GIGO Measurement of Lead Depends on Chemistry of

















- Slides: 60

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out Sample Prep Instrument Out put Signal (Data)

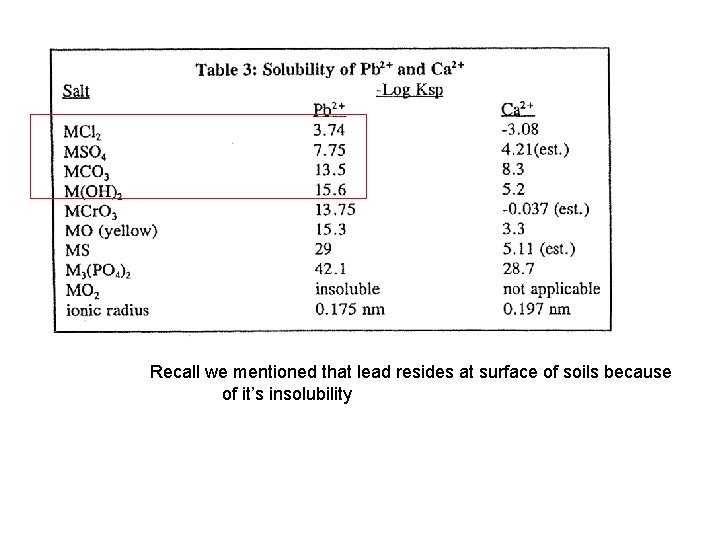
Recall we mentioned that lead resides at surface of soils because of it’s insolubility

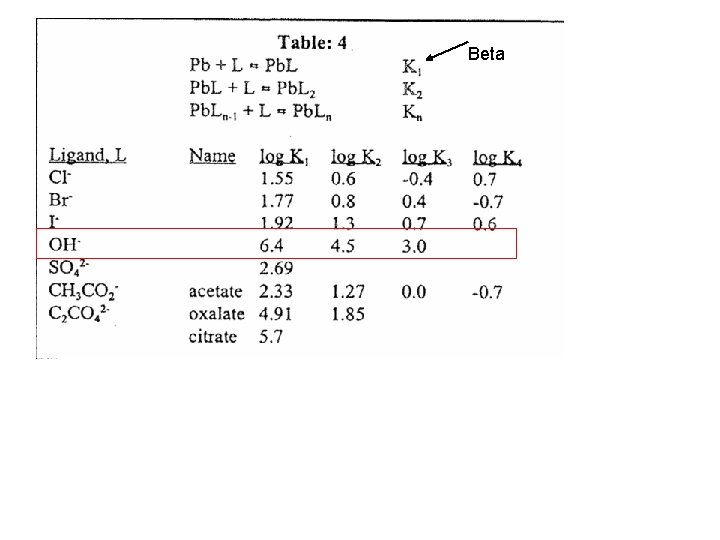
Beta

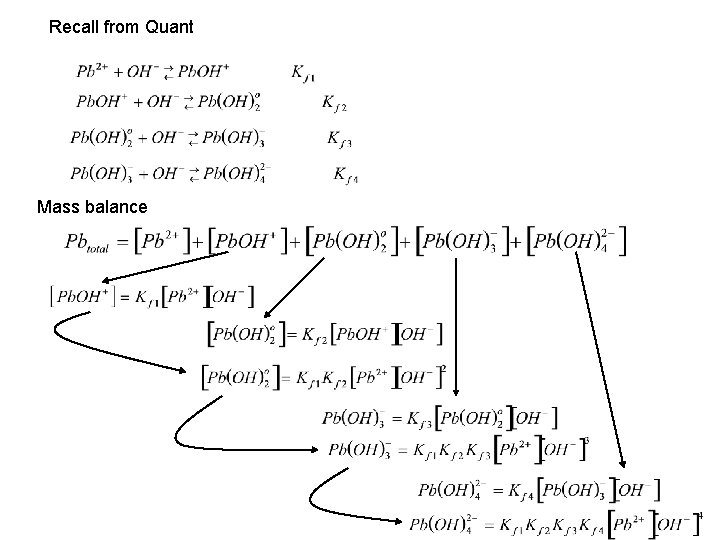
Recall from Quant Mass balance

Mass balance Make a definition to simplify the expression, and factor out terms



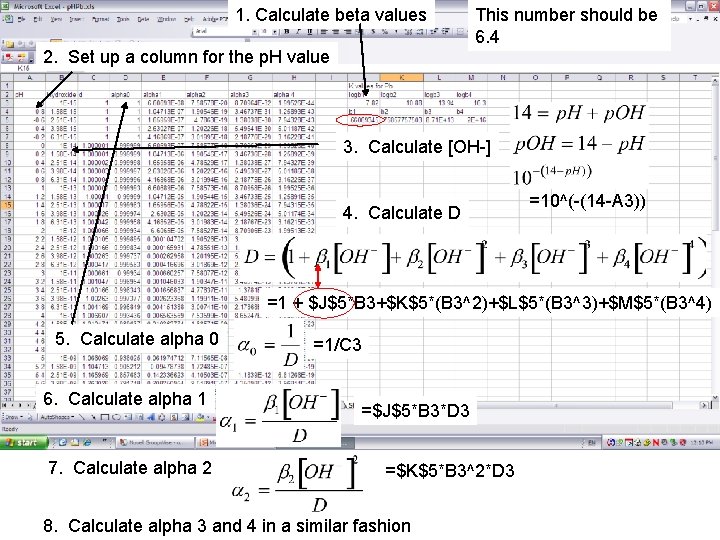
1. Calculate beta values This number should be 6. 4 2. Set up a column for the p. H value 3. Calculate [OH-] 4. Calculate D =10^(-(14 -A 3)) =1 + $J$5*B 3+$K$5*(B 3^2)+$L$5*(B 3^3)+$M$5*(B 3^4) 5. Calculate alpha 0 6. Calculate alpha 1 7. Calculate alpha 2 =1/C 3 =$J$5*B 3*D 3 =$K$5*B 3^2*D 3 8. Calculate alpha 3 and 4 in a similar fashion

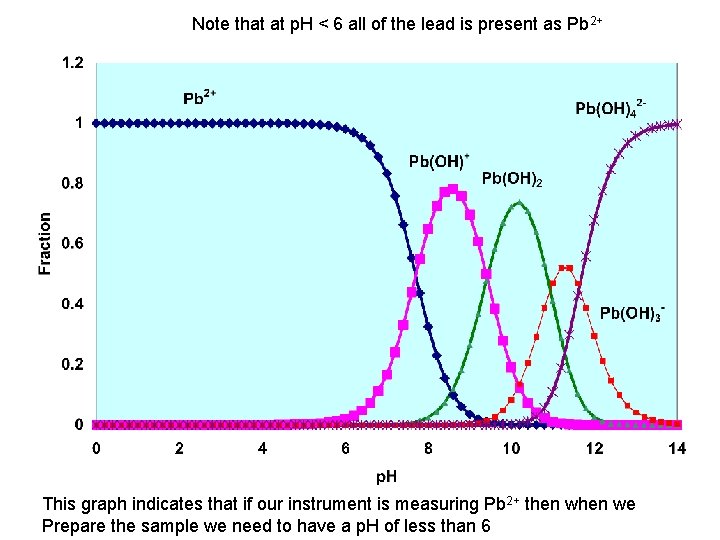
Note that at p. H < 6 all of the lead is present as Pb 2+ This graph indicates that if our instrument is measuring Pb 2+ then we Prepare the sample we need to have a p. H of less than 6

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out

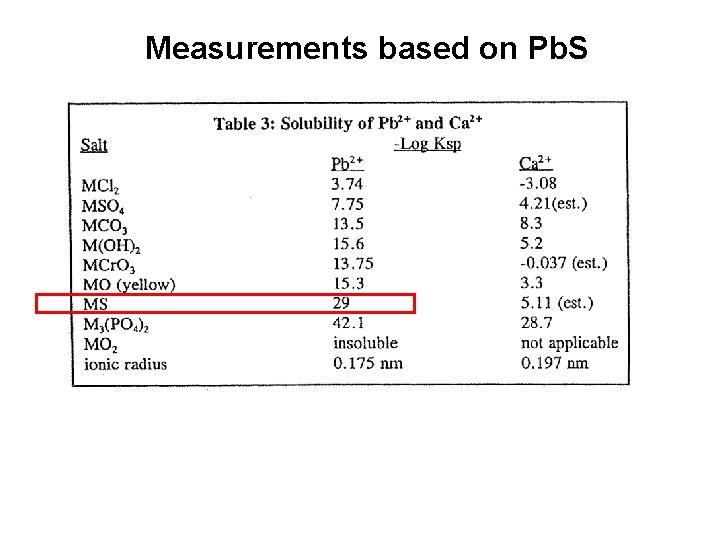
Measurements based on Pb. S

Measurements based on Pb. S 1820 Frederick Acum London

“ 1 part of acetate of lead May be detected by means of it in 20000 Parts of water. ”

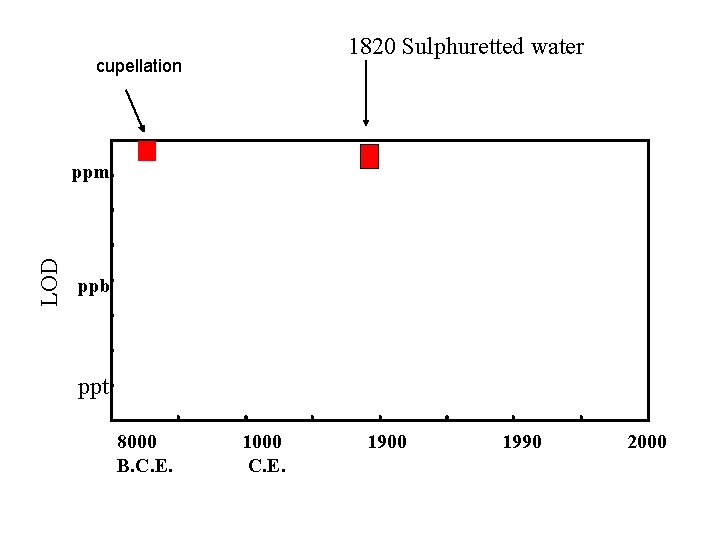
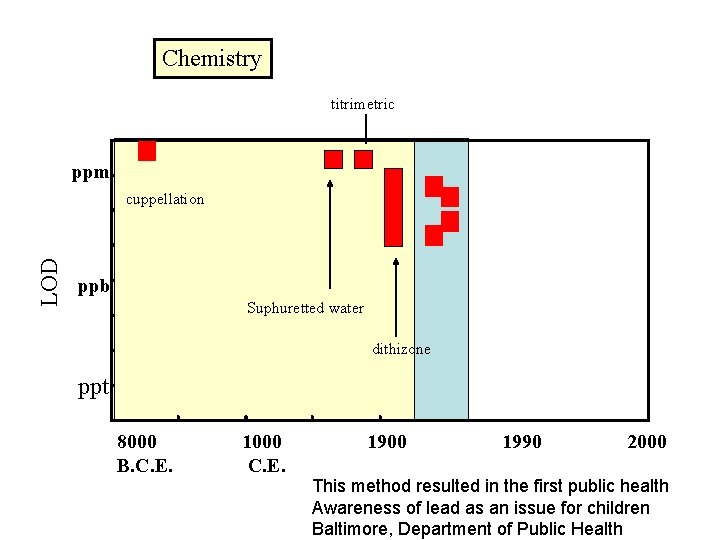
1820 Sulphuretted water cupellation LOD ppm ppb ppt 8000 B. C. E. 1000 C. E. 1900 1990 2000

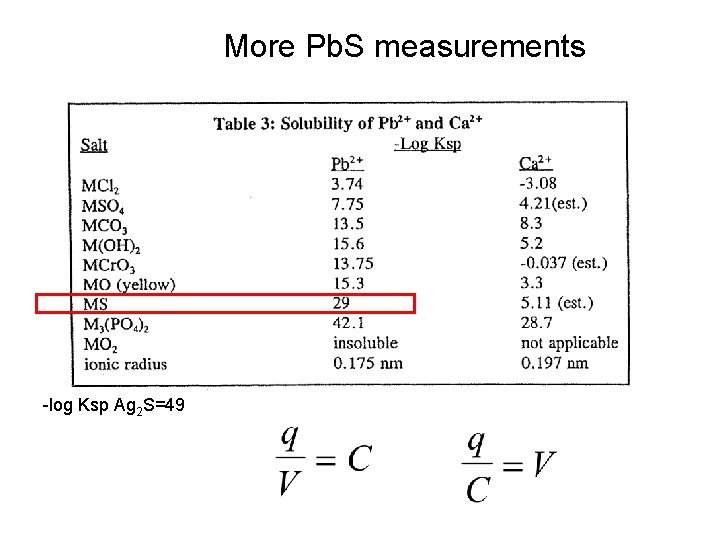
More Pb. S measurements -log Ksp Ag 2 S=49

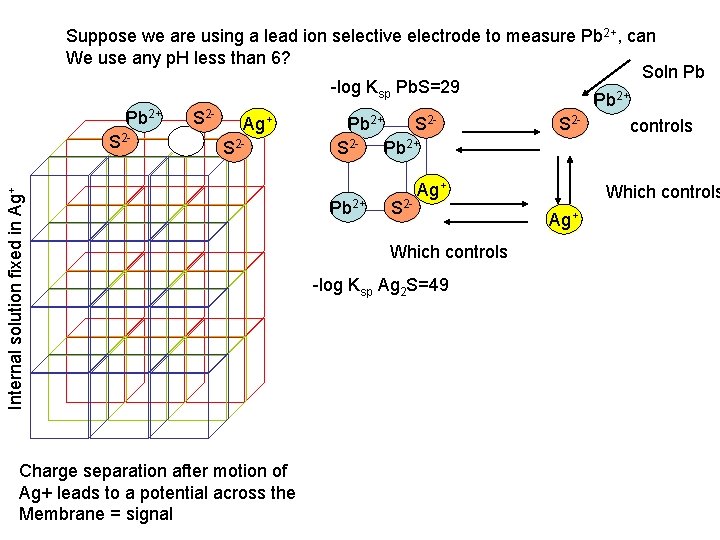
Internal solution fixed in Ag+ Suppose we are using a lead ion selective electrode to measure Pb 2+, can We use any p. H less than 6? Soln Pb -log Ksp Pb. S=29 Pb 2+ S 2 Ag+ Pb 2+ S 2 S 2 controls 2 S S 2 S 2 - Pb 2+ Charge separation after motion of Ag+ leads to a potential across the Membrane = signal Pb 2+ S 2 - Ag+ Which controls -log Ksp Ag 2 S=49 Which controls Ag+

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out

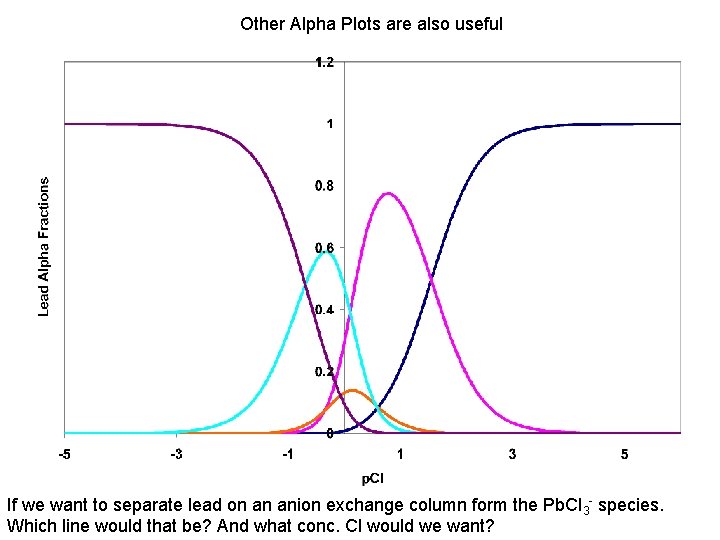
Other Alpha Plots are also useful If we want to separate lead on an anion exchange column form the Pb. Cl 3 - species. Which line would that be? And what conc. Cl would we want?

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out

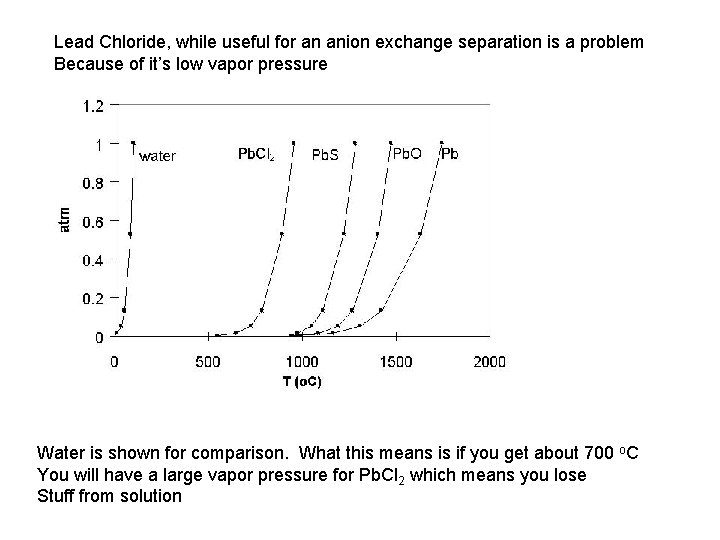
Lead Chloride, while useful for an anion exchange separation is a problem Because of it’s low vapor pressure Water is shown for comparison. What this means is if you get about 700 o. C You will have a large vapor pressure for Pb. Cl 2 which means you lose Stuff from solution

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of preparing the sample Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out only instrument you have is a…. . UV-Vis Spectrophotometer UV-Vis monitors valence shell electrons Need to convert Pb to something that a. Has UV-Vis activity b. That can be selective toward Pb binding c. That can be separated from other binding metals

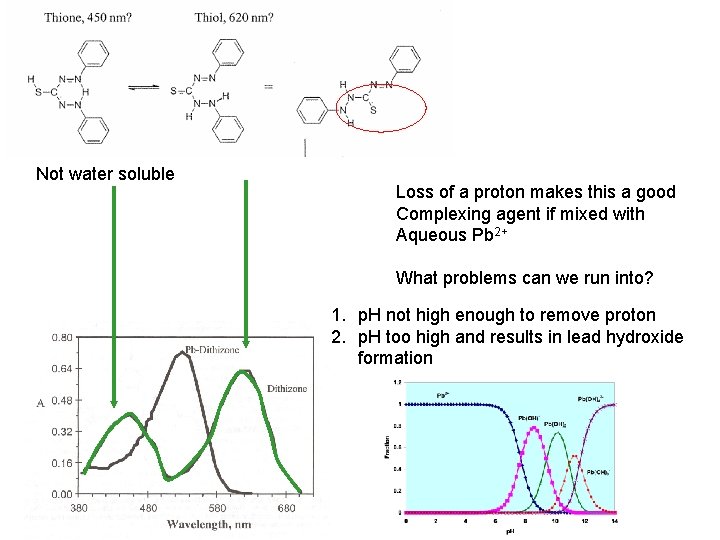
Not water soluble Loss of a proton makes this a good Complexing agent if mixed with Aqueous Pb 2+ What problems can we run into? 1. p. H not high enough to remove proton 2. p. H too high and results in lead hydroxide formation


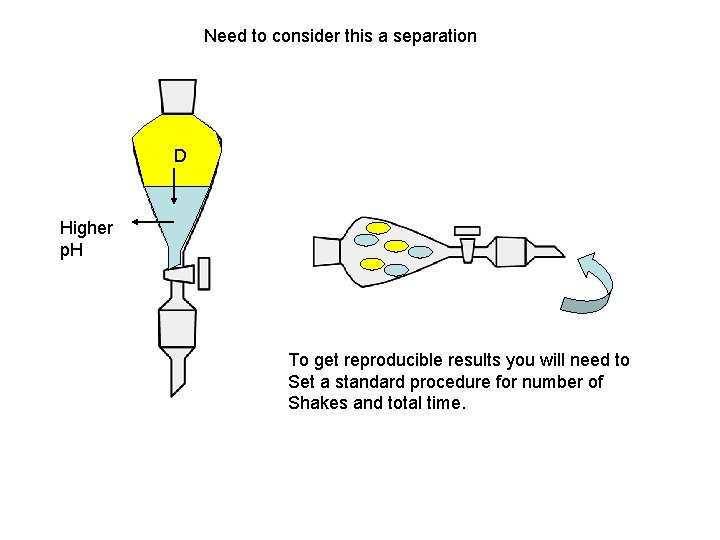
Need to consider this a separation D Higher p. H To get reproducible results you will need to Set a standard procedure for number of Shakes and total time.

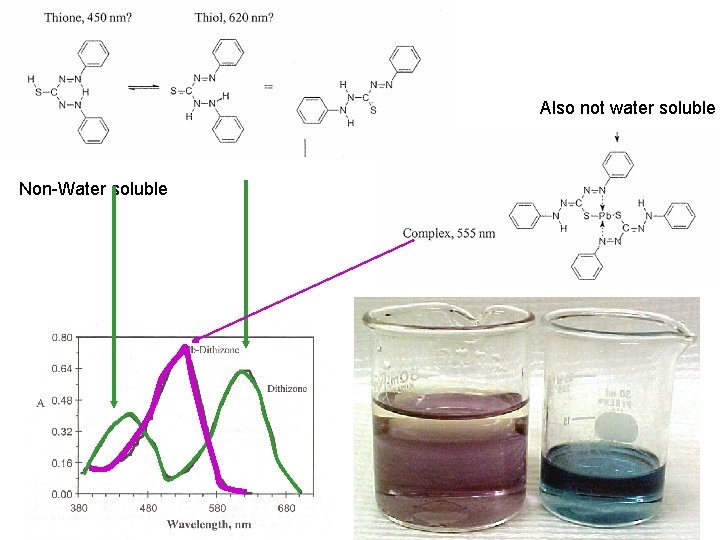
Also not water soluble Non-Water soluble

Other Considerations? False Positives Any other metals (including Mg 2+!!!) can cause a color change The chalk used to line the interior of your Protective gloves can cause false positives Solution? Selectively complex other metals and leave behind the lead!!!!

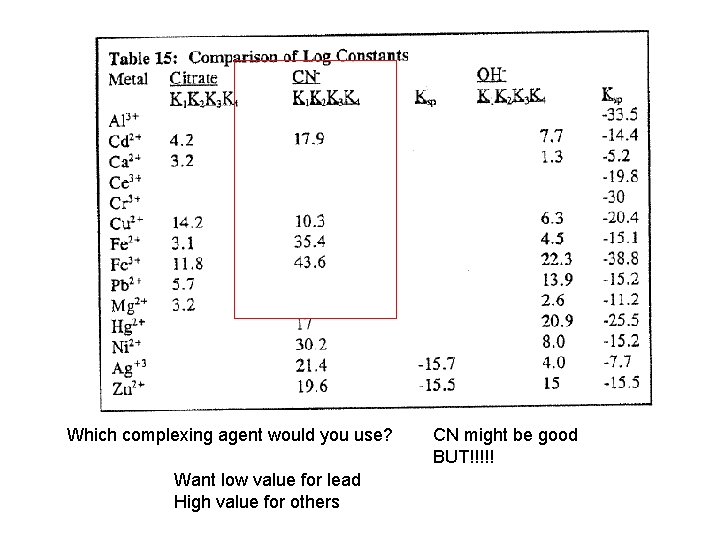
Which complexing agent would you use? Want low value for lead High value for others CN might be good BUT!!!!!

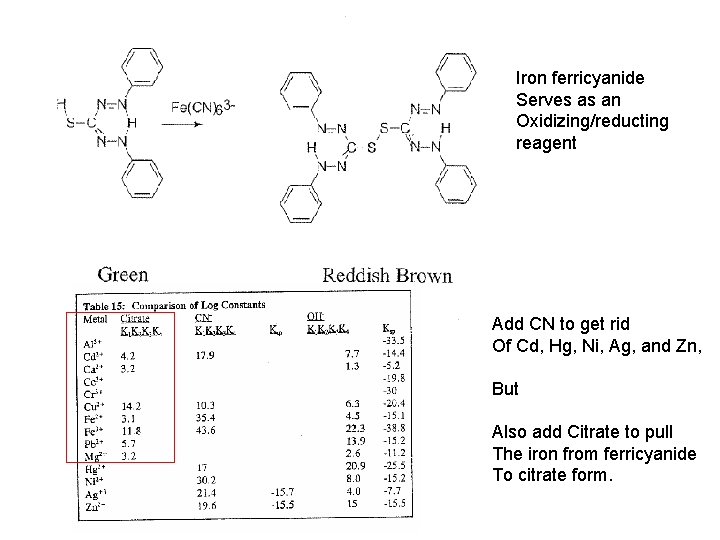
Iron ferricyanide Serves as an Oxidizing/reducting reagent Add CN to get rid Of Cd, Hg, Ni, Ag, and Zn, But Also add Citrate to pull The iron from ferricyanide To citrate form.

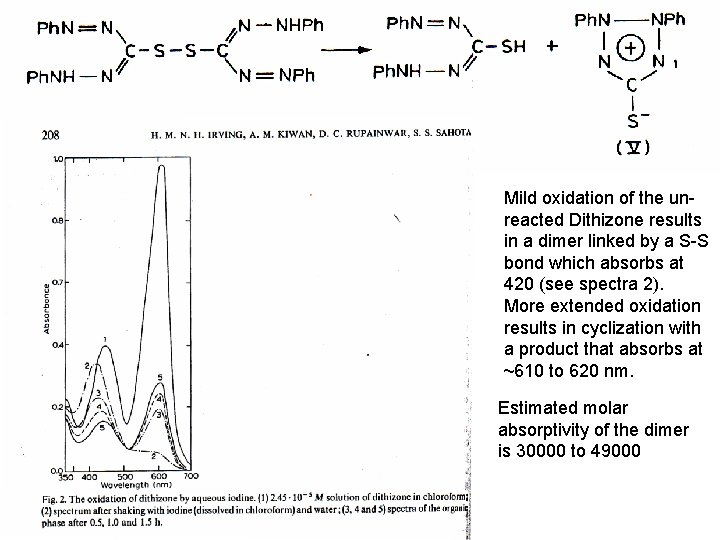
Mild oxidation of the unreacted Dithizone results in a dimer linked by a S-S bond which absorbs at 420 (see spectra 2). More extended oxidation results in cyclization with a product that absorbs at ~610 to 620 nm. Estimated molar absorptivity of the dimer is 30000 to 49000

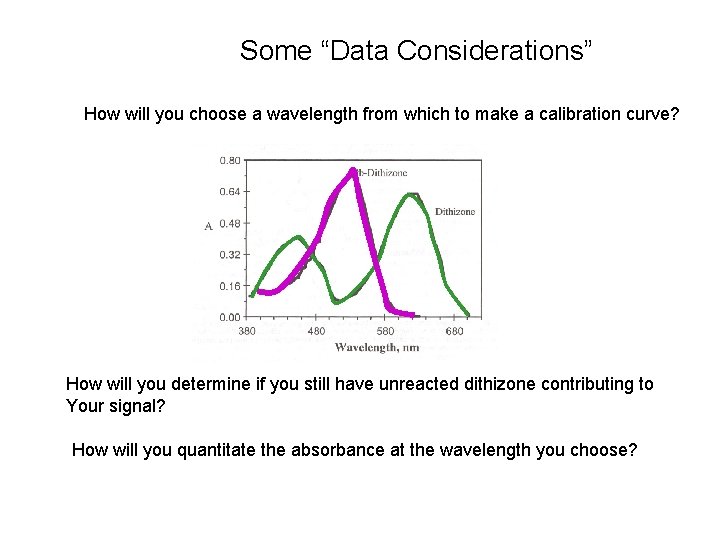
Some “Data Considerations” How will you choose a wavelength from which to make a calibration curve? How will you determine if you still have unreacted dithizone contributing to Your signal? How will you quantitate the absorbance at the wavelength you choose?

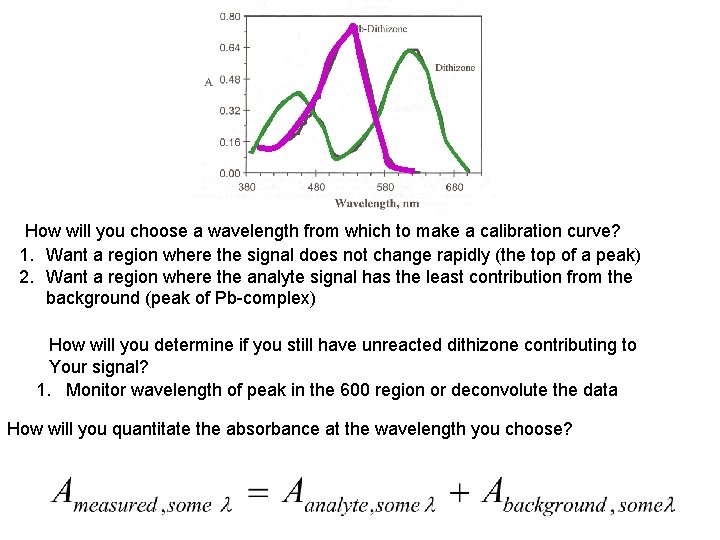
How will you choose a wavelength from which to make a calibration curve? 1. Want a region where the signal does not change rapidly (the top of a peak) 2. Want a region where the analyte signal has the least contribution from the background (peak of Pb-complex) How will you determine if you still have unreacted dithizone contributing to Your signal? 1. Monitor wavelength of peak in the 600 region or deconvolute the data How will you quantitate the absorbance at the wavelength you choose?

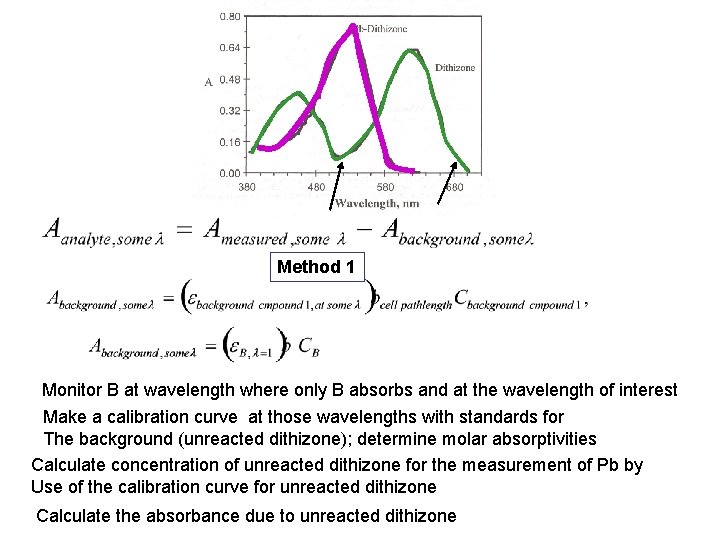
Method 1 Monitor B at wavelength where only B absorbs and at the wavelength of interest Make a calibration curve at those wavelengths with standards for The background (unreacted dithizone); determine molar absorptivities Calculate concentration of unreacted dithizone for the measurement of Pb by Use of the calibration curve for unreacted dithizone Calculate the absorbance due to unreacted dithizone


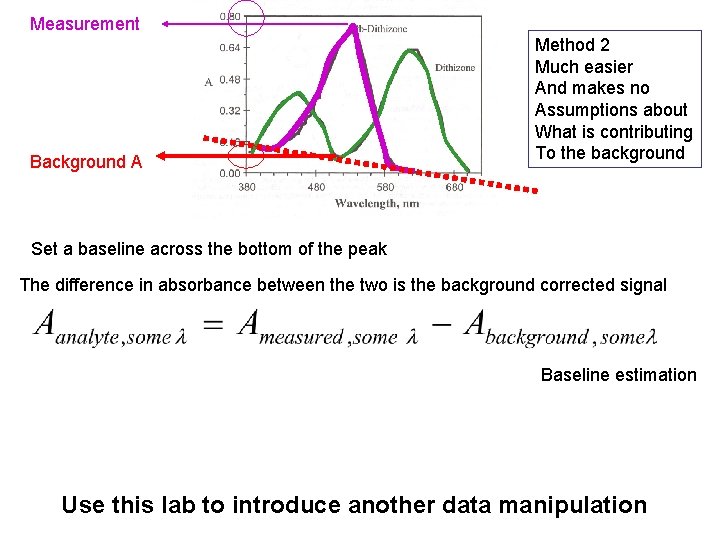
Measurement Background A Method 2 Much easier And makes no Assumptions about What is contributing To the background Set a baseline across the bottom of the peak The difference in absorbance between the two is the background corrected signal Baseline estimation Use this lab to introduce another data manipulation

Use this lab to introduce another data manipulation Method 3 – assess contribution by assuming Gaussians

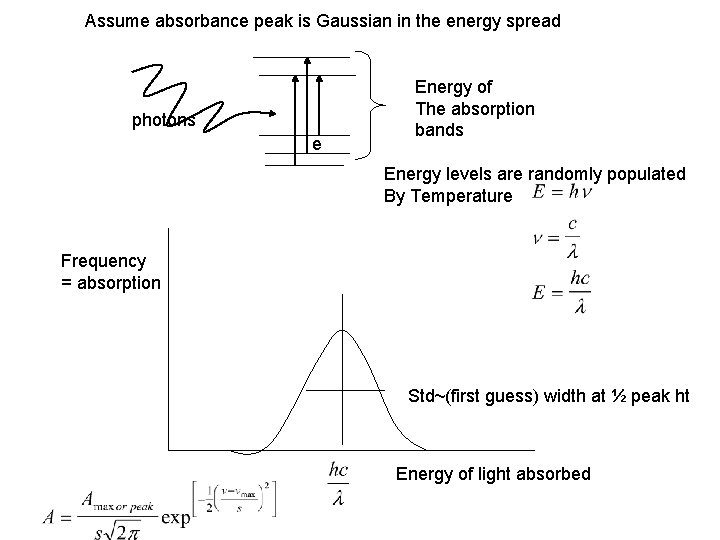
Assume absorbance peak is Gaussian in the energy spread photons e Energy of The absorption bands Energy levels are randomly populated By Temperature Frequency = absorption Std~(first guess) width at ½ peak ht Energy of light absorbed

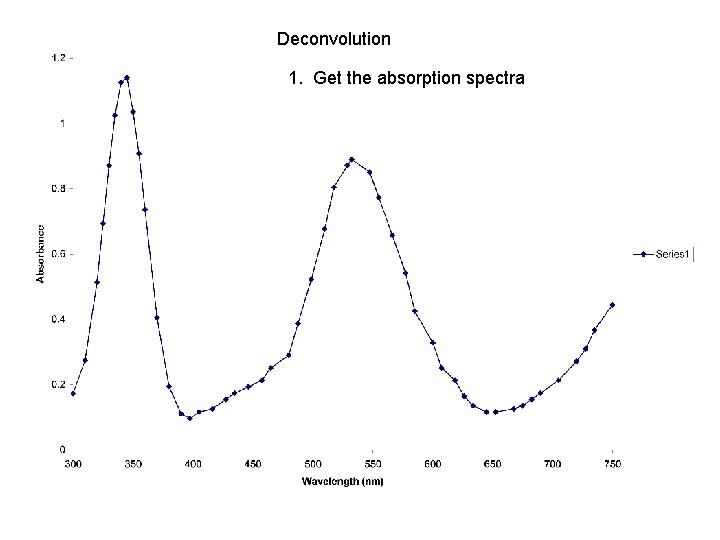
Deconvolution 1. Get the absorption spectra

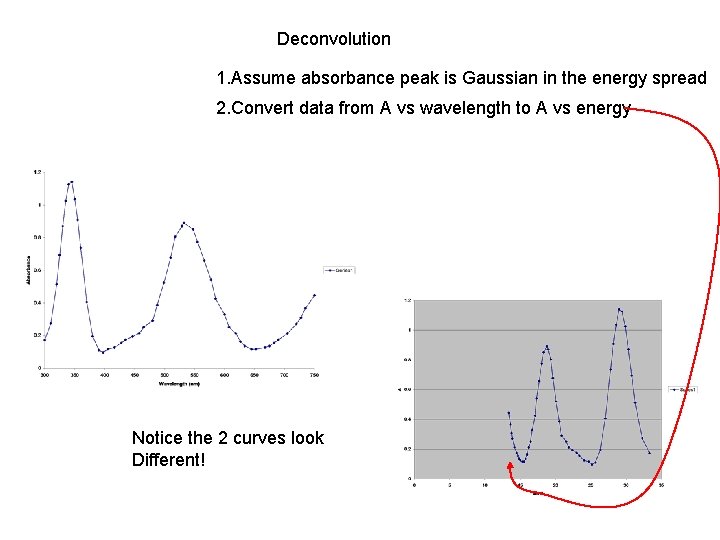
Deconvolution 1. Assume absorbance peak is Gaussian in the energy spread 2. Convert data from A vs wavelength to A vs energy Notice the 2 curves look Different!

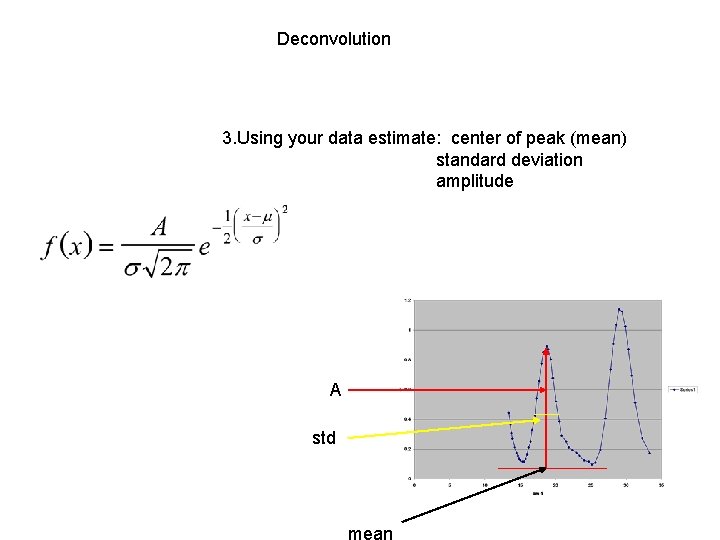
Deconvolution 3. Using your data estimate: center of peak (mean) standard deviation amplitude A std mean

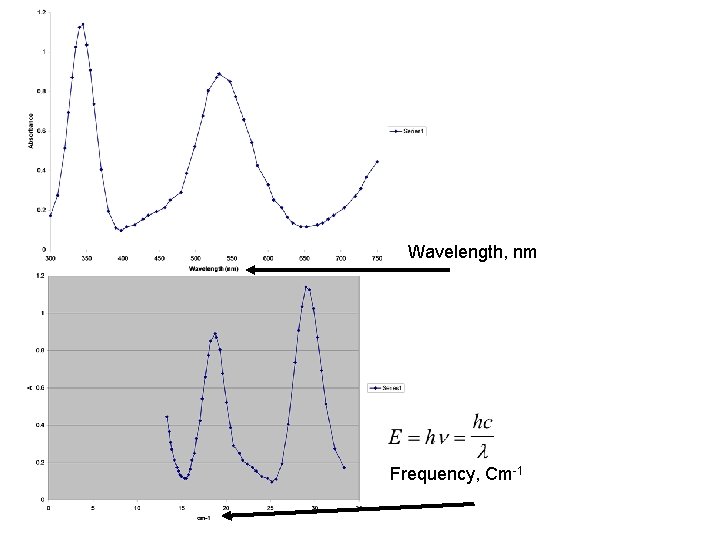
Wavelength, nm Frequency, Cm-1

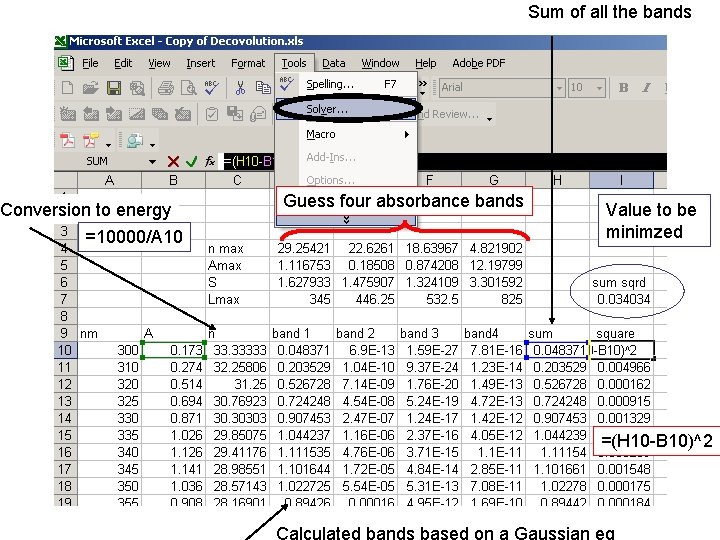
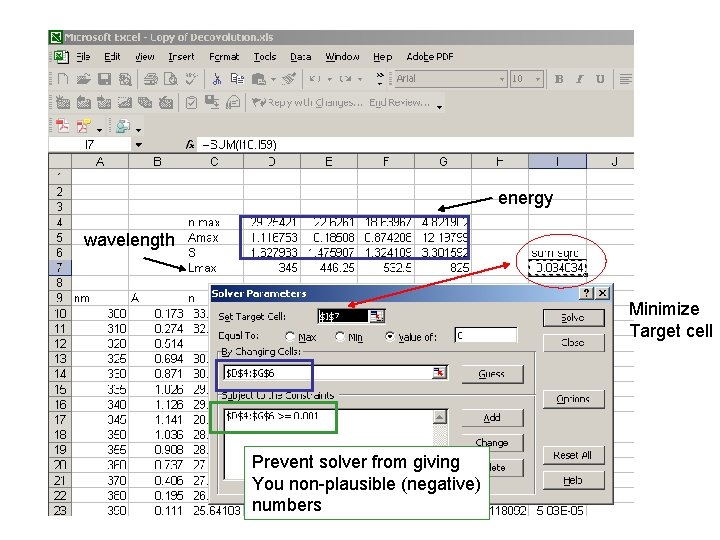
Sum of all the bands Conversion to energy =10000/A 10 Guess four absorbance bands Value to be minimzed =(H 10 -B 10)^2 Calculated bands based on a Gaussian eq

energy wavelength Minimize Target cell Prevent solver from giving You non-plausible (negative) numbers

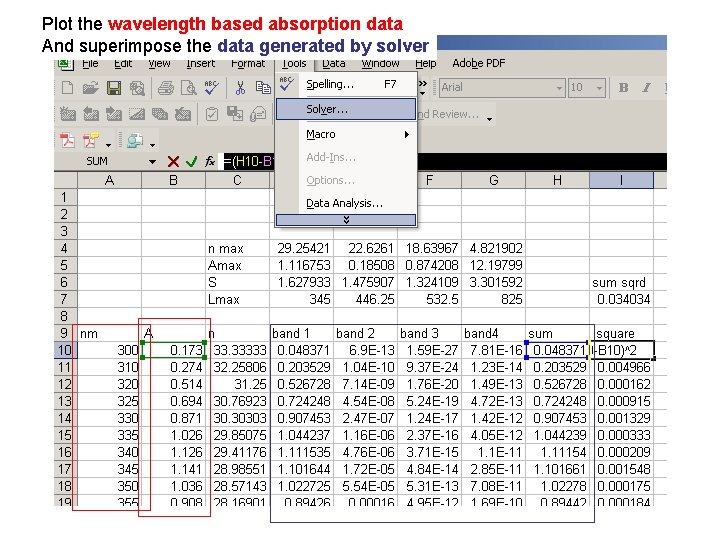
Plot the wavelength based absorption data And superimpose the data generated by solver

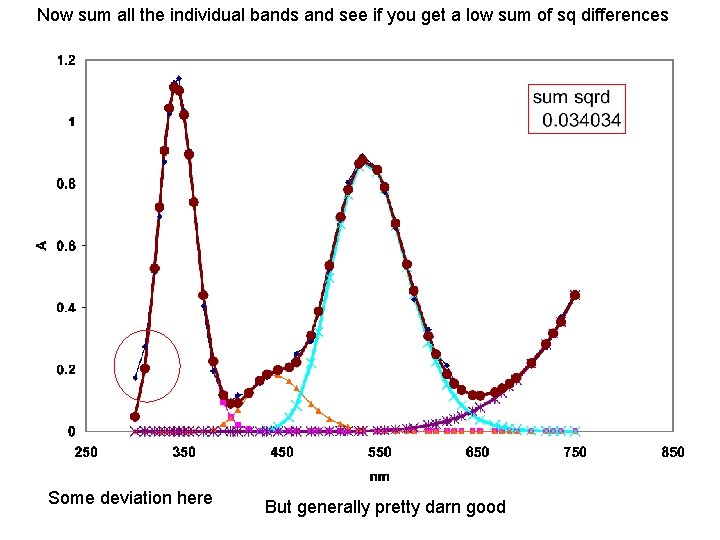
Now sum all the individual bands and see if you get a low sum of sq differences Some deviation here But generally pretty darn good

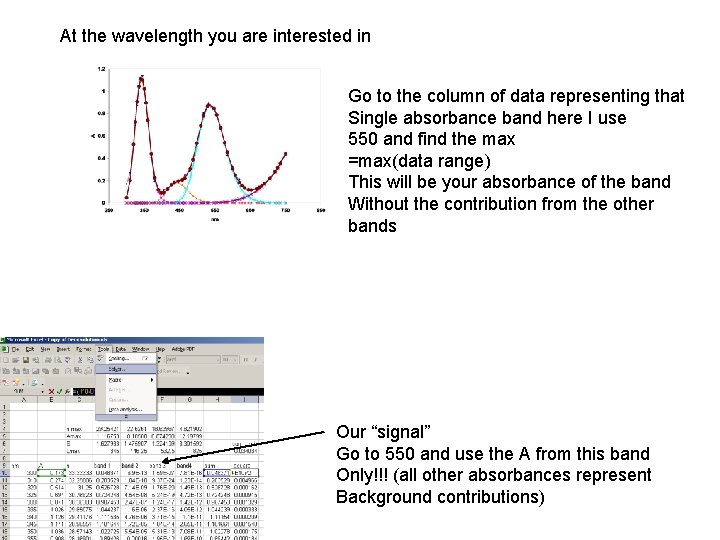
At the wavelength you are interested in Go to the column of data representing that Single absorbance band here I use 550 and find the max =max(data range) This will be your absorbance of the band Without the contribution from the other bands Our “signal” Go to 550 and use the A from this band Only!!! (all other absorbances represent Background contributions)

Chemistry titrimetric ppm LOD cuppellation ppb Suphuretted water dithizone ppt 8000 B. C. E. 1000 C. E. 1900 1990 2000 This method resulted in the first public health Awareness of lead as an issue for children Baltimore, Department of Public Health

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out Convert lead to some compound which can Be measured by some instrument (what ever happens to Be available in your lab) Suppose you only have a fluorimeter!

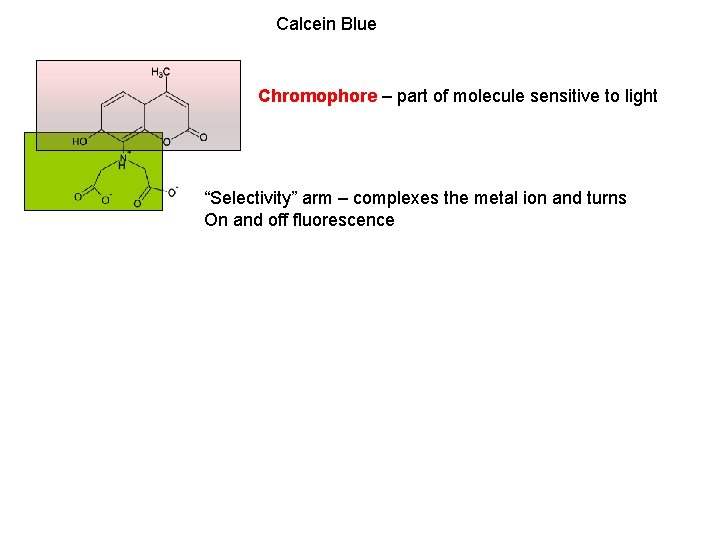
Calcein Blue Chromophore – part of molecule sensitive to light “Selectivity” arm – complexes the metal ion and turns On and off fluorescence

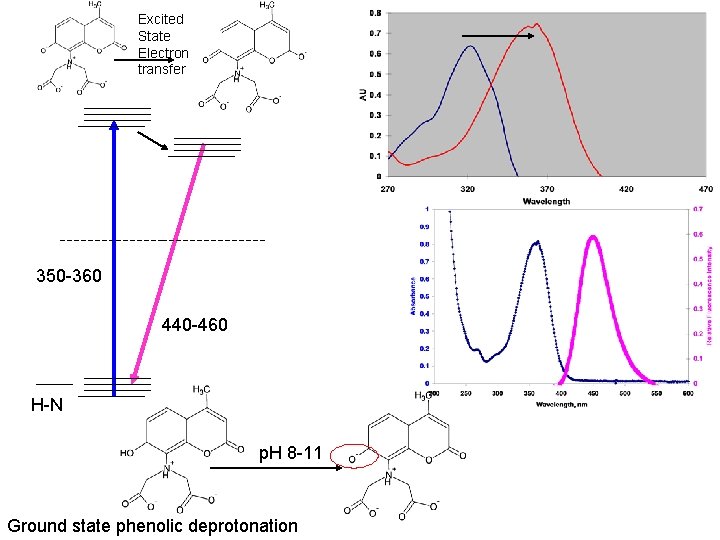
Excited State Proton transfer 480 -490 nm emission 18 -33 ns duration 320 nm excitation p. H 6 -8 Carboxyl groups only deprotonated Note role of resonance here Absorbance spectra Emission Spectra, excitation at 320

Excited State Electron transfer 350 -360 440 -460 H-N p. H 8 -11 Ground state phenolic deprotonation

Key point so far – Excitation is p. H dependent Therefore the emission location and intensity is also p. H dependent If the solution is fluctuating in p. H will not get a linear working curve. Since you have to control p. H for the chemical signal, need to also consider The role of p. H in the form of the lead that is present.

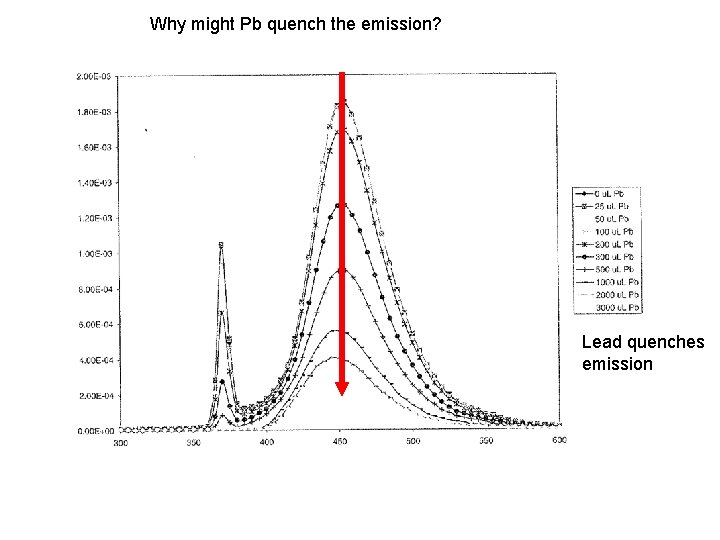
Why might Pb quench the emission? Lead quenches emission

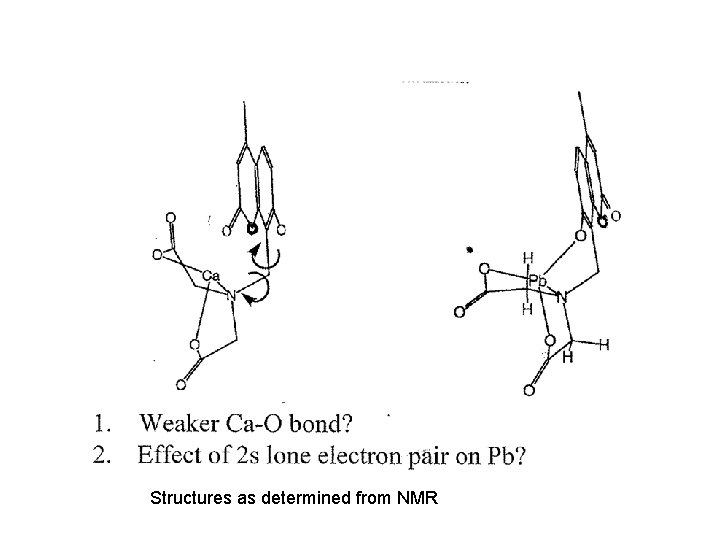
Structures as determined from NMR

GIGO Measurement of Lead Depends on: Chemistry of Lead for Separations Chemistry of Lead for Creating a signal Chemistry of Lead for Creating Background Chemistry of Lead for the Stability of the Signal Garbage In = Garbage Out Convert lead to some compound which can Be measured by some instrument (what ever happens to Be available in your lab) Suppose you only have an IR!

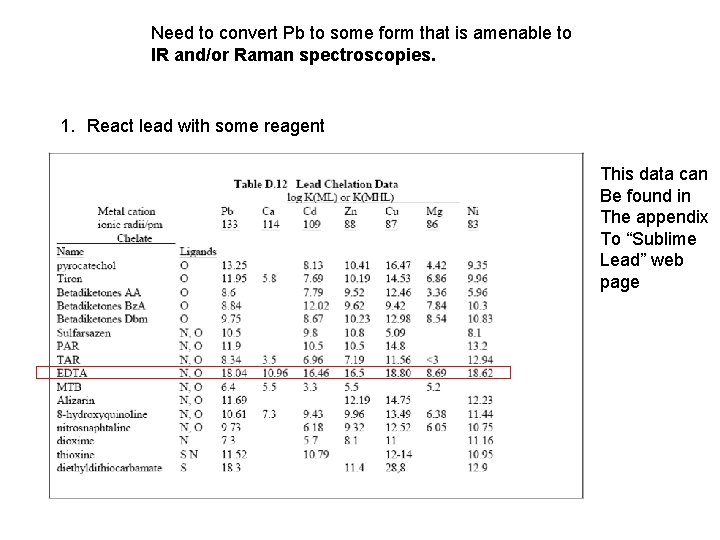
Need to convert Pb to some form that is amenable to IR and/or Raman spectroscopies. 1. React lead with some reagent This data can Be found in The appendix To “Sublime Lead” web page

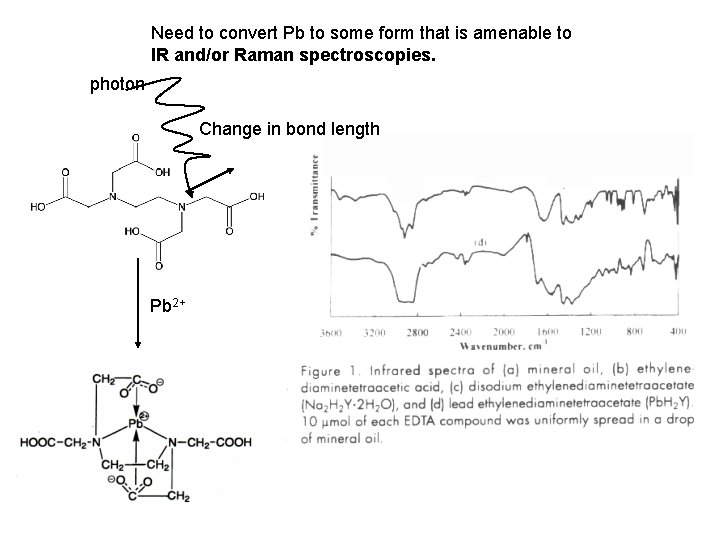
Need to convert Pb to some form that is amenable to IR and/or Raman spectroscopies. photon Change in bond length Pb 2+

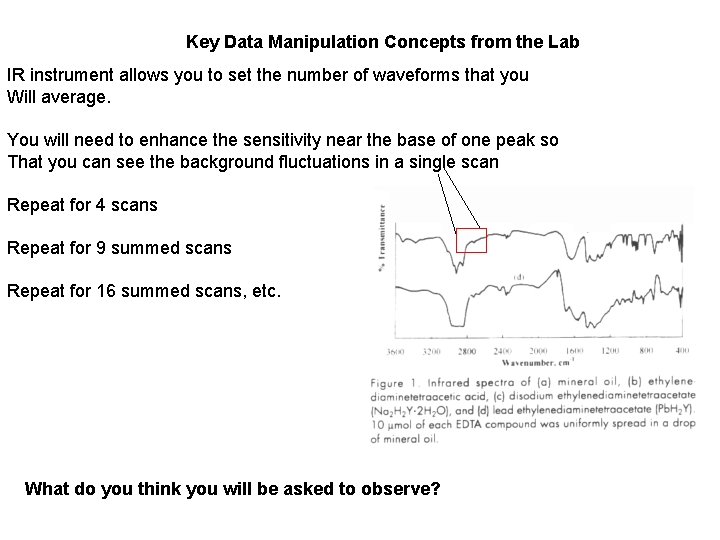
Key Data Manipulation Concepts from the Lab IR instrument allows you to set the number of waveforms that you Will average. You will need to enhance the sensitivity near the base of one peak so That you can see the background fluctuations in a single scan Repeat for 4 scans Repeat for 9 summed scans Repeat for 16 summed scans, etc. What do you think you will be asked to observe?

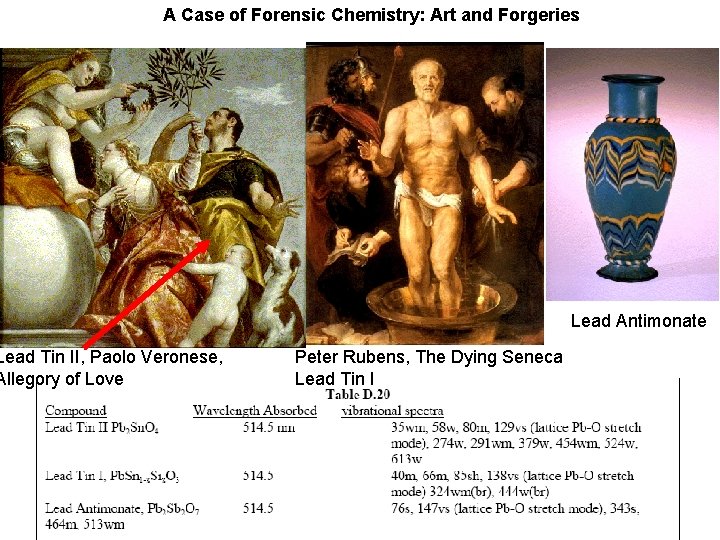
A Case of Forensic Chemistry: Art and Forgeries Lead Tin II, Paolo Veronese, Allegory of Love Lead Antimonate Peter Rubens, The Dying Seneca Lead Tin I

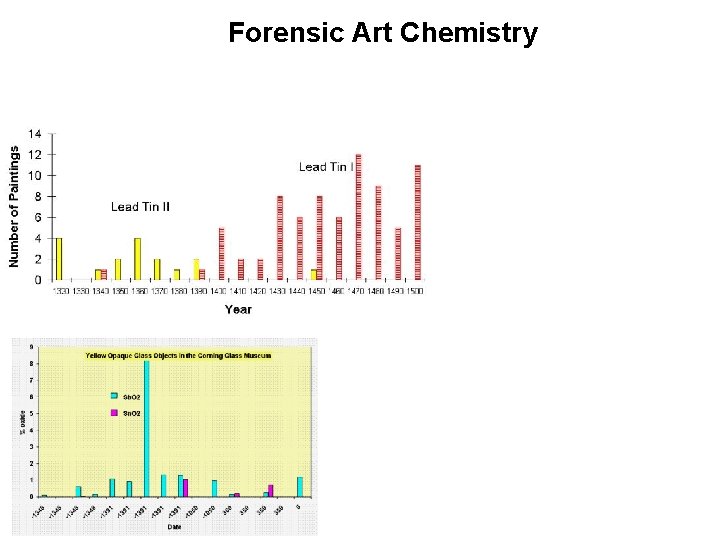
Forensic Art Chemistry

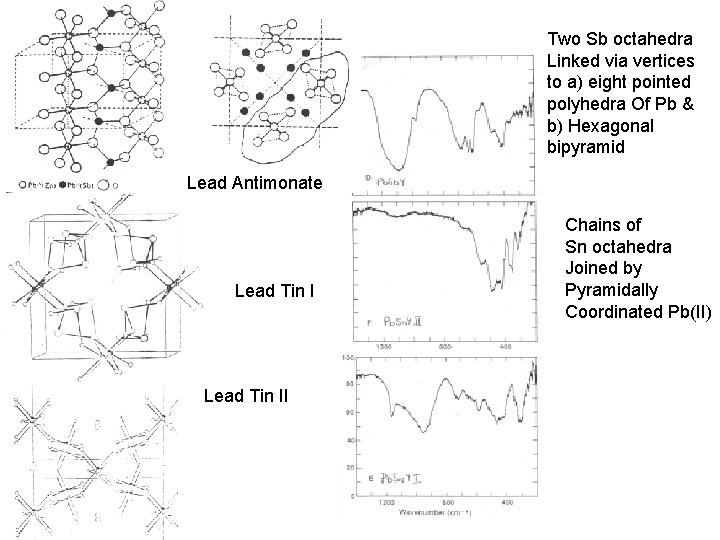
Two Sb octahedra Linked via vertices to a) eight pointed polyhedra Of Pb & b) Hexagonal bipyramid Lead Antimonate Lead Tin II Chains of Sn octahedra Joined by Pyramidally Coordinated Pb(II)
 Gigo heredia
Gigo heredia Precision of measurement depends on *
Precision of measurement depends on * Lead magnesium niobate
Lead magnesium niobate Lead line depth measurement
Lead line depth measurement 3 scientific measurement
3 scientific measurement Ib chemistry measurement and data processing worksheets
Ib chemistry measurement and data processing worksheets Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry The amount of light that is reflected depends on: *
The amount of light that is reflected depends on: * Composition of matter flow chart
Composition of matter flow chart Tirtw
Tirtw Custodial model of organizational behavior
Custodial model of organizational behavior Examples of inert and labile complexes
Examples of inert and labile complexes Clasp brake
Clasp brake The rate of weathering depends upon the area's ____
The rate of weathering depends upon the area's ____ Infiltration capacity of soil depends upon *
Infiltration capacity of soil depends upon * The part of a shadow surrounding the darkest part
The part of a shadow surrounding the darkest part Steel roof frame design
Steel roof frame design Earthquake intensity depends primarily on the height of
Earthquake intensity depends primarily on the height of The atomic mass of an element depends upon the _____.
The atomic mass of an element depends upon the _____. Independent variable is what axis
Independent variable is what axis Resistivity depends on
Resistivity depends on The size of the jhsc usually depends on
The size of the jhsc usually depends on Carnot cycle efficiency depends upon
Carnot cycle efficiency depends upon Refraction of light
Refraction of light The method used for transferring a patient depends on
The method used for transferring a patient depends on A student's grade depends on how much she studies
A student's grade depends on how much she studies The velocity factor of a transmission line depends on
The velocity factor of a transmission line depends on The success of using delayed rewards depends on:
The success of using delayed rewards depends on: Hydraulic jump depends upon
Hydraulic jump depends upon Chapter 9 natural laws and car control
Chapter 9 natural laws and car control Electrode movement
Electrode movement Fire fighting methods
Fire fighting methods Holonomic and rheonomic
Holonomic and rheonomic Example of electrical energy
Example of electrical energy Right to evaluation
Right to evaluation Degree of dissociation of electrolyte depends on
Degree of dissociation of electrolyte depends on What does the sun eat
What does the sun eat Thermal energy depends on
Thermal energy depends on The end depends on the beginning
The end depends on the beginning What is momentum in physics
What is momentum in physics Depends with whom
Depends with whom A student's grade depends on how much she studies
A student's grade depends on how much she studies Momentum is affected by what two factors
Momentum is affected by what two factors Thermal energy depends on
Thermal energy depends on Bipolar nrz and rz
Bipolar nrz and rz The design of modern x-ray telescopes depends on
The design of modern x-ray telescopes depends on Economy of a multiple effect evaporator depends upon the
Economy of a multiple effect evaporator depends upon the Reflection of sound experiment
Reflection of sound experiment Steady state error formula
Steady state error formula Xenolite material
Xenolite material Startling statement lead examples
Startling statement lead examples Crm lead rotator
Crm lead rotator Which of the following is a pronatalist pressure
Which of the following is a pronatalist pressure Paragraph writing strategy
Paragraph writing strategy Jubilee lead academy
Jubilee lead academy Ecg lead placement
Ecg lead placement The isothermal compressibility of lead at 293 k
The isothermal compressibility of lead at 293 k Dynamics 365 round robin lead assignment
Dynamics 365 round robin lead assignment Lead time in community medicine
Lead time in community medicine Lead shapes
Lead shapes