THE CHEMISTRY OF LIFE LIFE DEPENDS ON CHEMISTRY















- Slides: 46

THE CHEMISTRY OF LIFE

LIFE DEPENDS ON CHEMISTRY • Millions of chemical reactions occur in living organisms every day. • Organisms rely on chemical reactions in order to function. • Food must be broken down, gases must be exchanged, molecules must be built.

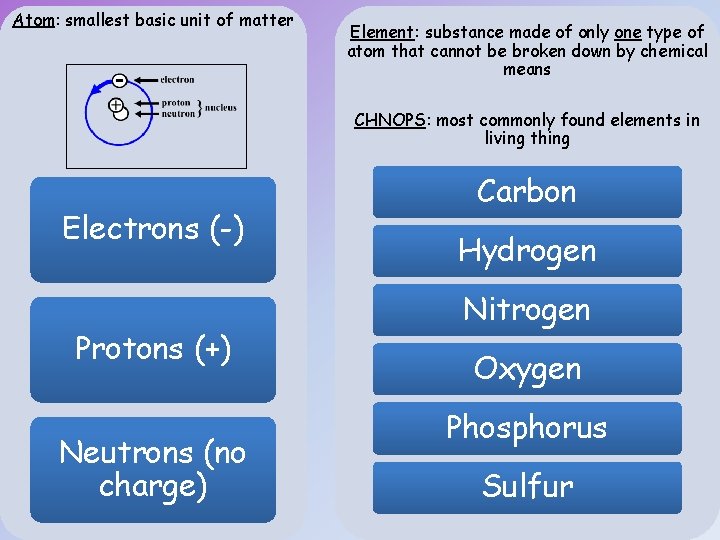
Atom: smallest basic unit of matter Element: substance made of only one type of atom that cannot be broken down by chemical means CHNOPS: most commonly found elements in living thing Electrons (-) Protons (+) Neutrons (no charge) Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur

PERIODIC TABLE OF ELEMENTS • There are more than 100 known elements • Each is represented by a 1, 2, or 3 letter symbol

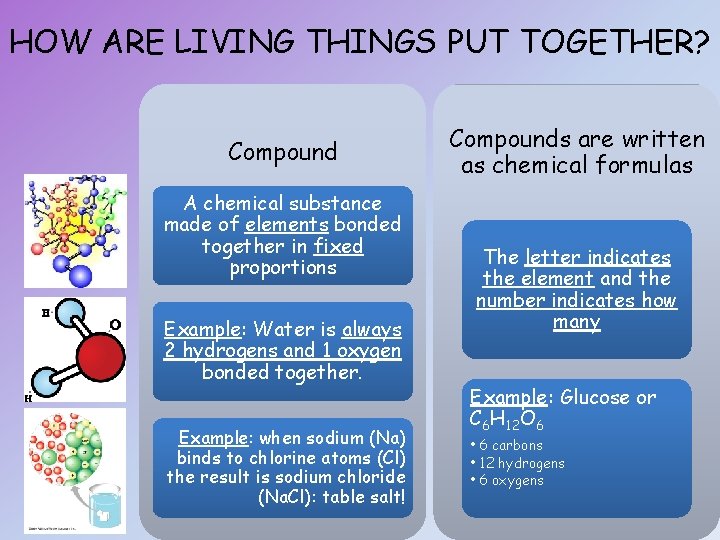
HOW ARE LIVING THINGS PUT TOGETHER? Compound A chemical substance made of elements bonded together in fixed proportions Example: Water is always 2 hydrogens and 1 oxygen bonded together. Example: when sodium (Na) binds to chlorine atoms (Cl) the result is sodium chloride (Na. Cl): table salt! Compounds are written as chemical formulas The letter indicates the element and the number indicates how many Example: Glucose or C 6 H 12 O 6 • 6 carbons • 12 hydrogens • 6 oxygens

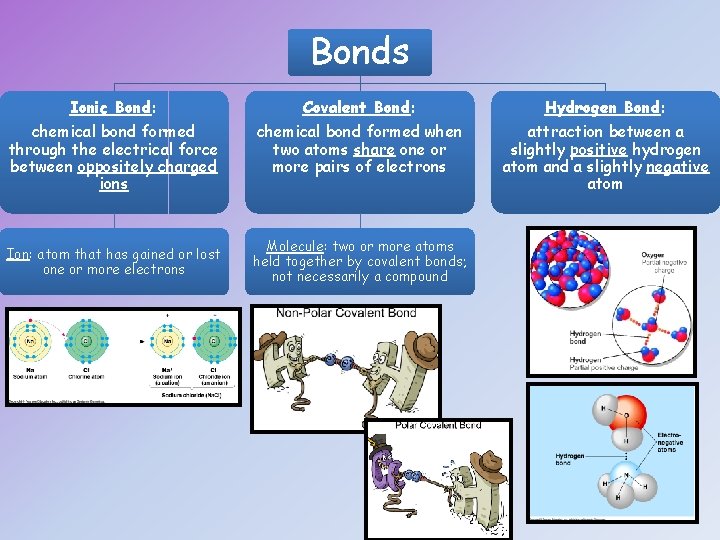
Bonds Ionic Bond: Covalent Bond: Hydrogen Bond: chemical bond formed through the electrical force between oppositely charged ions chemical bond formed when two atoms share one or more pairs of electrons attraction between a slightly positive hydrogen atom and a slightly negative atom Ion: atom that has gained or lost one or more electrons Molecule: two or more atoms held together by covalent bonds; not necessarily a compound

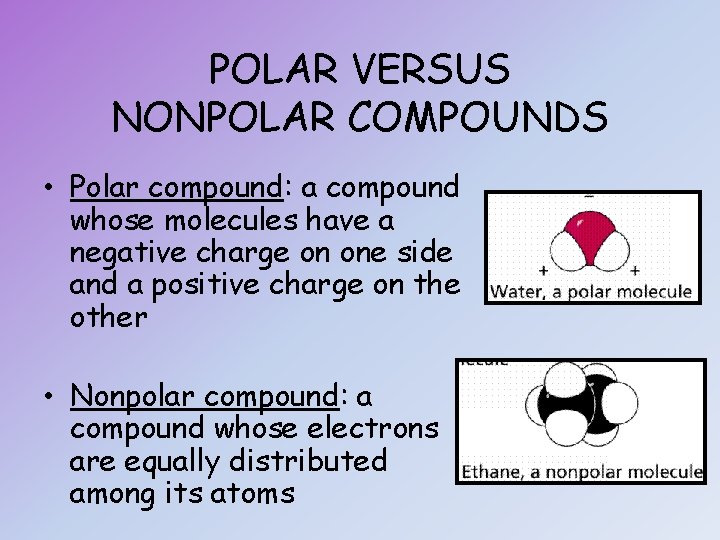
POLAR VERSUS NONPOLAR COMPOUNDS • Polar compound: a compound whose molecules have a negative charge on one side and a positive charge on the other • Nonpolar compound: a compound whose electrons are equally distributed among its atoms

WATER IN LIVING THINGS • Nearly 70% of your body is water! • 2/3 of the molecules in your body are water molecules! • Why is water sooooo important?

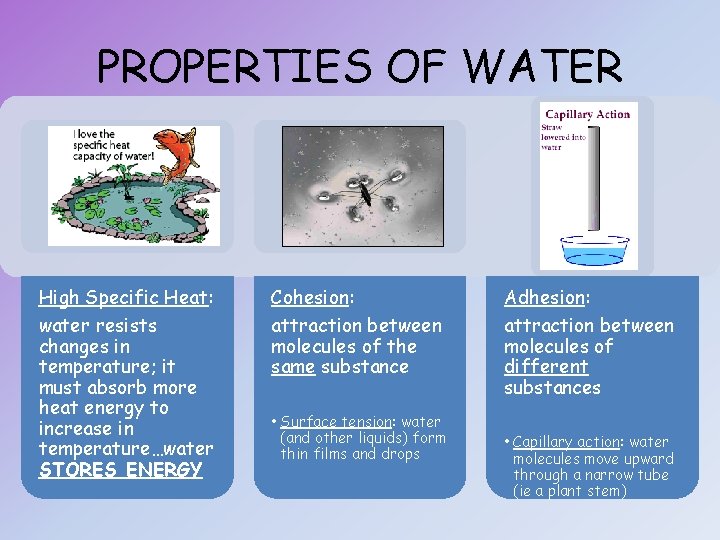
PROPERTIES OF WATER High Specific Heat: Cohesion: Adhesion: water resists changes in temperature; it must absorb more heat energy to increase in temperature…water STORES ENERGY attraction between molecules of the same substance attraction between molecules of different substances • Surface tension: water (and other liquids) form thin films and drops • Capillary action: water molecules move upward through a narrow tube (ie a plant stem)

HOW MANY DROPS OF WATER CAN FIT ON A PENNY?

WATER POLARITY • Properties of Water • Enables many substances to dissolve in water • Ionic compounds and polar molecules dissolve best in water • Nonpolar molecules do not dissolve well in water (ie oil)

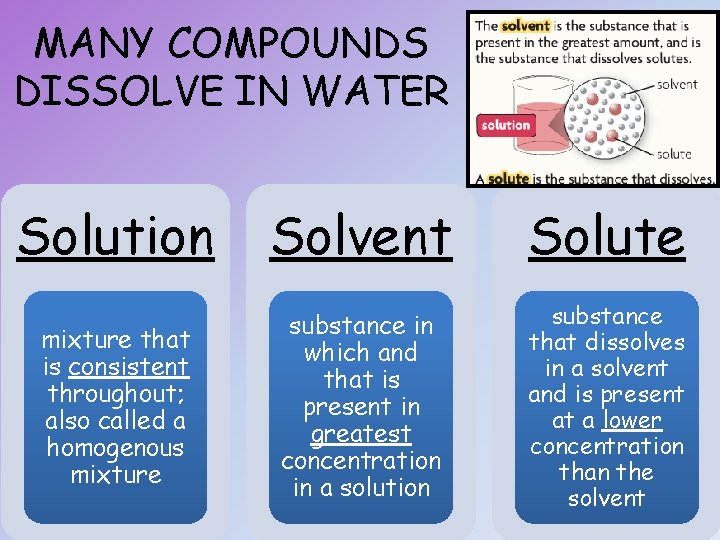
MANY COMPOUNDS DISSOLVE IN WATER Solution Solvent Solute mixture that is consistent throughout; also called a homogenous mixture substance in which and that is present in greatest concentration in a solution substance that dissolves in a solvent and is present at a lower concentration than the solvent

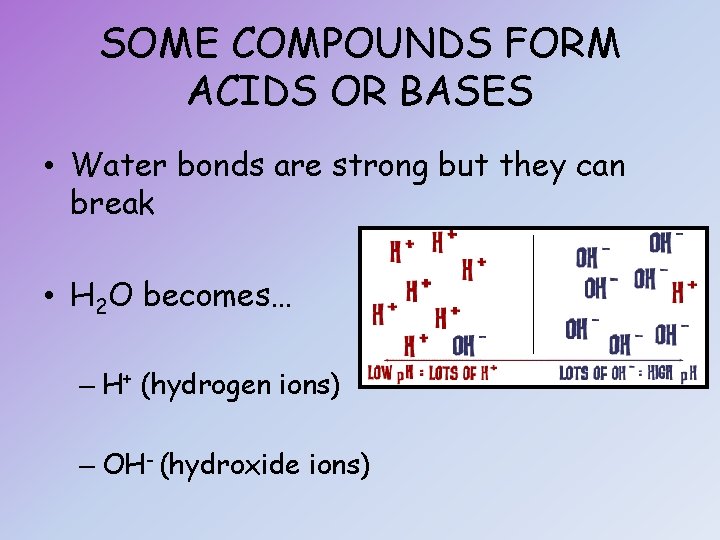
SOME COMPOUNDS FORM ACIDS OR BASES • Water bonds are strong but they can break • H 2 O becomes… – H+ (hydrogen ions) – OH- (hydroxide ions)

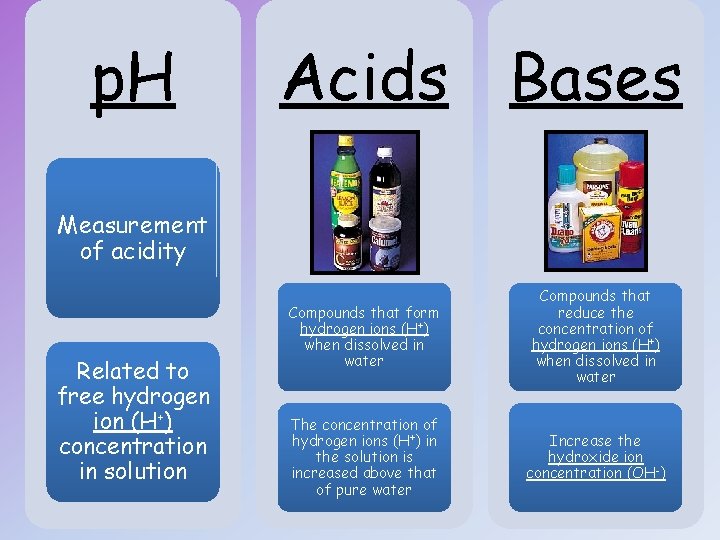
p. H Acids Bases Measurement of acidity Related to free hydrogen ion (H+) concentration in solution Compounds that form hydrogen ions (H+) when dissolved in water Compounds that reduce the concentration of hydrogen ions (H+) when dissolved in water The concentration of hydrogen ions (H+) in the solution is increased above that of pure water Increase the hydroxide ion concentration (OH-)

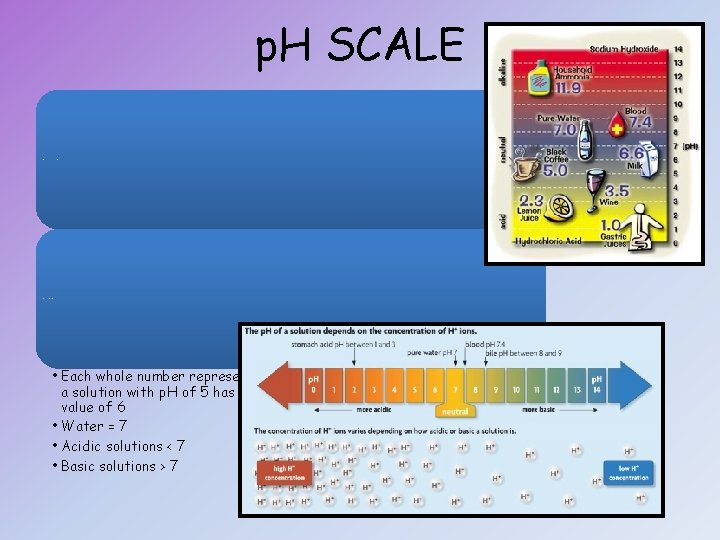
p. H SCALE Based on the concentration of H+ in solutions p. H between 0 and 14 • Each whole number represents a factor of 10 on the scale: for example, a solution with p. H of 5 has 10 X as many hydrogen ions as one with a p. H value of 6 • Water = 7 • Acidic solutions < 7 • Basic solutions > 7

CLASSWORK/HOMEWORK • Solutions, Acids and Bases worksheet (front and back)

CARBON-BASED MOLECULES • Most matter in your body that is not water is made of organic compounds carbon • Organic compounds: contain atoms that are covalently bonded to other elements

CARBON ATOMS HAVE UNIQUE BONDING PROPERTIES • Carbon forms the backbone or basic structure of all large organic molecules – Carbon can bond with up to 4 other atoms at once. – Carbon can form single, double or triple bonds. – Carbon bonds easily with other carbon atoms to form the backbone of large organic molecules – Carbon can bond with many different elements such as H, N, O, P, S • Monomer: molecular subunit of a polymer… a ”building block” • Polymer: large, carbon-based molecule formed by monomers

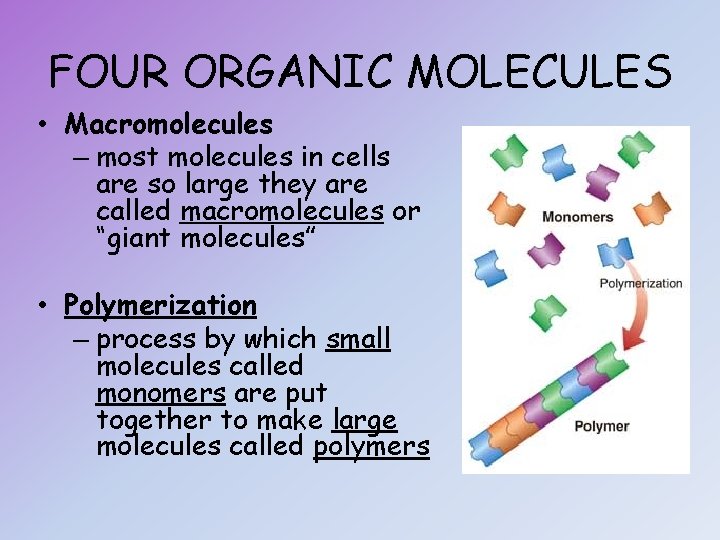
FOUR ORGANIC MOLECULES • Macromolecules – most molecules in cells are so large they are called macromolecules or “giant molecules” • Polymerization – process by which small molecules called monomers are put together to make large molecules called polymers

FOUR MAIN TYPES OF CARBONBASED MOLECULES ARE FOUND IN LIVING THINGS • Carbohydrates • Lipids • Nucleic acids • Proteins

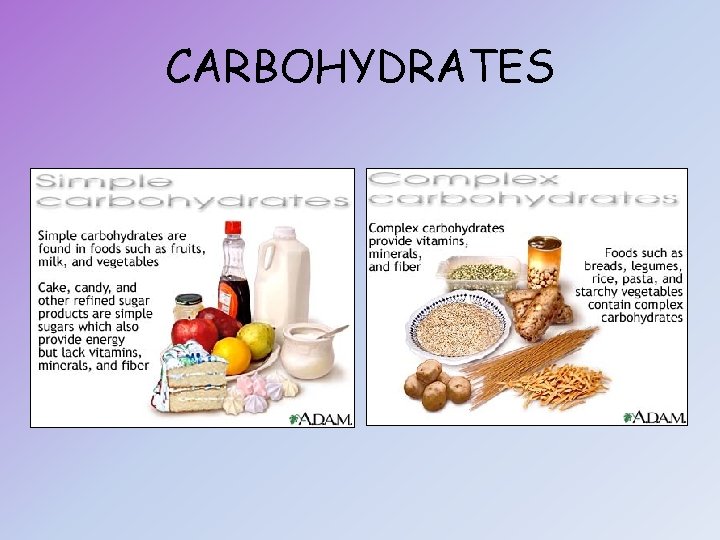
CARBOHYDRATES

CARBOHYDRATES Composition: carbon, hydrogen and oxygen atoms 1: 2: 1 Monomers: • “Monosaccharides” • Simple sugars: glucose (C 6 H 12 O 6) and fructose Examples: • Disaccharides: double sugars formed when two monosaccharides are joined • Polysaccharides: chains of 3 or more monosaccharides (ie starch) • Starch: made by plants • Glycogen: made by animals • Cellulose: provides structural support for plants; humans cannot digest it Function: • Key source of energy, found in most foods • Glucose is the main source of energy in cells

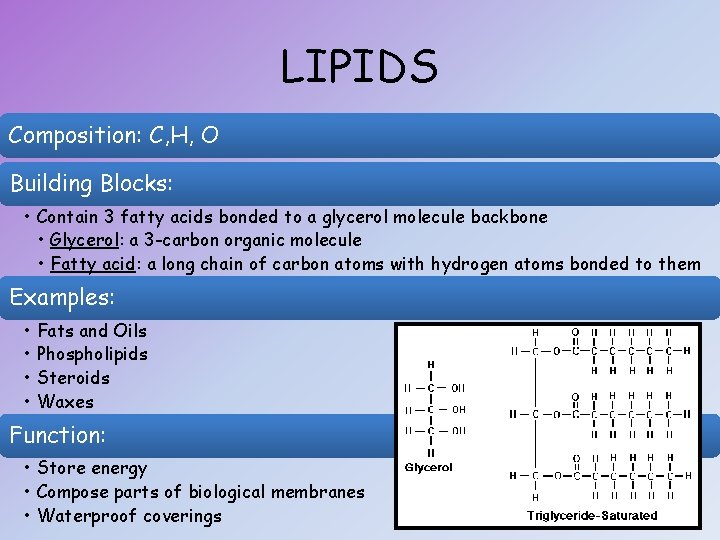
LIPIDS Composition: C, H, O Building Blocks: • Contain 3 fatty acids bonded to a glycerol molecule backbone • Glycerol: a 3 -carbon organic molecule • Fatty acid: a long chain of carbon atoms with hydrogen atoms bonded to them Examples: • • Fats and Oils Phospholipids Steroids Waxes Function: • Store energy • Compose parts of biological membranes • Waterproof coverings

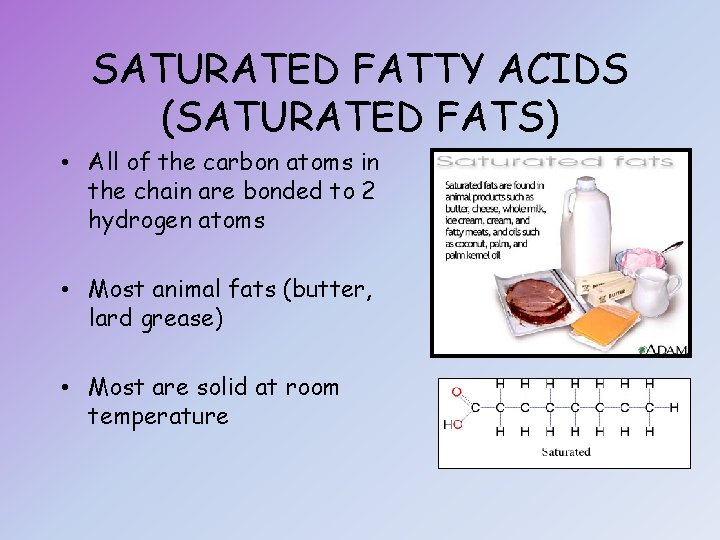
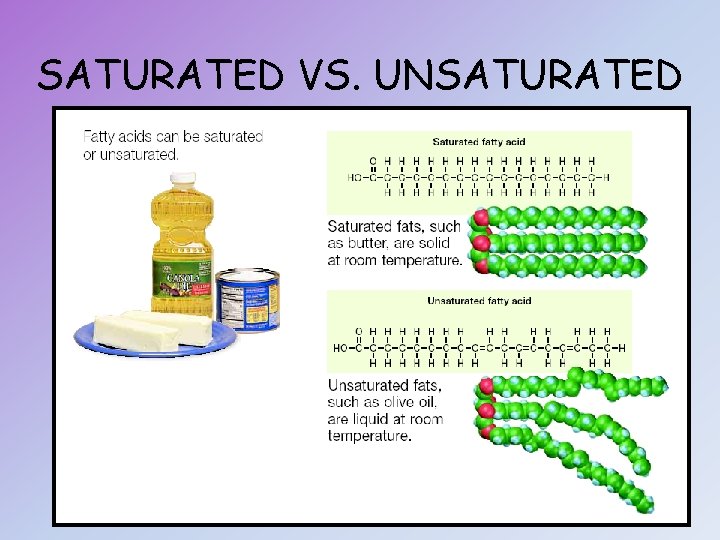
SATURATED FATTY ACIDS (SATURATED FATS) • All of the carbon atoms in the chain are bonded to 2 hydrogen atoms • Most animal fats (butter, lard grease) • Most are solid at room temperature

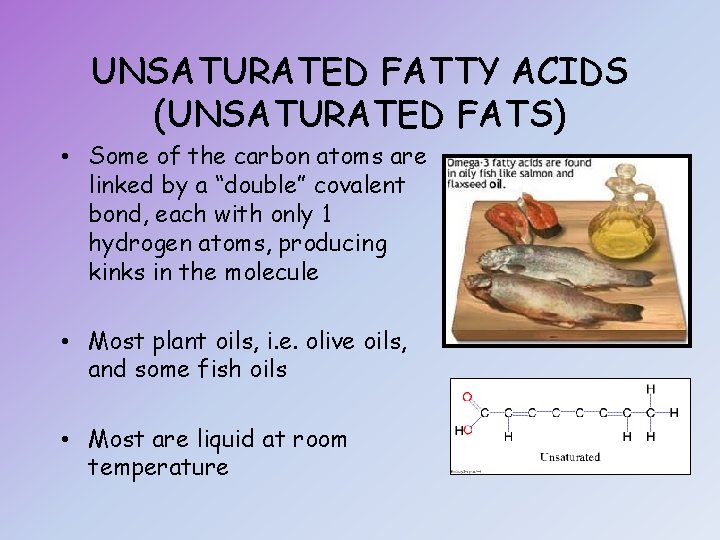
UNSATURATED FATTY ACIDS (UNSATURATED FATS) • Some of the carbon atoms are linked by a “double” covalent bond, each with only 1 hydrogen atoms, producing kinks in the molecule • Most plant oils, i. e. olive oils, and some fish oils • Most are liquid at room temperature

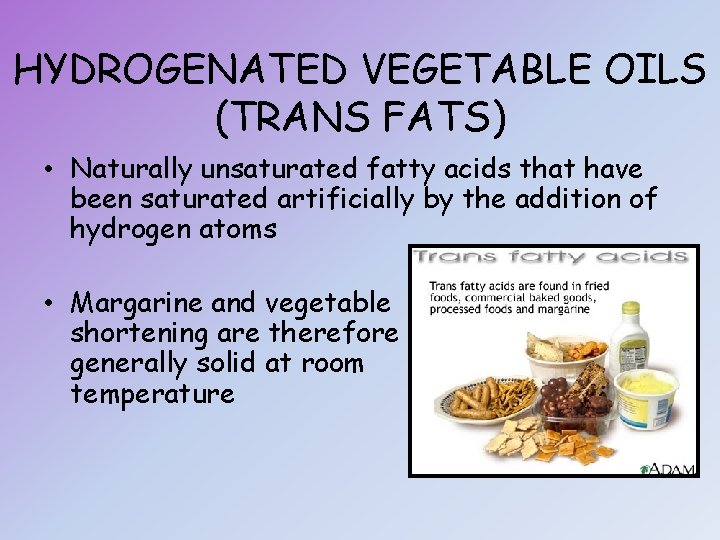
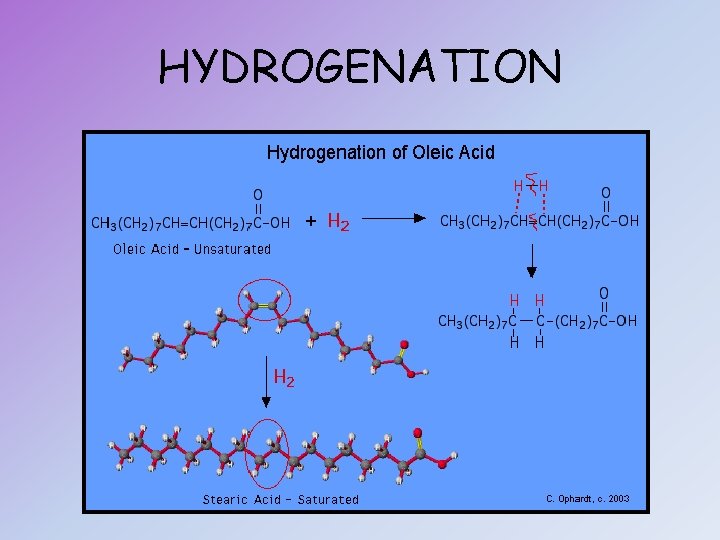
HYDROGENATED VEGETABLE OILS (TRANS FATS) • Naturally unsaturated fatty acids that have been saturated artificially by the addition of hydrogen atoms • Margarine and vegetable shortening are therefore generally solid at room temperature

SATURATED VS. UNSATURATED

HYDROGENATION

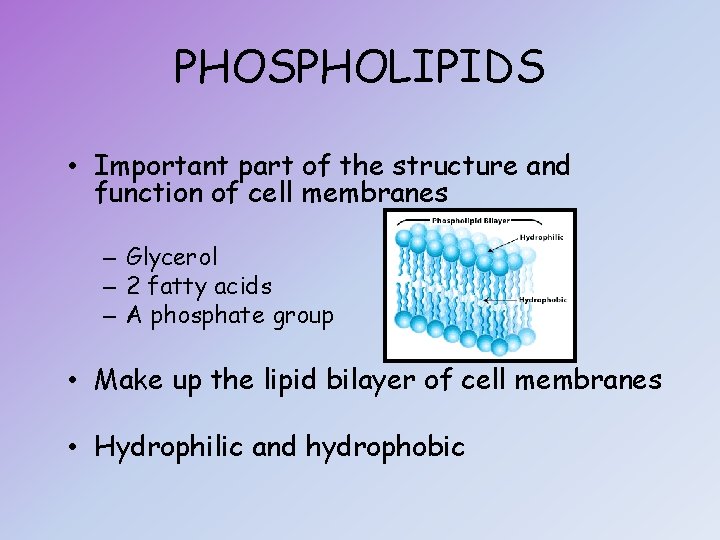
PHOSPHOLIPIDS • Important part of the structure and function of cell membranes – Glycerol – 2 fatty acids – A phosphate group • Make up the lipid bilayer of cell membranes • Hydrophilic and hydrophobic

STEROIDS AND WAXES • Steroids: include cholesterol, found in animal cell membranes • Waxes: are used to coat and protect things in nature. • Steroids occur in animals in hormones • Bees…plants…your ears! • A four-ring structure, one with five carbons and three with six carbons in the rings

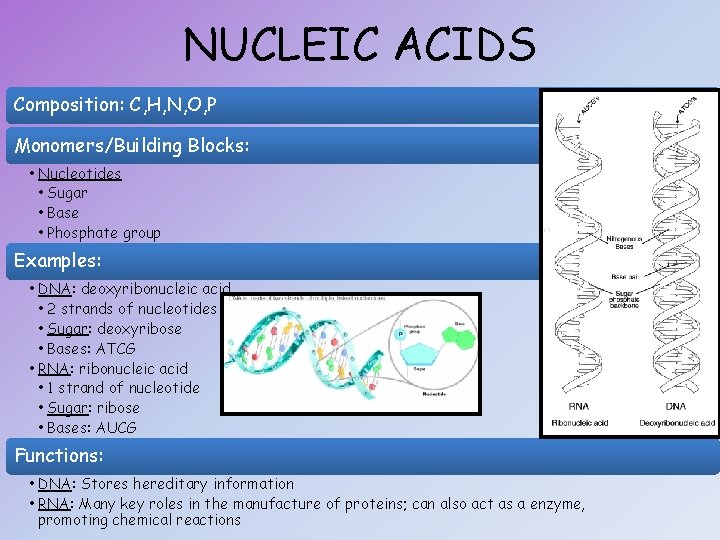
NUCLEIC ACIDS Composition: C, H, N, O, P Monomers/Building Blocks: • Nucleotides • Sugar • Base • Phosphate group Examples: • DNA: deoxyribonucleic acid • 2 strands of nucleotides • Sugar: deoxyribose • Bases: ATCG • RNA: ribonucleic acid • 1 strand of nucleotide • Sugar: ribose • Bases: AUCG Functions: • DNA: Stores hereditary information • RNA: Many key roles in the manufacture of proteins; can also act as a enzyme, promoting chemical reactions

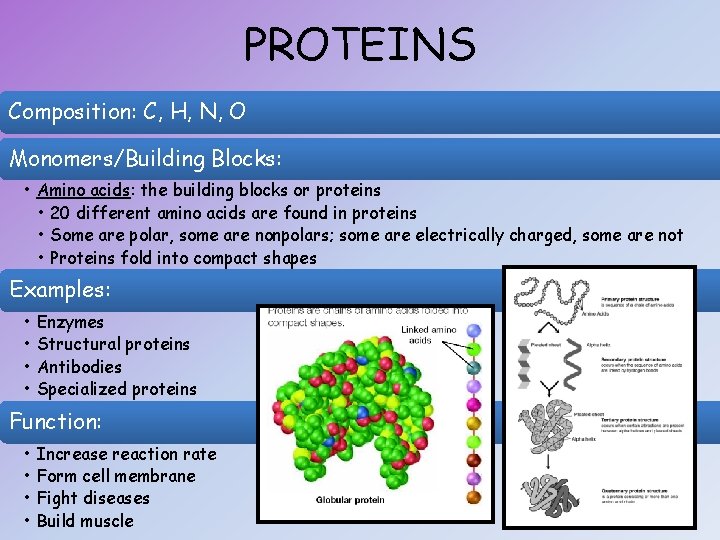
PROTEINS Composition: C, H, N, O Monomers/Building Blocks: • Amino acids: the building blocks or proteins • 20 different amino acids are found in proteins • Some are polar, some are nonpolars; some are electrically charged, some are not • Proteins fold into compact shapes Examples: • • Enzymes Structural proteins Antibodies Specialized proteins Function: • • Increase reaction rate Form cell membrane Fight diseases Build muscle

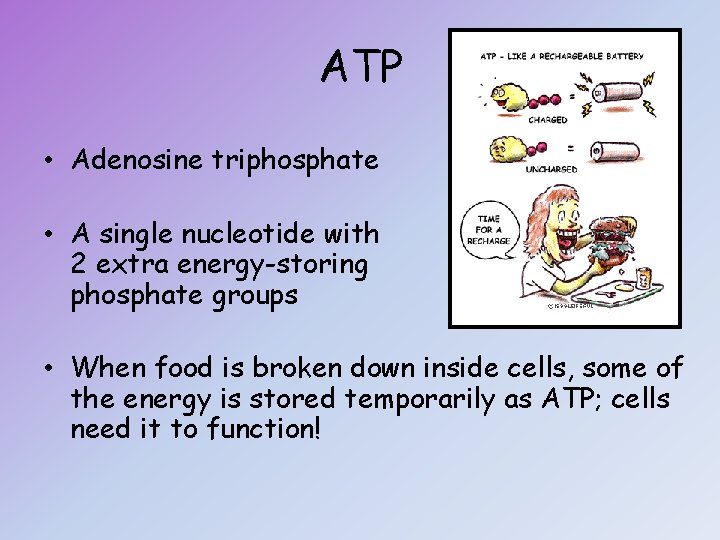
ATP • Adenosine triphosphate • A single nucleotide with 2 extra energy-storing phosphate groups • When food is broken down inside cells, some of the energy is stored temporarily as ATP; cells need it to function!

IN SUMMARY…


CLASSWORK/HOMEWORK • Chemistry of Life packet • Frayer Model • Organic Molecules worksheet (front and back) • Investigating Organic molecules (cut-out)

BONDS BREAK AND FORM DURING CHEMICAL REACTIONS • Chemical reaction: process by which substances change into different substances through the breaking or forming of chemical bonds • Reactants: the starting materials for chemical reactions • Products: the newly formed substances Reactants Products means “changes to” or “forms” Na. Cl Na+ + Cl- • Equilibrium: condition in which reactants and products of a chemical reaction are formed at the same time

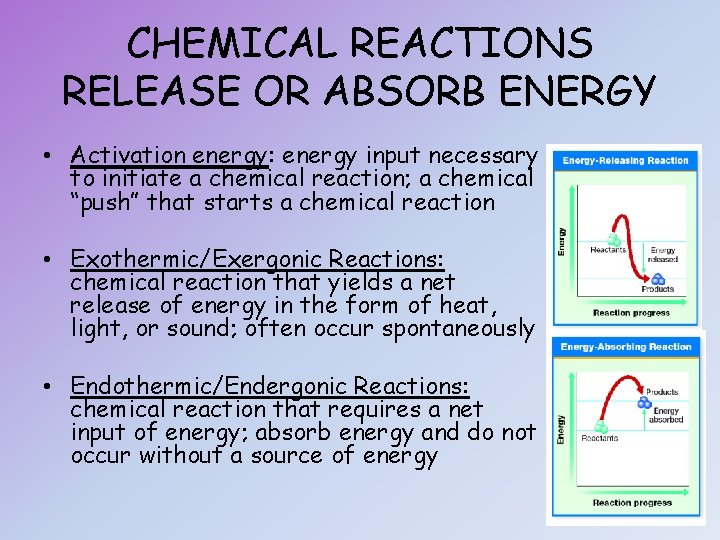
CHEMICAL REACTIONS RELEASE OR ABSORB ENERGY • Activation energy: energy input necessary to initiate a chemical reaction; a chemical “push” that starts a chemical reaction • Exothermic/Exergonic Reactions: chemical reaction that yields a net release of energy in the form of heat, light, or sound; often occur spontaneously • Endothermic/Endergonic Reactions: chemical reaction that requires a net input of energy; absorb energy and do not occur without a source of energy

ENZYMES • Without enzymes some cell processes would take too long! • Enzyme: protein that catalyzes chemical reactions for organisms • Enzymes work by lowering the activation energy of the reaction. • Enzymes temporarily bind to reactants to help them react so the shape of the enzyme is very important. • Enzymes are not altered during the reaction so they can catalyze the same reaction over and over. • p. H and temperature affected how well enzymes work because they change the shape of the protein.

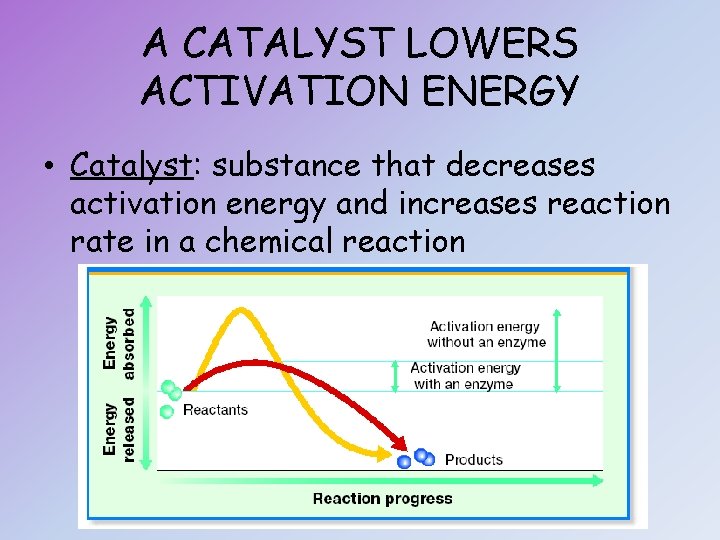
A CATALYST LOWERS ACTIVATION ENERGY • Catalyst: substance that decreases activation energy and increases reaction rate in a chemical reaction

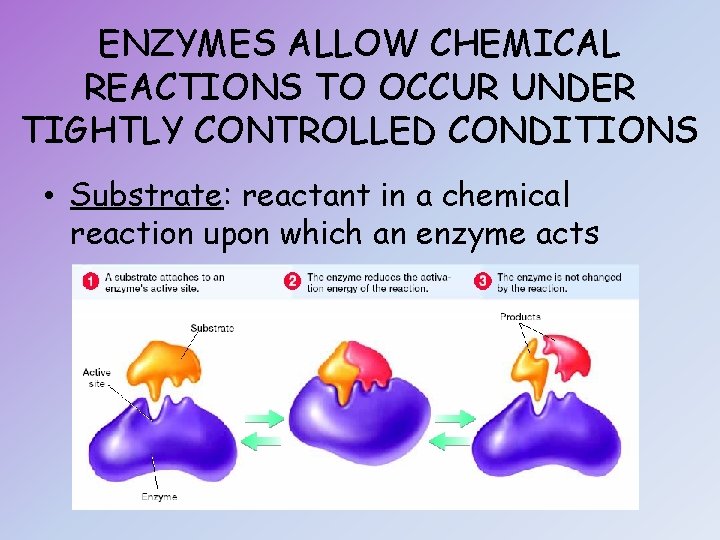
ENZYMES ALLOW CHEMICAL REACTIONS TO OCCUR UNDER TIGHTLY CONTROLLED CONDITIONS • Substrate: reactant in a chemical reaction upon which an enzyme acts

ENZYME SPECIFICITY • Substrate: a substance on which an enzyme acts during a chemical reaction • Enzymes only act on specific substrates • Active site: the site on an enzyme that attaches to the substrate • Video

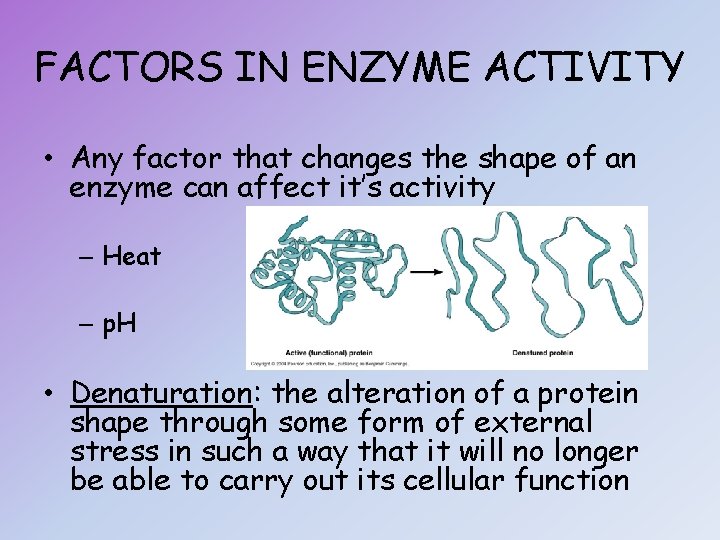
FACTORS IN ENZYME ACTIVITY • Any factor that changes the shape of an enzyme can affect it’s activity – Heat – p. H • Denaturation: the alteration of a protein shape through some form of external stress in such a way that it will no longer be able to carry out its cellular function

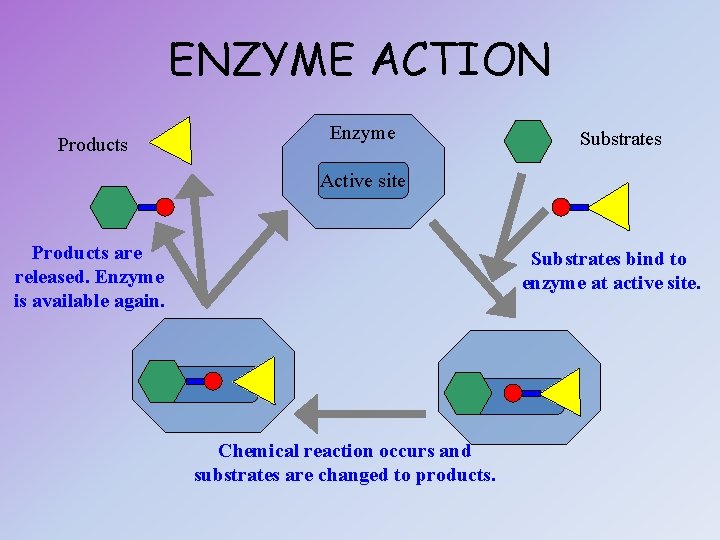
ENZYME ACTION Products Enzyme Substrates Active site Products are released. Enzyme is available again. Substrates bind to enzyme at active site. Chemical reaction occurs and substrates are changed to products.

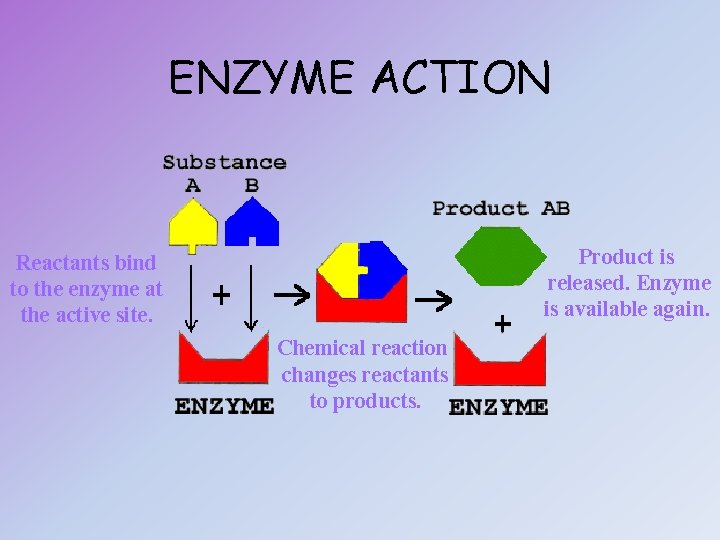
ENZYME ACTION Product is released. Enzyme is available again. Reactants bind to the enzyme at the active site. Chemical reaction changes reactants to products.

CLASSWORK/HOMEWORK • Practice with Reactions and Energy worksheet
 Life depends on the sun
Life depends on the sun Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Soils in ____ contain little organic material and are thin.
Soils in ____ contain little organic material and are thin. The method used for transferring a patient depends on
The method used for transferring a patient depends on Thermal energy depends on
Thermal energy depends on How to measure pitch of sound
How to measure pitch of sound