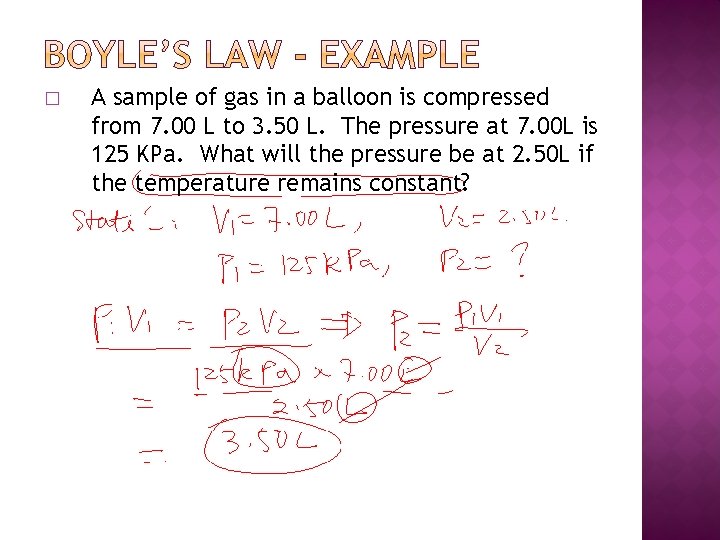
� A sample of gas in a balloon is compressed from 7. 00 L to 3. 50 L. The pressure at 7. 00 L is 125 KPa. What will the pressure be at 2. 50 L if the temperature remains constant?

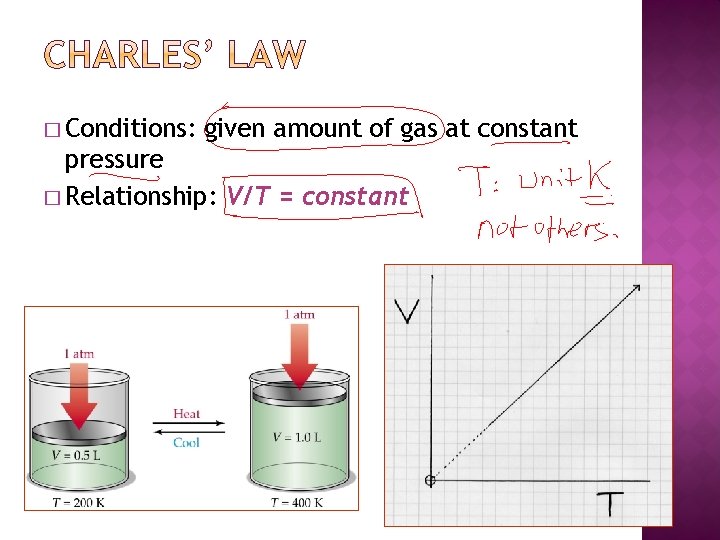
� Conditions: given amount of gas at constant pressure � Relationship: V/T = constant

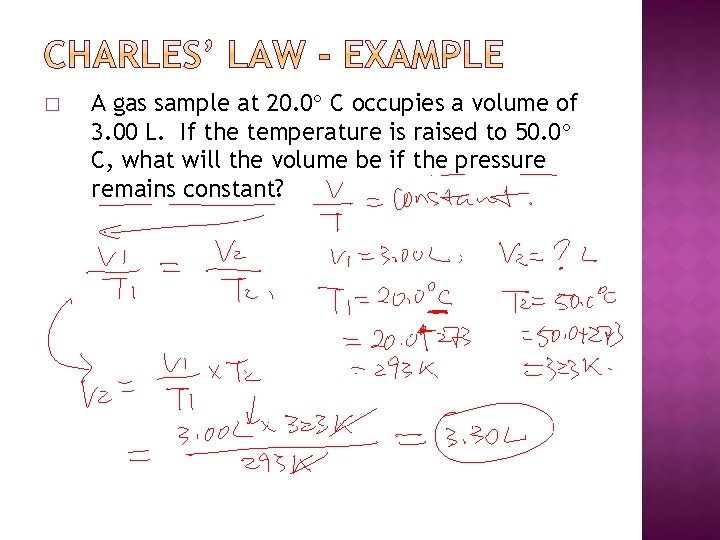
� A gas sample at 20. 0 C occupies a volume of 3. 00 L. If the temperature is raised to 50. 0 C, what will the volume be if the pressure remains constant?

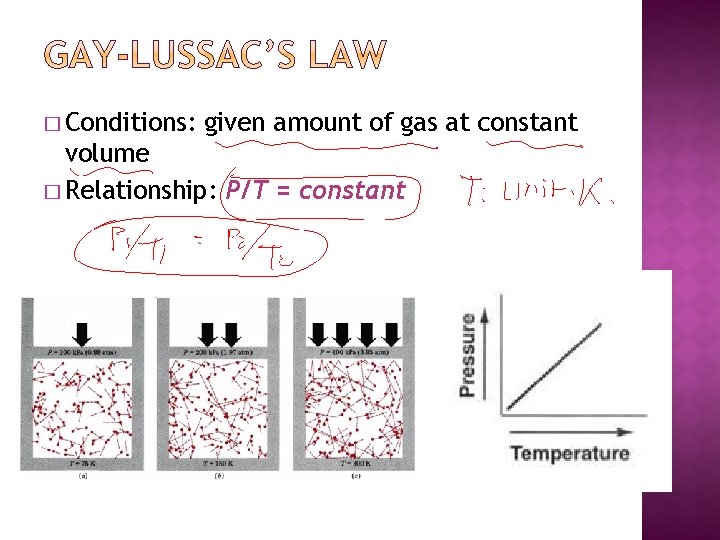
� Conditions: given amount of gas at constant volume � Relationship: P/T = constant

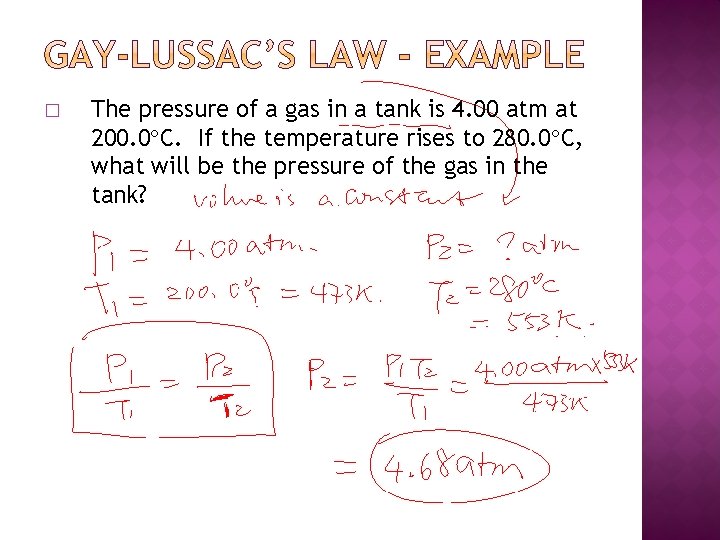
� The pressure of a gas in a tank is 4. 00 atm at 200. 0 C. If the temperature rises to 280. 0 C, what will be the pressure of the gas in the tank?

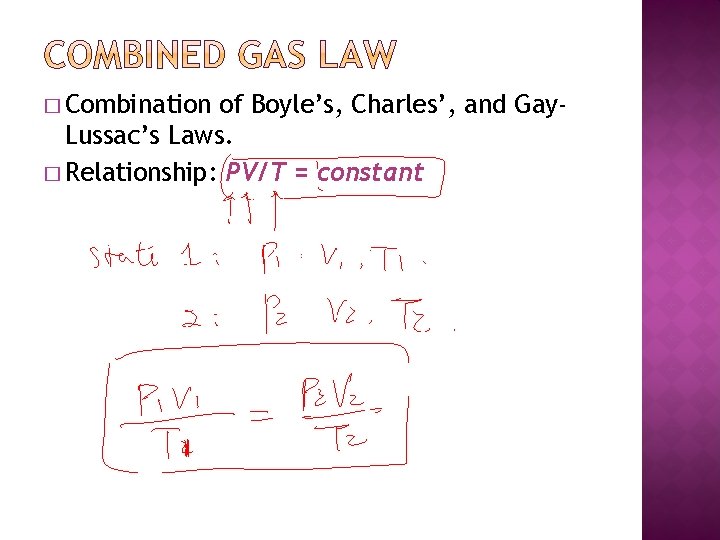
� Combination of Boyle’s, Charles’, and Gay. Lussac’s Laws. � Relationship: PV/T = constant

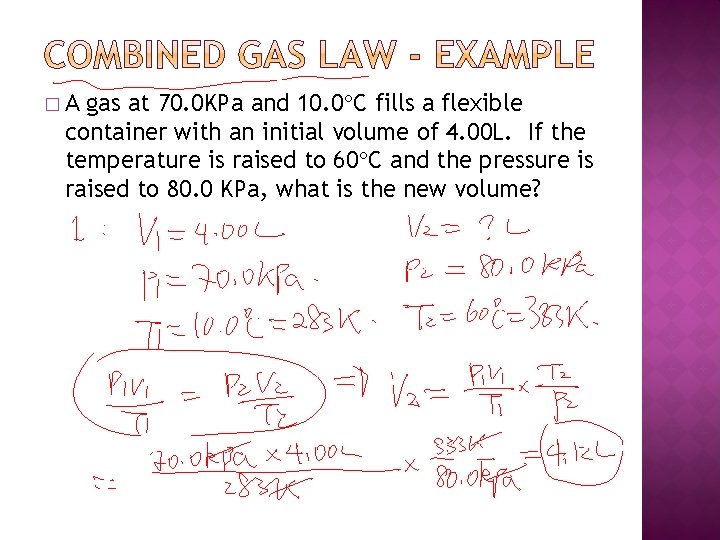
�A gas at 70. 0 KPa and 10. 0 C fills a flexible container with an initial volume of 4. 00 L. If the temperature is raised to 60 C and the pressure is raised to 80. 0 KPa, what is the new volume?

� Carefully read the problem and list all the known and unknown parameters. � Use the 1 and 2 for designations of start and end states, respectively. Carefully label each parameter with 1 or 2 subscript to keep track. � If any of the three parameter is held constant, that parameter would have the same value for both states 1 and 2. Then the identical parameter can be cancelled from the combined gas law and individual gas laws reconstitute.

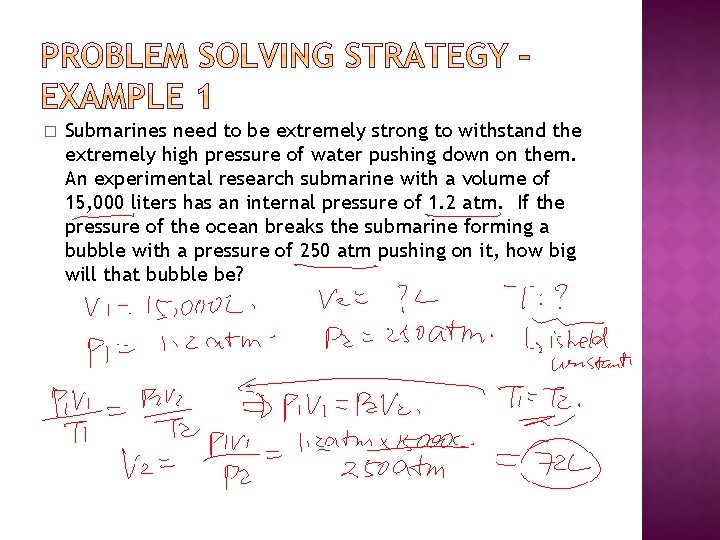
� Submarines need to be extremely strong to withstand the extremely high pressure of water pushing down on them. An experimental research submarine with a volume of 15, 000 liters has an internal pressure of 1. 2 atm. If the pressure of the ocean breaks the submarine forming a bubble with a pressure of 250 atm pushing on it, how big will that bubble be?

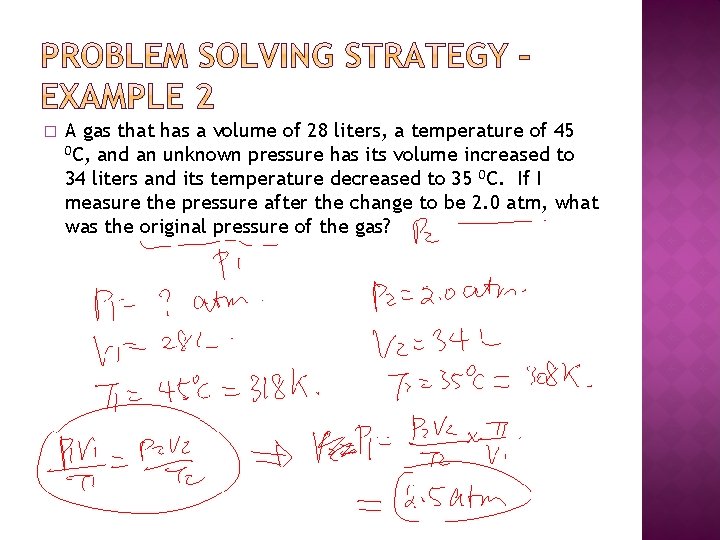
� A gas that has a volume of 28 liters, a temperature of 45 0 C, and an unknown pressure has its volume increased to 34 liters and its temperature decreased to 35 0 C. If I measure the pressure after the change to be 2. 0 atm, what was the original pressure of the gas?

1. A gas takes up a volume of 17 liters, has a pressure of 2. 3 atm, and a temperature of 299 K. If I raise the temperature to 350 K and lower the pressure to 1. 5 atm, what is the new volume of the gas? 2. A gas has a temperature of 14 0 C, and a volume of 4. 5 liters. If the temperature is raised to 29 0 C and the pressure is not changed, what is the new volume of the gas? 3. If I have 21 liters of gas held at a pressure of 78 atm and a temperature of 900 K, what will be the volume of the gas if I decrease the pressure to 45 atm and decrease the temperature to 750 K?

1. A gas takes up a volume of 17 liters, has a pressure of 2. 3 atm, and a temperature of 299 K. If I raise the temperature to 350 K and lower the pressure to 1. 5 atm, what is the new volume of the gas? [30. 5 L] 2. A gas has a temperature of 14 0 C, and a volume of 4. 5 liters. If the temperature is raised to 29 0 C and the pressure is not changed, what is the new volume of the gas? [4. 74 L] 3. If I have 21 liters of gas held at a pressure of 78 atm and a temperature of 900 K, what will be the volume of the gas if I decrease the pressure to 45 atm and decrease the temperature to 750 K? [30. 3 L]