Why is Water Important to Living Things Water














- Slides: 23


Why is Water Important to Living Things? • Water is the substance that makes possible life as we know it here on Earth • Covers ¾ of the Earth’s Surface • Makes up 70 -95% of cells; most cells are surrounded by water • Only common substance to exist in all three physical states of matter – Solid, Liquid, and Gas – in the natural environment.

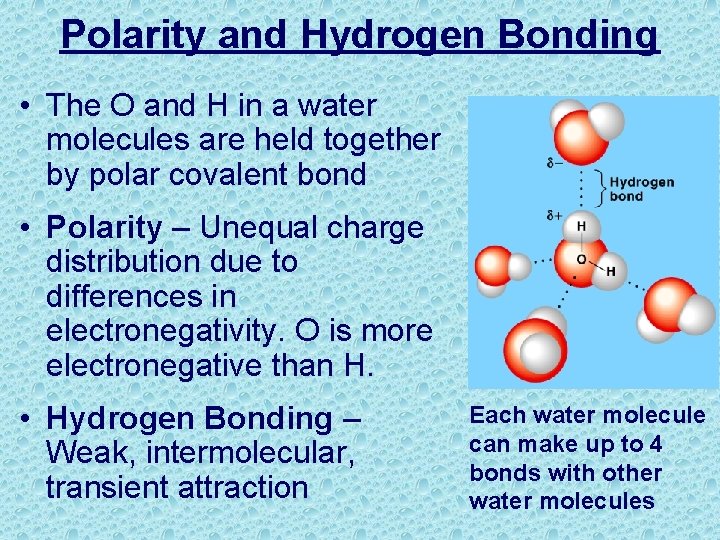
Polarity and Hydrogen Bonding • The O and H in a water molecules are held together by polar covalent bond • Polarity – Unequal charge distribution due to differences in electronegativity. O is more electronegative than H. • Hydrogen Bonding – Weak, intermolecular, transient attraction Each water molecule can make up to 4 bonds with other water molecules

Cohesion, Adhesion • Cohesion – Hydrogen bonds hold water molecules together. One water molecule can bond with up to 4 other water molecules at one time. • Adhesion – Allow water molecules to adhere to hydrophilic surfaces. Benefit to Life • Transport of water through the xylem of plants against gravity.

Water Cohesion/Adhesion • Allows water to move as continuous column upward through stems of plants

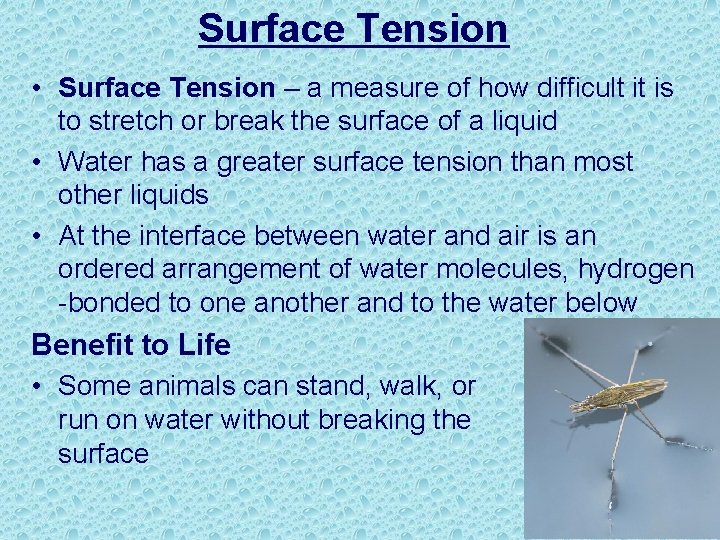
Surface Tension • Surface Tension – a measure of how difficult it is to stretch or break the surface of a liquid • Water has a greater surface tension than most other liquids • At the interface between water and air is an ordered arrangement of water molecules, hydrogen -bonded to one another and to the water below Benefit to Life • Some animals can stand, walk, or run on water without breaking the surface

Heat and Temperature • Anything that moves has kinetic energy, the energy of motion • The faster a molecule moves, the greater its kinetic energy. • Heat is a measure of the total quantity of kinetic energy due to molecular motion • Temperature measures the intensity of heat due to the average kinetic energy of the molecules – when the average speed of the molecules increases, a thermometer records this as a rise in temperature

High Specific Heat of Water • Amount of heat that must be absorbed or lost for 1 g of substance to change its temp. by 1 o. C • For water, the specific heat is 1 cal/g/o. C • Water resists changes in its temp. • Heat is absorbed to break H-bonds; released when bonds form.

High Specific Heat Benefit to Life • Large bodies of water (i. e. oceans) can absorb and store a huge amount of heat. In winter, heat lost to the air can warm it and make coastal areas milder in climate than inland regions. • Stabilizes temperatures – favorable environment for life – keeps land/water temperatures within limits that permit life. • Living things are mostly water – resists change in temperature and maintains somewhat constant temp.

High Heat of Vaporization • Energy required to change 1 g of substance from a liquid to a gas. • Water has a high heat of vap. – it takes a lot of energy to vaporize it. (580 cal at 25 o. C for 1 g of water) • An increase in heat breaks H-bonds releasing molecules to a gaseous state. Benefit to Life • The solar heat absorbed by tropical seas causes evaporation of water. As the moist air travels towards the poles it condenses to form rain

Evaporative Cooling • Water molecules with high kinetic energy (the “hottest” ones) evaporate (change from a liquid to a gas); remaining molecules are cooler. Benefit to Life • Stabilizes temperatures in lake and ponds. • Prevent terrestrial organisms from overheating. • High humidity prevents evaporation and sweat and makes us more uncomfortable.

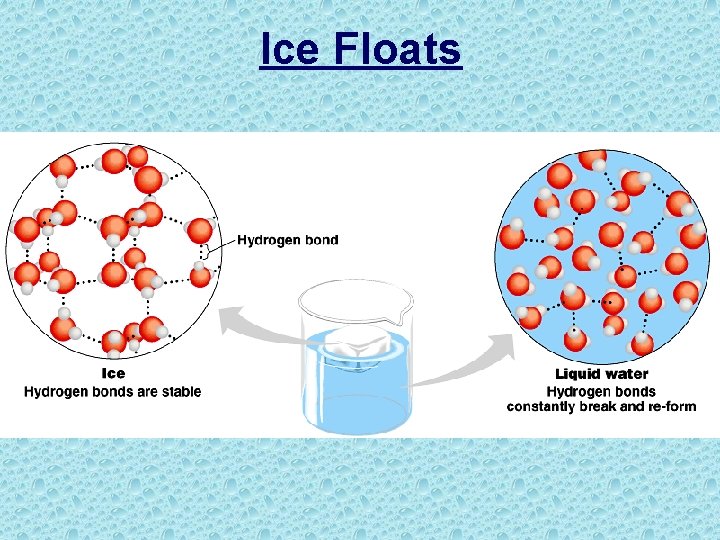
Ice Floats • Water expands as it freezes; less dense as a solid (fewer molecules for same volume) • Hydrogen bonds in ice keep the molecules far enough apart to make ice less dense than liquid water at 4 o. C or above. Benefit to Life • Floating ice insulates bodies of water so they don’t freeze solid – keeps the water below the ice from the colder air

Ice Floats

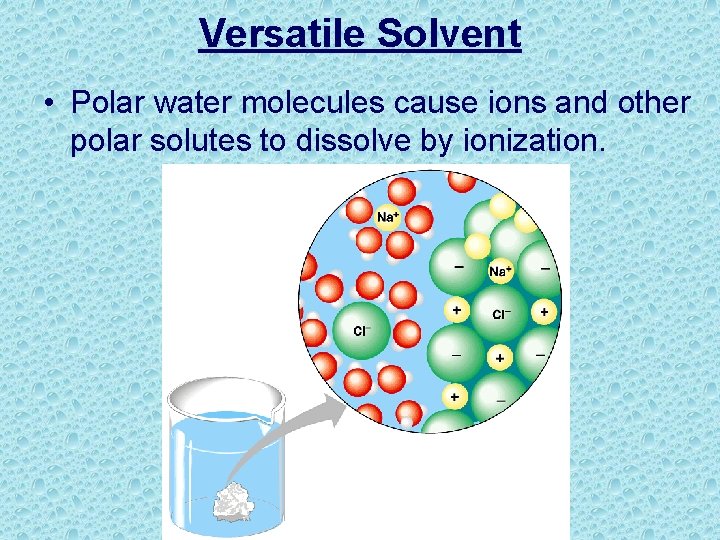
Versatile Solvent • Polar water molecules cause ions and other polar solutes to dissolve by ionization.

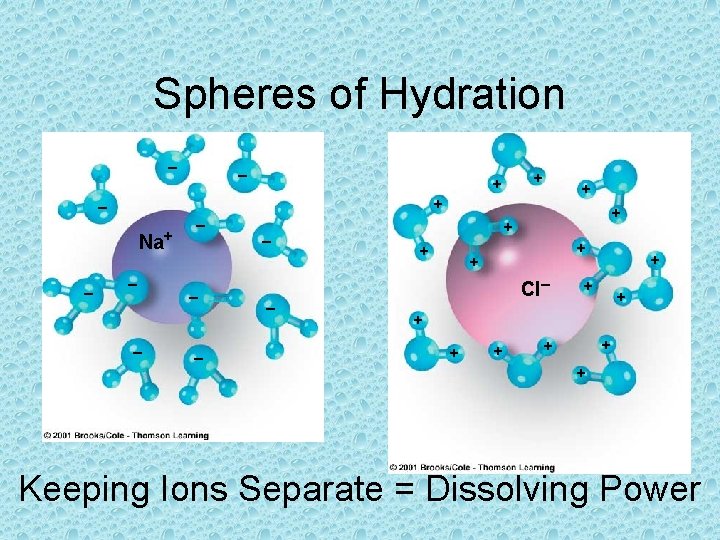
Spheres of Hydration – + + – Na+ – – – – – + + + Cl– + + + + + Keeping Ions Separate = Dissolving Power

Versatile Solvent • Solution – a liquid that is a completely homogeneous mixture of two or more substances • Solvent – the dissolving agent of a solution • Solute – the substance that is dissolved • Aqueous solution- solution where water is the solvent Benefit to Life • Most chemical reactions in living things involve solutes dissolved in water

To Dissolve or Not to Dissolve? • Hydrophilic: A substance with an affinity/able to dissolve in water. – What kind of substances? • Hydrophobic: A substance that repels water/don’t dissolve. – What kind of substances?

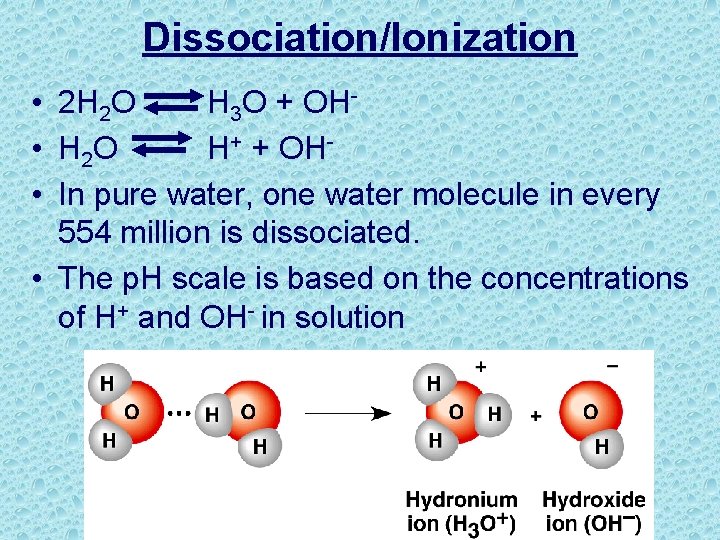
Dissociation/Ionization • 2 H 2 O H 3 O + OH • H 2 O H+ + OH • In pure water, one water molecule in every 554 million is dissociated. • The p. H scale is based on the concentrations of H+ and OH- in solution

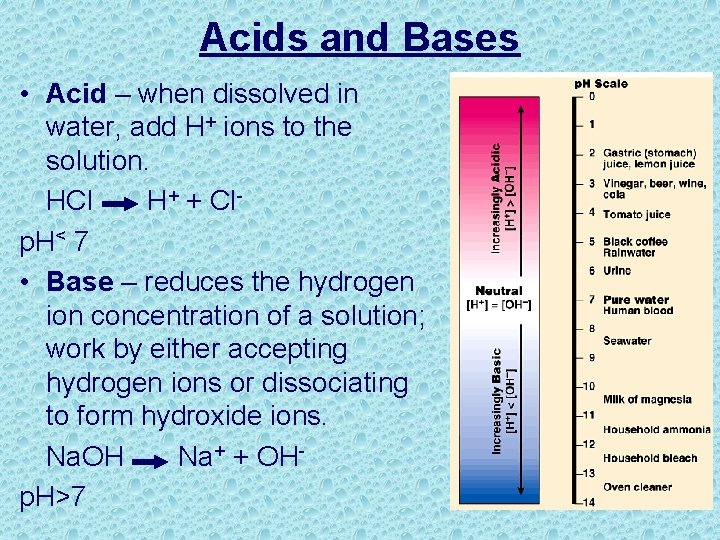
Acids and Bases • Acid – when dissolved in water, add H+ ions to the solution. HCl H+ + Clp. H< 7 • Base – reduces the hydrogen ion concentration of a solution; work by either accepting hydrogen ions or dissociating to form hydroxide ions. Na. OH Na+ + OHp. H>7

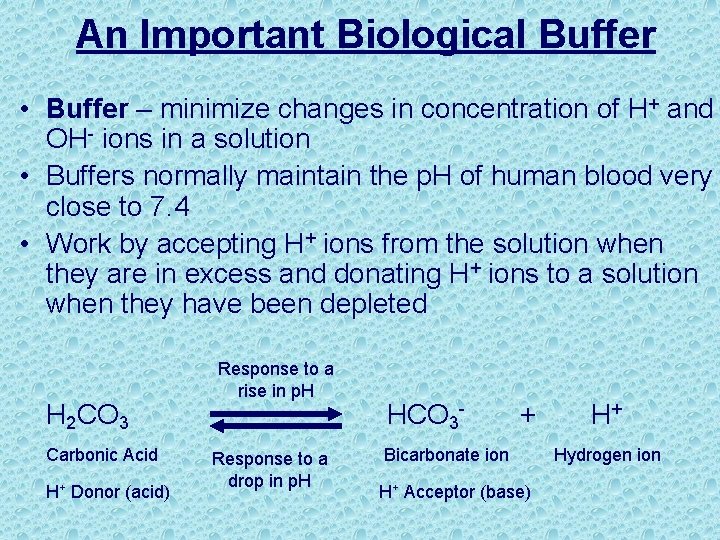
An Important Biological Buffer • Buffer – minimize changes in concentration of H+ and OH- ions in a solution • Buffers normally maintain the p. H of human blood very close to 7. 4 • Work by accepting H+ ions from the solution when they are in excess and donating H+ ions to a solution when they have been depleted H 2 CO 3 Carbonic Acid H+ Donor (acid) Response to a rise in p. H Response to a drop in p. H HCO 3 - + Bicarbonate ion H+ Acceptor (base) H+ Hydrogen ion

Carbonic Acid. Bicarbonate Buffer System • When blood p. H rises, carbonic acid dissociates to form bicarbonate and H+ H 2 C 03 -----> HC 03 - + H+ • When blood p. H drops, bicarbonate binds H+ to form carbonic acid HC 03 - + H+ -----> H 2 C 03

Acid Precipitation • Mostly sulfur oxides and nitrogen oxides dissolved in rainwater. • Come from fossil fuels burned in factories and automobiles • Aquatic animal’s egg and young are vulnerable to low p. H levels – can alter the structure of biochemical molecules and prevent them from carrying out essential chemical processes of life.

Acid Precipitation
 Why is water important to living things
Why is water important to living things Why are enzymes so important to living things
Why are enzymes so important to living things Pyramid hesd
Pyramid hesd The smallest living unit of all living things is
The smallest living unit of all living things is 7 life processes of living things
7 life processes of living things Pictures
Pictures The 8 levels of classification
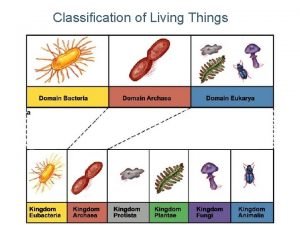
The 8 levels of classification Why do scientists classify organisms?
Why do scientists classify organisms? Water and water and water water
Water and water and water water What is b
What is b Is mold living or nonliving
Is mold living or nonliving Living non living dead
Living non living dead Dont ask
Dont ask Newspaper article format
Newspaper article format Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Desert living and nonliving things
Desert living and nonliving things What are the 5 kingdoms of living organisms
What are the 5 kingdoms of living organisms Living things grow
Living things grow Living things meaning
Living things meaning Ecosystems examples
Ecosystems examples Organic compounds made by living things
Organic compounds made by living things Living things grow images
Living things grow images Jackal linnaean system
Jackal linnaean system