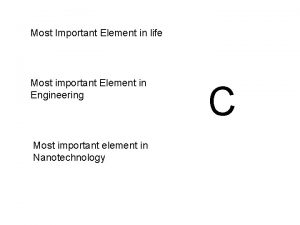
CHEMISTRY OF LIFE Why is chemistry important Chemistry







- Slides: 30

CHEMISTRY OF LIFE Why is chemistry important? Chemistry is related to Biology because all the processes that go on in our world and in your body are a result of chemical reactions. - photosynthesis - digestion - metabolism Biochemistry: chemistry of living organisms

Everything around us is made up of matter. Matter: anything that has mass and volume Mass: amount of matter in an object Volume: amount of space and object takes up Weight: force of gravity on mass

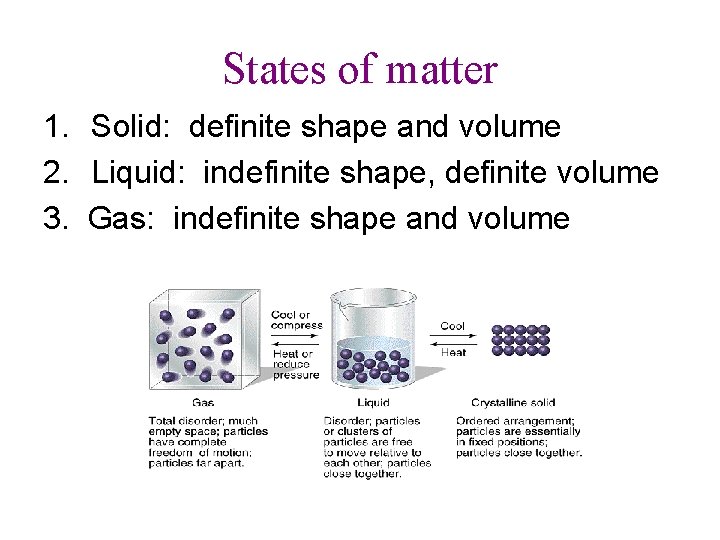
States of matter 1. Solid: definite shape and volume 2. Liquid: indefinite shape, definite volume 3. Gas: indefinite shape and volume

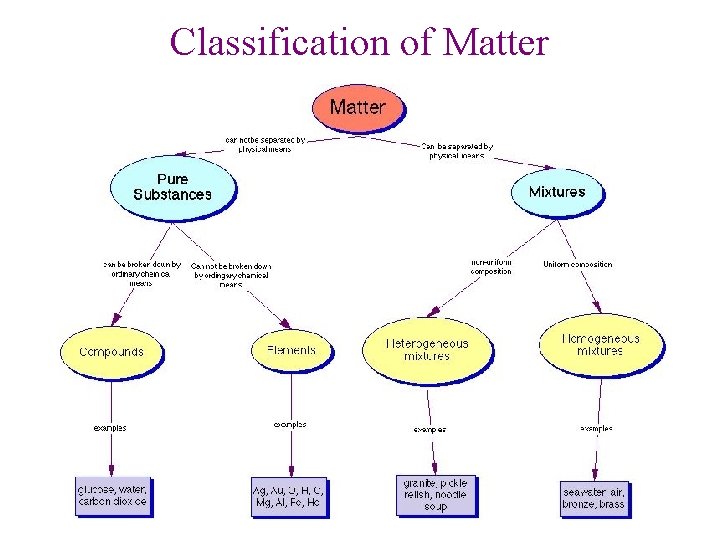
Classification of Matter

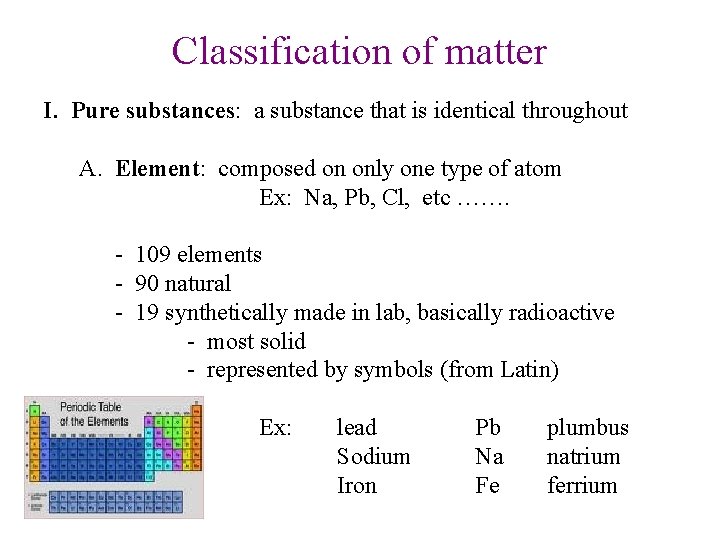
Classification of matter I. Pure substances: a substance that is identical throughout A. Element: composed on only one type of atom Ex: Na, Pb, Cl, etc ……. - 109 elements - 90 natural - 19 synthetically made in lab, basically radioactive - most solid - represented by symbols (from Latin) Ex: lead Sodium Iron Pb Na Fe plumbus natrium ferrium

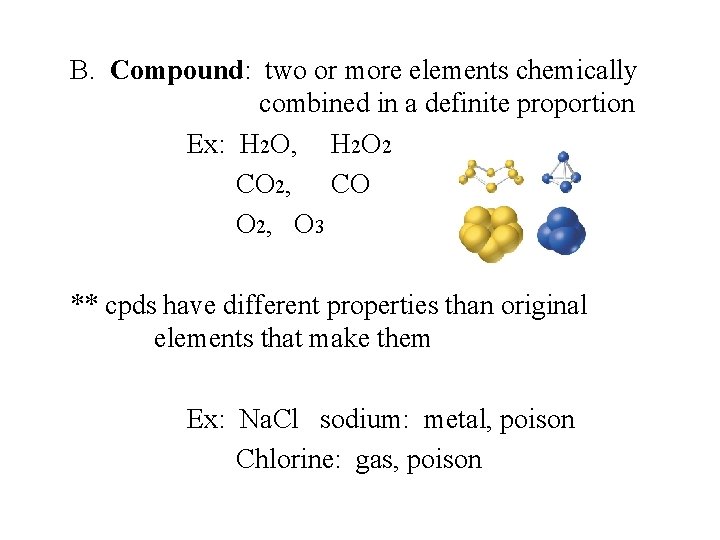
B. Compound: two or more elements chemically combined in a definite proportion Ex: H 2 O, H 2 O 2 CO 2, CO O 2, O 3 ** cpds have different properties than original elements that make them Ex: Na. Cl sodium: metal, poison Chlorine: gas, poison

II. Mixture: two or more substances (element or compound) mixed but not chemically combined - original substances keep their identities and can be separated out by physical means Ex: salt water – boil off water and collect salt Ex of mixtures: salad dressing rocks, sand, water, blood, earth’s atmosphere (O, N, CO 2, Ar, etc)

Two types Mixtures A. Solution: mixture where substances are equally distributed and appear as one substance Components of a solution Solute: substance being dissolved Solvent: substance doing dissolving ex: ice tea mix in water ex: 0. 85% Na. Cl in plasma (water component of blood) Aqueous solution: water is UNIVERSAL SOLVENT when two same states of matter: solvent is substance in larger amount

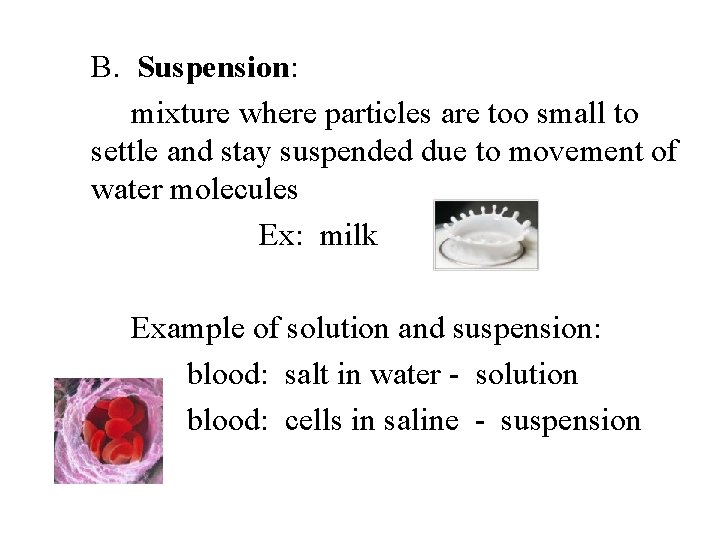
B. Suspension: mixture where particles are too small to settle and stay suspended due to movement of water molecules Ex: milk Example of solution and suspension: blood: salt in water - solution blood: cells in saline - suspension

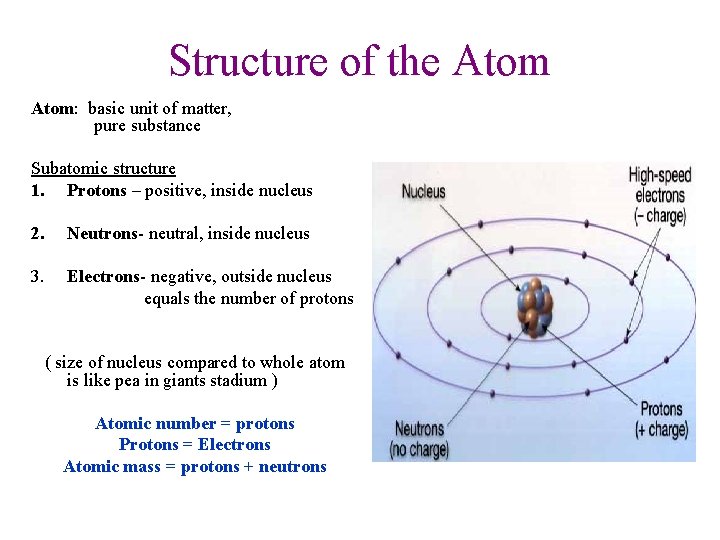
Structure of the Atom: basic unit of matter, pure substance Subatomic structure 1. Protons – positive, inside nucleus 2. Neutrons- neutral, inside nucleus 3. Electrons- negative, outside nucleus equals the number of protons ( size of nucleus compared to whole atom is like pea in giants stadium ) Atomic number = protons Protons = Electrons Atomic mass = protons + neutrons

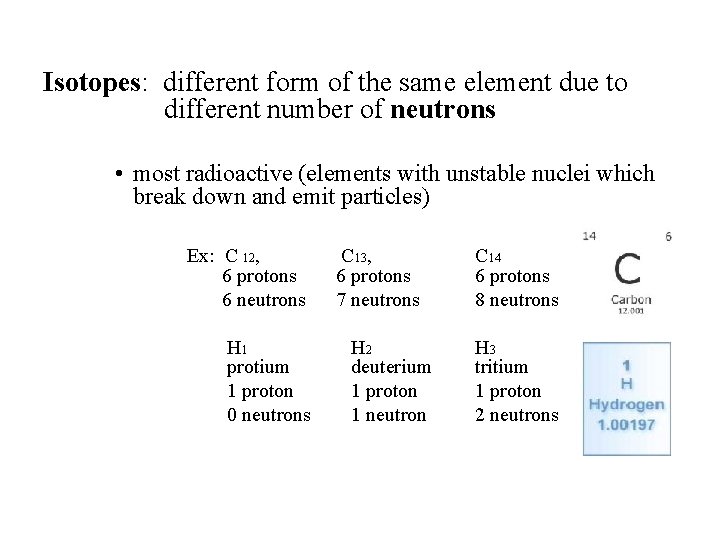
Isotopes: different form of the same element due to different number of neutrons • most radioactive (elements with unstable nuclei which break down and emit particles) Ex: C 12, 6 protons 6 neutrons H 1 protium 1 proton 0 neutrons C 13, 6 protons 7 neutrons H 2 deuterium 1 proton 1 neutron C 14 6 protons 8 neutrons H 3 tritium 1 proton 2 neutrons

Uses of Isotopes - Study age of fossils and rocks C 14 - Radiation therapy: cobalt 60, cancer carbon 14, brain tumors - Medical tests: Thallium, stress tests Iron 59, blood circulation Iodine, thyroid tests cobalt 60

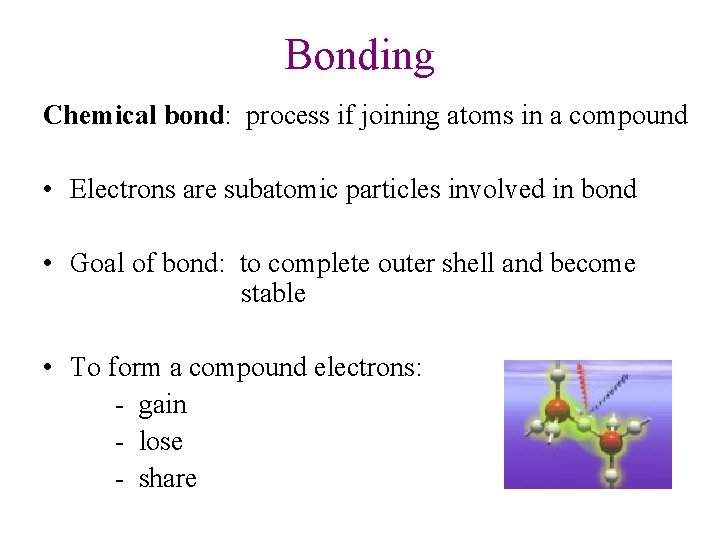
Bonding Chemical bond: process if joining atoms in a compound • Electrons are subatomic particles involved in bond • Goal of bond: to complete outer shell and become stable • To form a compound electrons: - gain - lose - share

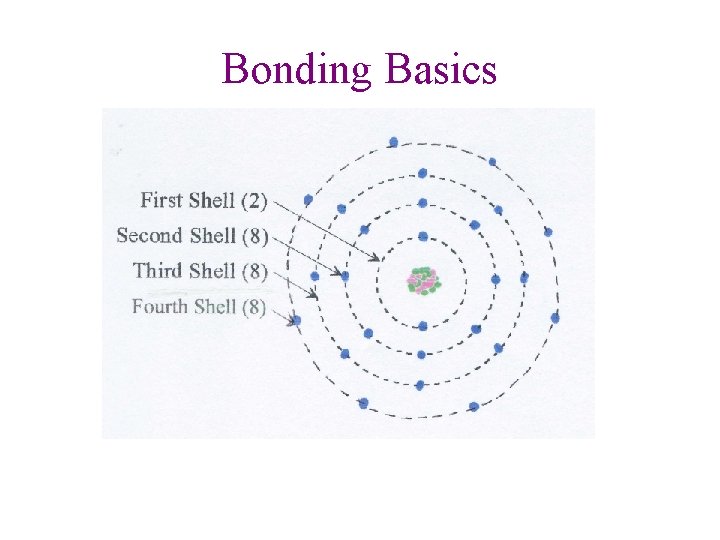
Bonding Basics

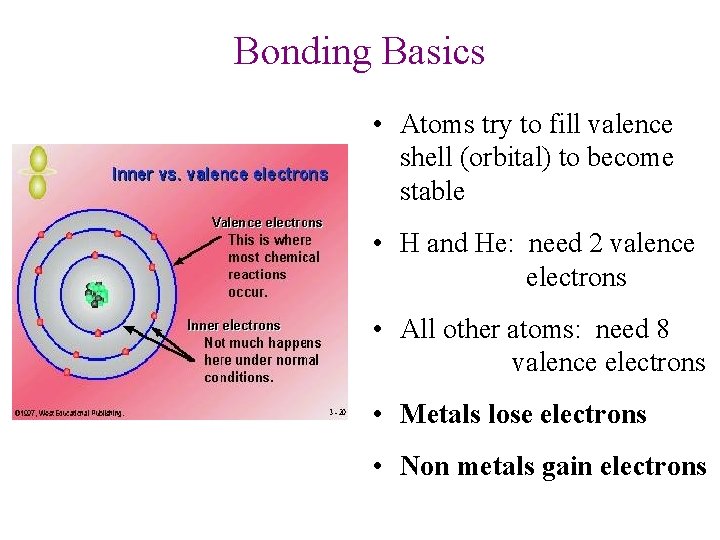
Bonding Basics • Atoms try to fill valence shell (orbital) to become stable • H and He: need 2 valence electrons • All other atoms: need 8 valence electrons • Metals lose electrons • Non metals gain electrons

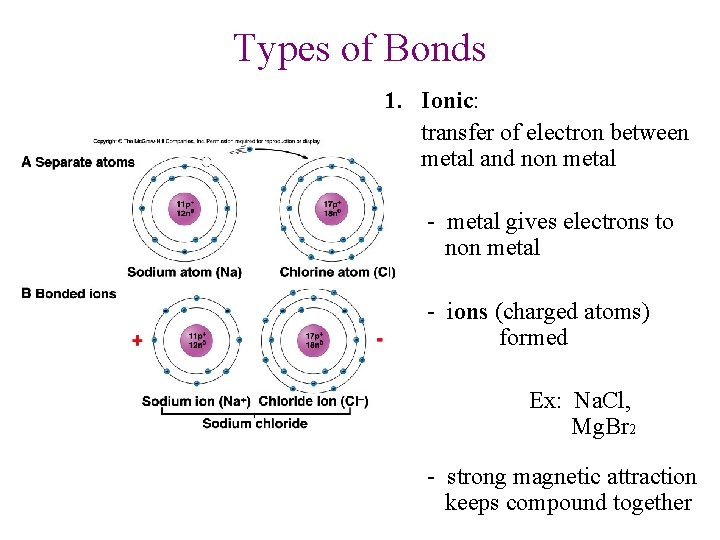
Types of Bonds 1. Ionic: transfer of electron between metal and non metal - metal gives electrons to non metal - ions (charged atoms) formed Ex: Na. Cl, Mg. Br 2 - strong magnetic attraction keeps compound together

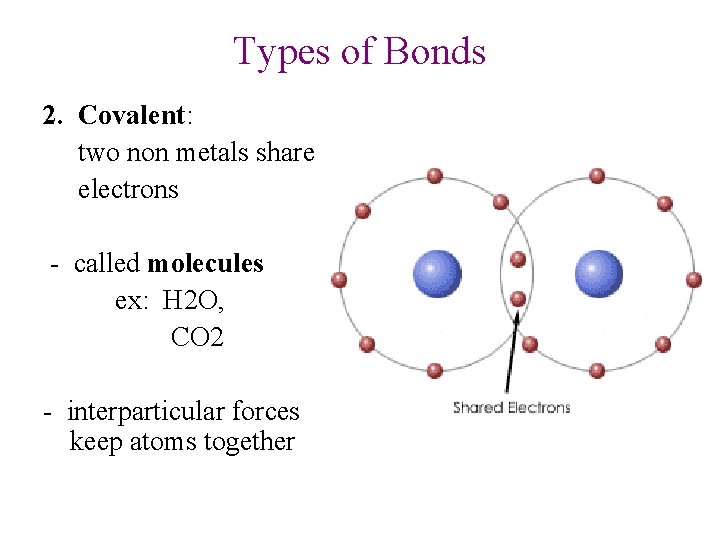
Types of Bonds 2. Covalent: two non metals share electrons - called molecules ex: H 2 O, CO 2 - interparticular forces keep atoms together

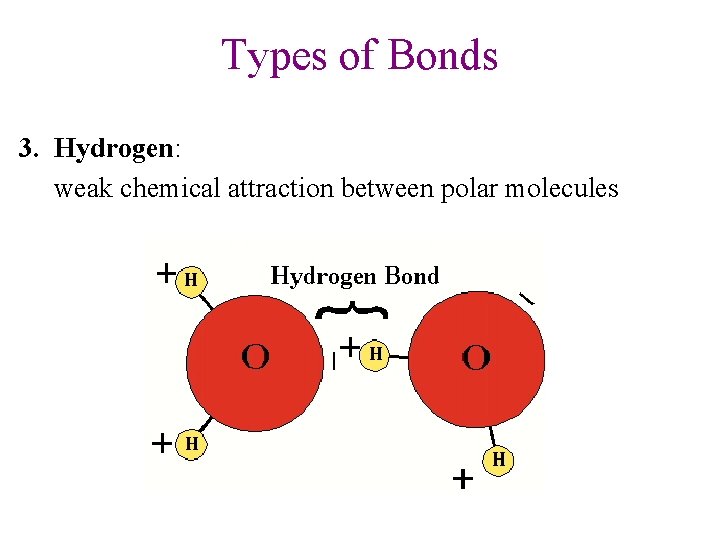
Types of Bonds 3. Hydrogen: weak chemical attraction between polar molecules

WHENEVER BOND IS FORMED A CHEMICAL CHANGE TAKES PLACE Chemical reaction (change): process in which a chemical change occurs due to bonding Ex: decaying of apples digestion of food burning of coal decomposition of plants rusting of iron EVERYTHING THAT OCCURS IN LIVING ORGANISMS IS A RESULT OF CHEMICAL REACTIONS.

WATER AND SOLUTIONS • universal solvent in organisms • one of few liquid compounds found naturally on earth, most solid • expands in solid form • covers > 75% of earth • most abundant compound in living organisms (human body ~ 70%) • most important compound in organisms - cells surrounded by it - filled with it - cellular events occur in it) - carries or dissolves other substances

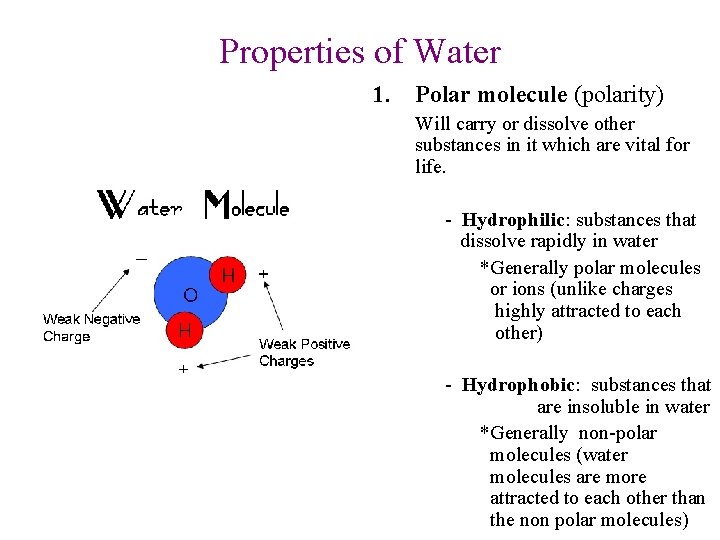
Properties of Water 1. Polar molecule (polarity) Will carry or dissolve other substances in it which are vital for life. - Hydrophilic: substances that dissolve rapidly in water *Generally polar molecules or ions (unlike charges highly attracted to each other) - Hydrophobic: substances that are insoluble in water *Generally non-polar molecules (water molecules are more attracted to each other than the non polar molecules)

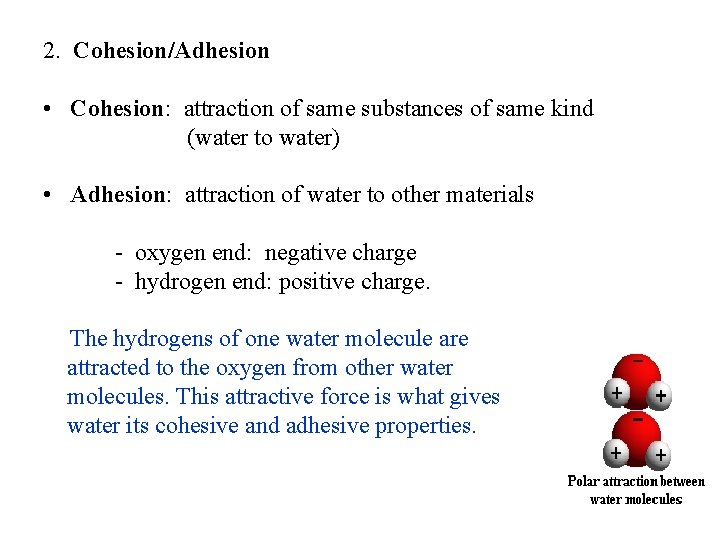
2. Cohesion/Adhesion • Cohesion: attraction of same substances of same kind (water to water) • Adhesion: attraction of water to other materials - oxygen end: negative charge - hydrogen end: positive charge. The hydrogens of one water molecule are attracted to the oxygen from other water molecules. This attractive force is what gives water its cohesive and adhesive properties.

3. Surface Tension: cohesion of water molecules at the surface of a body of water - Each molecule in the water drop is attracted to the other water molecules in the drop. - This causes the water to pull itself into a shape with the smallest amount of surface area, a bead (sphere). - All the water molecules on the surface of the bead are creating surface tension. (like a large group of people tightly holding hands)

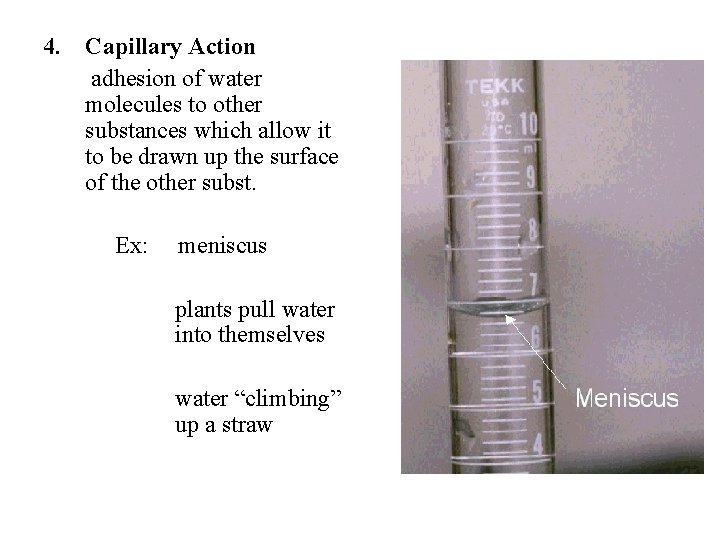
4. Capillary Action adhesion of water molecules to other substances which allow it to be drawn up the surface of the other subst. Ex: meniscus plants pull water into themselves water “climbing” up a straw

5. Stores heat efficiently - absorbs a lot of energy before it will raise its temp. - retains its heat longer than many other substances - this property keeps temperature fluctuations to a minimum in order support life on land in water

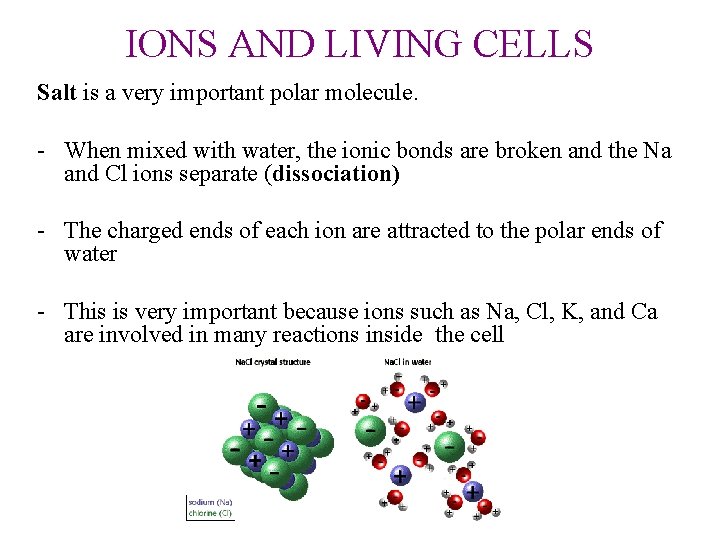
IONS AND LIVING CELLS Salt is a very important polar molecule. - When mixed with water, the ionic bonds are broken and the Na and Cl ions separate (dissociation) - The charged ends of each ion are attracted to the polar ends of water - This is very important because ions such as Na, Cl, K, and Ca are involved in many reactions inside the cell

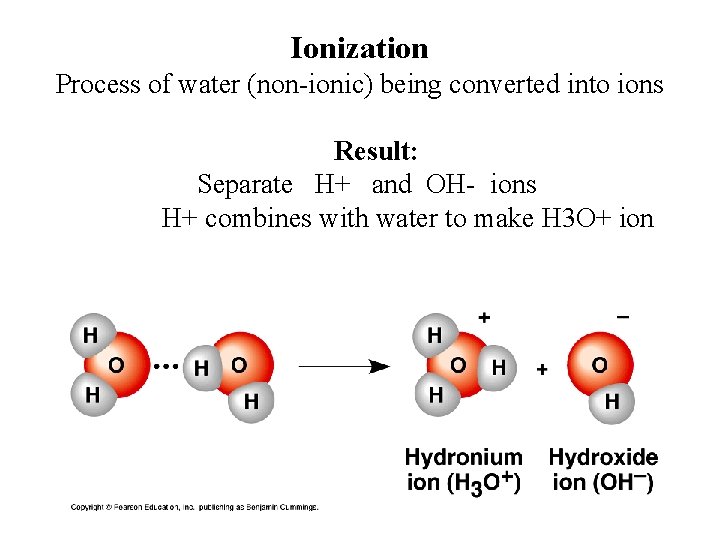
Ionization Process of water (non-ionic) being converted into ions Result: Separate H+ and OH- ions H+ combines with water to make H 3 O+ ion

p. H: number of H ions in a solution Acid: any compound that releases H ions into water - H 3 O+ (hydronium ion is formed) ex: hydrochloric acid in water HCl H+ + Cl- **** Most reactive ion due to no electrons- attacks bonds in many molecules Base: compound that releases OH- ions into water Ex: Na. OH Na+ + OH-

• Neutralization reaction: production of H 2 O from mixture of strong acid and base neutral acidic basic, alkaline H H H = > < OH OH OH Buffer solution which resists changes in p. H when small quantities of an acid or an alkali are added to it • Important in maintaining p. H in organisms

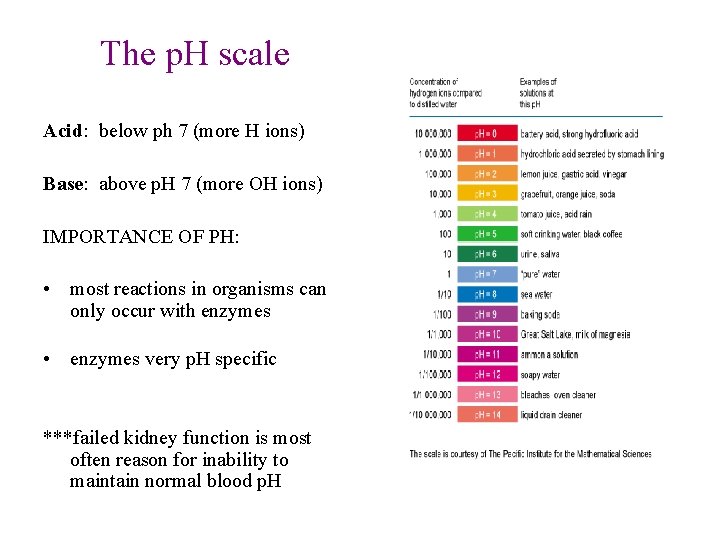
The p. H scale Acid: below ph 7 (more H ions) Base: above p. H 7 (more OH ions) IMPORTANCE OF PH: • most reactions in organisms can only occur with enzymes • enzymes very p. H specific ***failed kidney function is most often reason for inability to maintain normal blood p. H
 Hey hey bye bye
Hey hey bye bye Why is chemistry important
Why is chemistry important Why are life lessons important
Why are life lessons important Dont ask why why why
Dont ask why why why From most important to least important in writing
From most important to least important in writing Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Why is the resurrection important gcse
Why is the resurrection important gcse Why is footwork important in netball
Why is footwork important in netball Items that distort or prevent communication
Items that distort or prevent communication Why experience is important
Why experience is important Types of corporate strategy
Types of corporate strategy Why is water important to living things
Why is water important to living things Important of reading
Important of reading Physically diverse
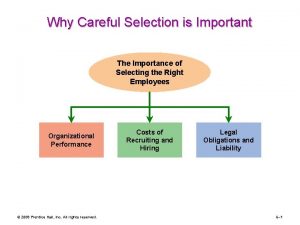
Physically diverse Why careful selection is important
Why careful selection is important Why are rivers important
Why are rivers important What is taxonomy and why is it important?
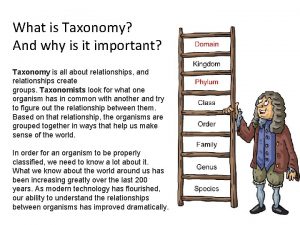
What is taxonomy and why is it important? Why is self-awareness important
Why is self-awareness important What is the passover
What is the passover What is advent
What is advent Why is ramadan important
Why is ramadan important Why are wetlands important
Why are wetlands important Managerial judgement workforce planning
Managerial judgement workforce planning Why is uml important
Why is uml important Why is tolerance important
Why is tolerance important Why is time management important
Why is time management important Importance of food quality
Importance of food quality Why is culture important
Why is culture important The importance of compliments
The importance of compliments 3 health triangle
3 health triangle