Scientific Measurement Chemistry Chapter 3 Measurements Are really









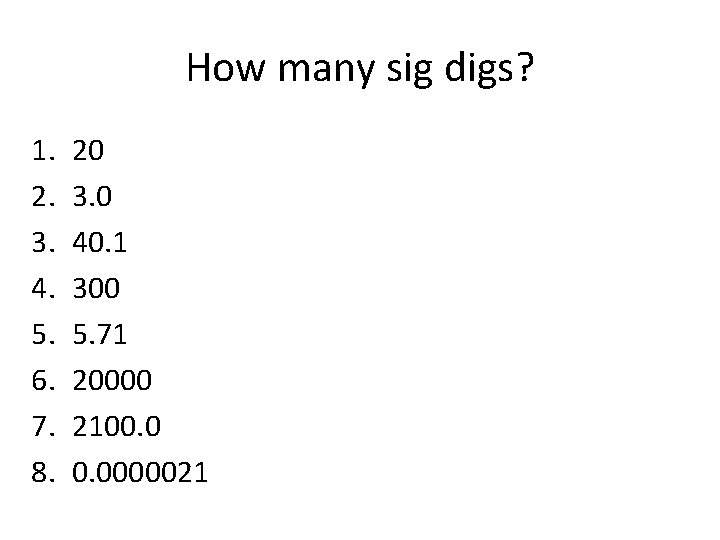
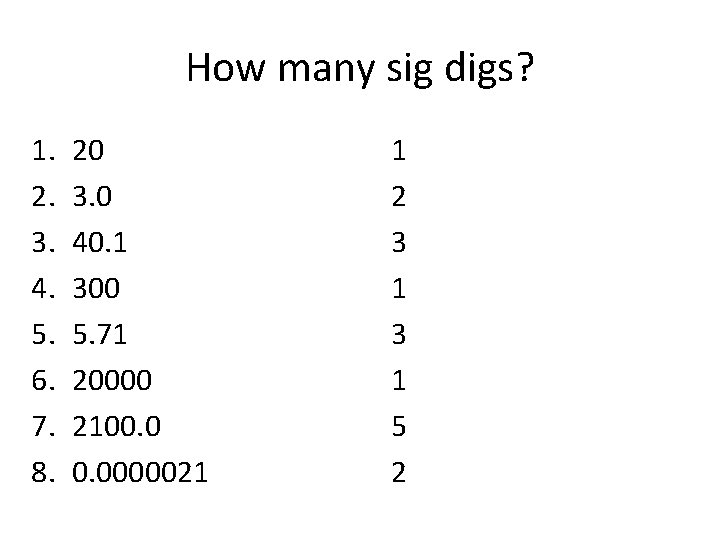


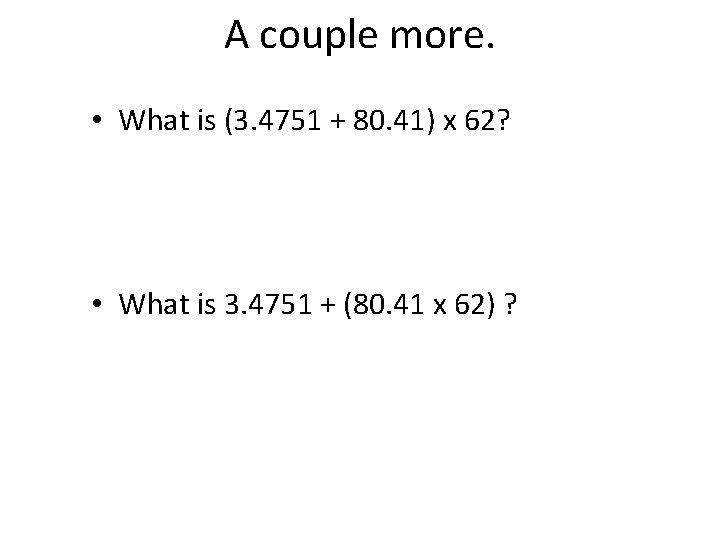








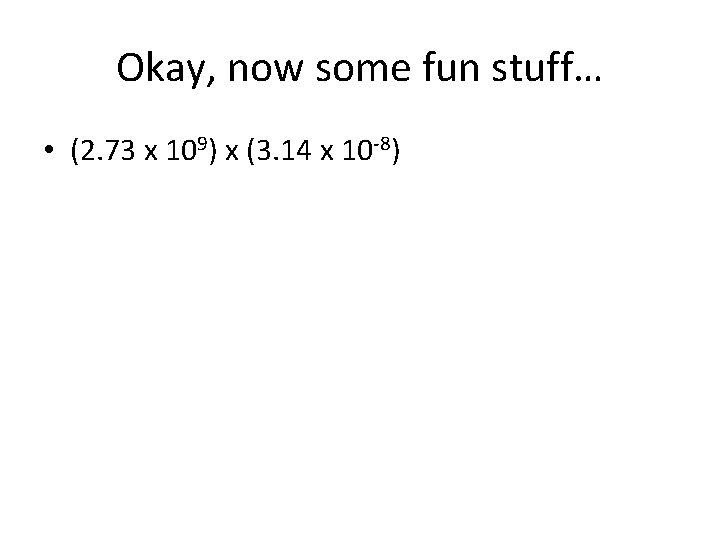














































- Slides: 83

Scientific Measurement Chemistry Chapter 3

Measurements • Are really, really important to sciences. • Important to be able to decide if measurements are correct. • Must have two parts: – A quantity – A unit • UNIT ARE VERY, VERY IMPORTANT!

• http: //www. youtube. com/watch? v=h. Qp. Q 0 hx VNTg

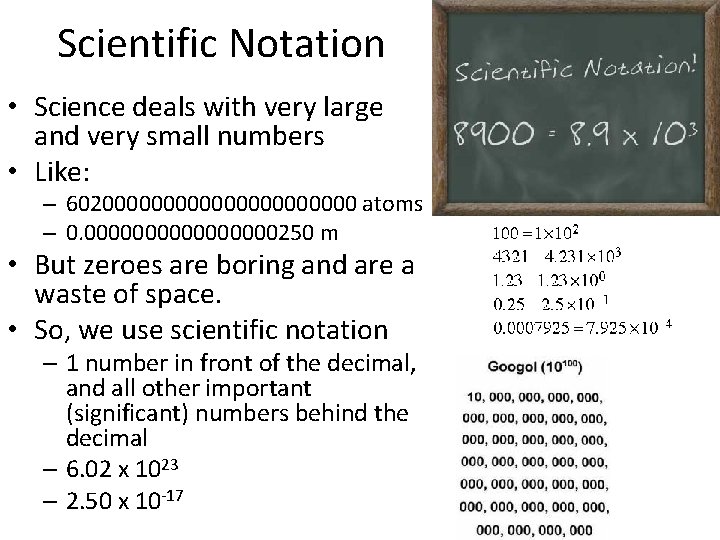
Scientific Notation • Science deals with very large and very small numbers • Like: – 60200000000000 atoms – 0. 00000000250 m • But zeroes are boring and are a waste of space. • So, we use scientific notation – 1 number in front of the decimal, and all other important (significant) numbers behind the decimal – 6. 02 x 1023 – 2. 50 x 10 -17

• http: //www. youtube. com/watch? v=0 l. FQOmb 6 m. Vs

Get the meter stick, Valz!

Significant Figures (or Digits) • How well you “know” and estimate your measurement – All the digits you are confident in, plus one that is estimated • Measurements must always be reported to the correct number of significant digits. – Answers depend on the correct number of digits to determine the most “honest” answer.

Counting significant digits 1. All zeroes before the first non-zero don’t count. • 0. 0000002 only has 1 sig digs 2. All non-zero numbers count. • 21343 has 5 sig digs 3. All zeroes between non-zeroes count • 2002 has 4 sig digs 4. All zeroes AFTER non-zeroes count IF there is a decimal • • • 200. 0 has 4 sig digs 200. has 3 sig digs (decimal at the end!) 200 has 1 sig digs

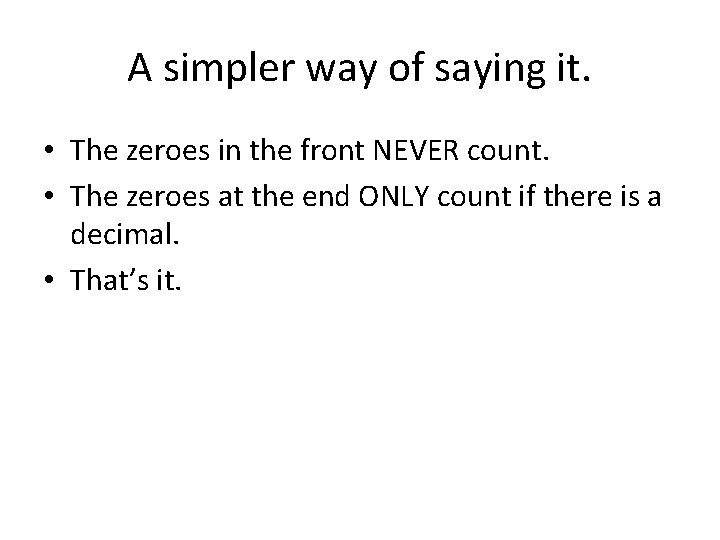
A simpler way of saying it. • The zeroes in the front NEVER count. • The zeroes at the end ONLY count if there is a decimal. • That’s it.

The Pacific/Atlantic Method • If the period is present (with a p) you start in the Pacific Ocean, on the left side (at the first nonzero), and count until you’re out of numbers. • If the period is absent (with an a) you start in the Atlantic, on the right side and don’t start counting until you hit the first non-zero.

Get some, scratch paper, yo. Number it 1 to 8.
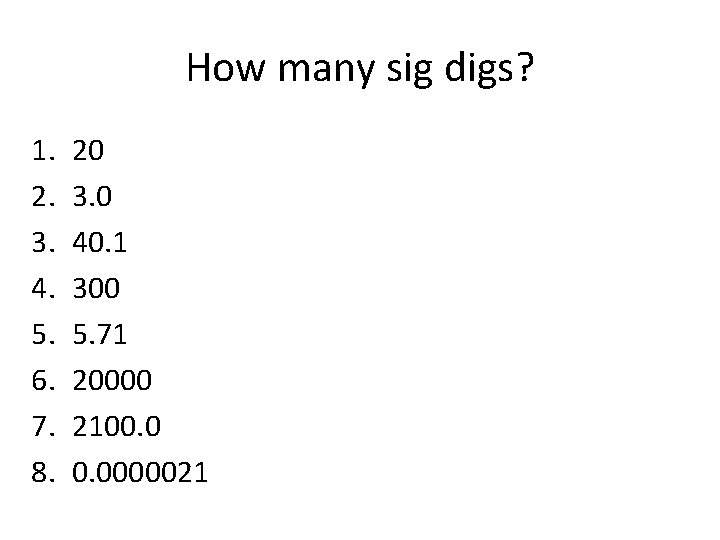
How many sig digs? 1. 2. 3. 4. 5. 6. 7. 8. 20 3. 0 40. 1 300 5. 71 20000 2100. 0000021
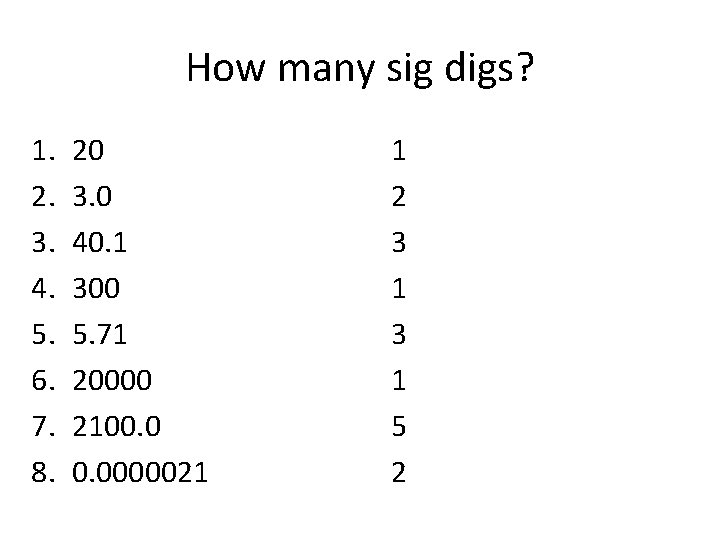
How many sig digs? 1. 2. 3. 4. 5. 6. 7. 8. 20 3. 0 40. 1 300 5. 71 20000 2100. 0000021 1 2 3 1 5 2

Rules for Math with Sig. Digs. • Multiply or Divide: – Use the LEAST number of significant digits from your measurements. • Add or Subtract – Use the LEAST significant place value (which has the highest place value). • When both: – Use whichever method gives you the least number of significant digits as the answer • When averaging: – The number of numbers is NOT a measurement, and therefore does NOT count when determining the number of significant digits.

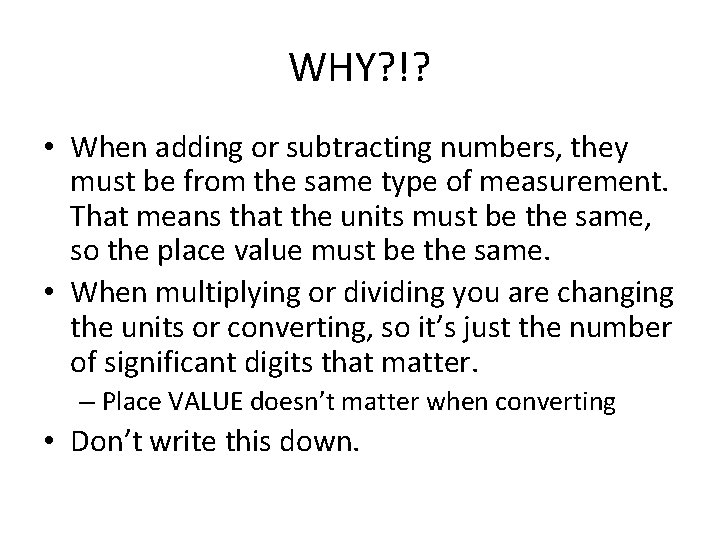
WHY? !? • When adding or subtracting numbers, they must be from the same type of measurement. That means that the units must be the same, so the place value must be the same. • When multiplying or dividing you are changing the units or converting, so it’s just the number of significant digits that matter. – Place VALUE doesn’t matter when converting • Don’t write this down.

Let’s try some… • What is 3. 3/1. 55 in correct significant digits? • What is 3. 161 – 0. 85 in correct significant digits?
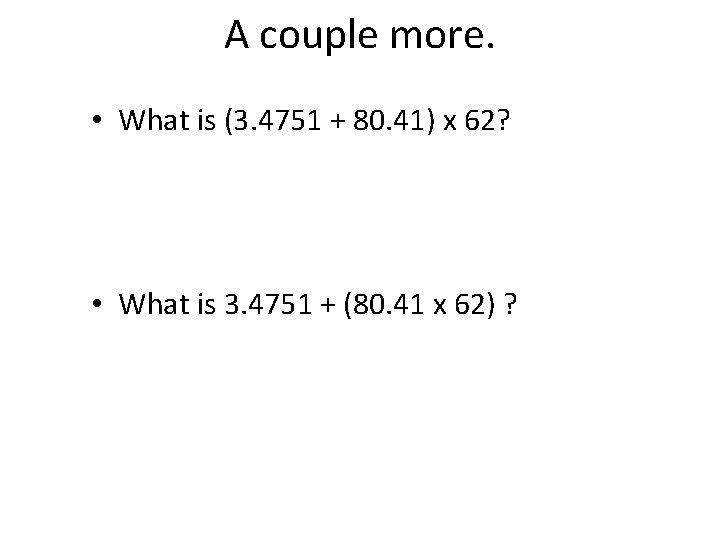
A couple more. • What is (3. 4751 + 80. 41) x 62? • What is 3. 4751 + (80. 41 x 62) ?

Back to scientific notation really quick…

Let’s try some out… • First, let’s convert 325100000 to scientific notation.

Let’s try some out… • First, let’s convert 325100000 to scientific notation. – 3. 251 x 108

Okay, how about this… • Convert 4. 78 x 10 -5 to standard notation.

Okay, how about this… • Convert 4. 78 x 10 -5 to standard notation. – 0. 0000478

Fine, do this one, smart guy. • Fix this number to proper scientific notation: 5541 x 105

Fine, do this one, smart guy. • Fix this number to proper scientific notation: 5541 x 105 – 5. 541 x 108

Doing maff with Scientific Notation • Addition/subtraction – Change the exponent on one of the numbers until they are the same – Then line up the decimals and subtract/add – Adjust the coefficients until there is again 1 digit in front of the decimal • Multiplication/division – Divide or multiply the coefficients – Divide or multiply the base 10 exponents – Adjust the coefficients again • YOU STILL FOLLOW THE RULES OF SIGNIFICANT DIGITS!!!1 ONE!1
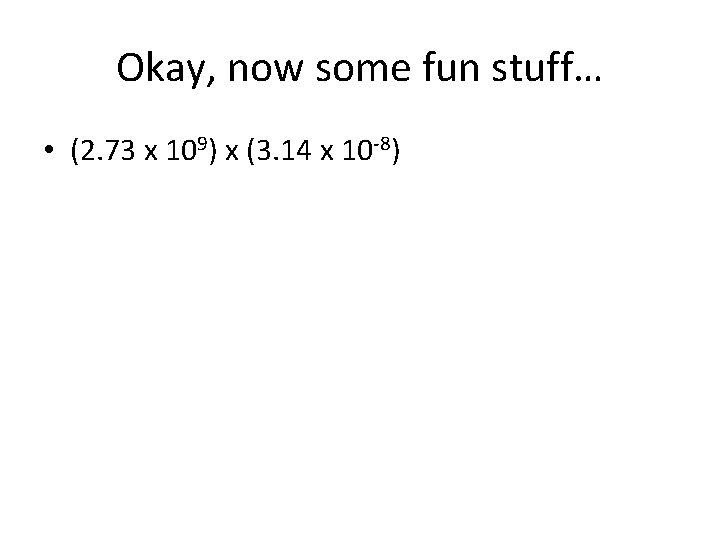
Okay, now some fun stuff… • (2. 73 x 109) x (3. 14 x 10 -8)

Okay, now some fun stuff… • (2. 73 x 109) x (3. 14 x 10 -8) – 8. 5722 x 101

Okay, now addition. • (2. 73 x 109) + (3. 14 x 108)

Okay, now addition. • (2. 73 x 109) + (3. 14 x 108) – 3. 044 x 109

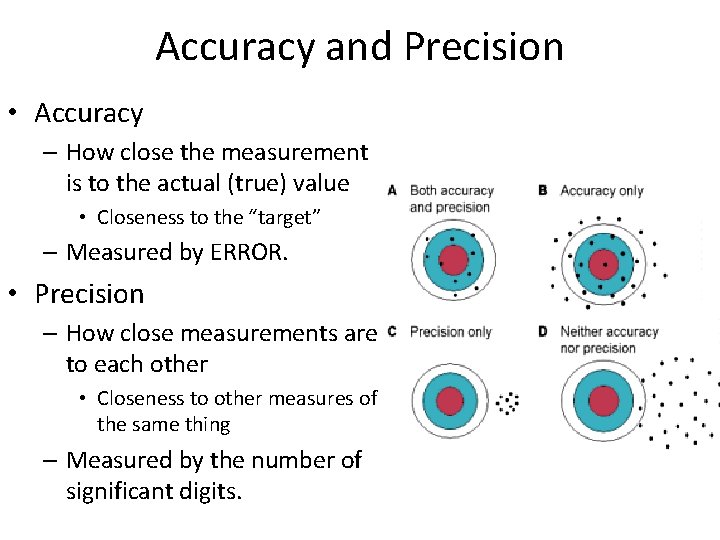
Accuracy and Precision • Accuracy – How close the measurement is to the actual (true) value • Closeness to the “target” – Measured by ERROR. • Precision – How close measurements are to each other • Closeness to other measures of the same thing – Measured by the number of significant digits.

• http: //www. youtube. com/watch? v=Tz. Ln. O 04 u O 30 • http: //www. youtube. com/watch? v=q. PC 1 Km. D Xeg. Y

Determining Error • Error is the difference between a measured (experimental) value and the accepted value – Error = experimental value – accepted value – % error = |Error|*100/accepted value

Practice • You measure the world’s largest duck to be 15 m long, but it is known to actually be 14 m long. – What is the error? – What is the percent error?

Practice • You measure the world’s largest duck to be 15 m long, but it is known to actually be 14 m long. – What is the error? • 15 -14 = 1 m – What is the percent error? • • |15 -14|*100/14 7. 1 % = 7% (with sig figs)

Okay, now your turn… • You have a 17. 1 kg sample of lead. It should displace 1. 508 liters of water. But when you measure it, it displaces exactly 1. 50 liters. What is your error and percent error?

3. 1 Quiz 1. Convert 3. 2 x 104 to standard notation. 2. Convert 0. 00079 to scientific notation. 3. Put 0. 000083 x 10 -4 in proper scientific notation. 4. How many significant digits are in 3, 000? 5. Solve and answer in correct significant digits: 3. 421 x 0. 411 + 9. 62

3. 1 Quiz answers 1. 32000 -4 2. 7. 9 x 10 3. 8. 3 x 10 -9 4. 1 5. 11. 0

SI: International System of Units (System International) • The basis for all measurements made in science (and in this class) • Uses 5 most commonly used units: • Meter (distance) • Kilogram (mass) • Kelvin (temperature) • Second (time) • Mole (amount) • All measurements can be reported as some SI measurement.

Prefixes in SI • SI uses prefixes to shorten measurements (much like scientific notation) • These are put in front of measurements to give a certain place value (multiple) of the unit. – Ex) Rather than 1000 m, you can write 1 km, because kilo means 1000. – Ex) Rather than writing 0. 000001 g, you can write 1 μg or 1 microgram, because micro means 10 -6


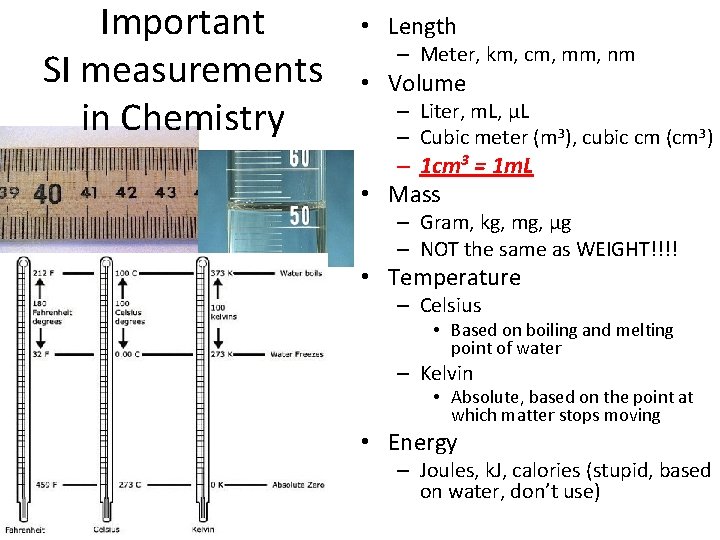
Important SI measurements in Chemistry • Length – Meter, km, cm, mm, nm • Volume – Liter, m. L, μL – Cubic meter (m 3), cubic cm (cm 3) – 1 cm 3 = 1 m. L • Mass – Gram, kg, mg, μg – NOT the same as WEIGHT!!!! • Temperature – Celsius • Based on boiling and melting point of water – Kelvin • Absolute, based on the point at which matter stops moving • Energy – Joules, k. J, calories (stupid, based on water, don’t use)

Volume’s two “sets of units” • Volume is 3 measures of length multiplied together. – m, km, cm, or mm 3 times • m 3, km 3, cm 3, or mm 3 • Volume can also be measure in terms of liters. – 1000 L = 1 m 3 – 1 L = 1000 cm 3 – 1 m. L = 1 cm 3 • This is the one that you NEED to know.

How to convert cubic units to liter units • Convert your original number to m. L or cm 3. • Then it is a 1 to 1 conversion (same number, just change the unit) • Then convert to the unit you want. • Ex) 40 L to mm 3 – 40 L = 40, 000 m. L – 40, 000 m. L = 40, 000 cm 3 – 1 cm = 10 mm • 1 cm = 103 mm 3 = 1000 mm 3 – 40, 000 cm 3 = 40, 000 mm 3

Temperature in SI • Celsius is a metric unit, but NOT an SI unit because it is not absolute. – It is based off of water, and is measured in degrees C = (°C) • We used Kelvin (K), which is measured from absolute zero – Where all matter has no movement. • To convert from C to K you just add 273. 15. – K = °C + 273. 15 – °C = K - 273. 15 • Still follow significant digits rules of place value for addition!

A problem? You’re the problem! • Convert 27°C to Kelvin.

A problem? You’re the problem! • Convert 27°C to Kelvin. – 273. 15 + 27 = 300. 15 K • 300. K with correct SD

A problem? You’re the problem! • Convert 27°C to Kelvin. – 273. 15 + 27 = 300. 15 K • 300. K with correct SD • Convert 395. 17 K to °C

A problem? You’re the problem! • Convert 27°C to Kelvin. – 273. 15 + 27 = 300. 15 K • 300. K with correct SD • Convert 395. 17 K to °C – 395. 17 – 273. 15 = 122. 02°C

Don’t worry about the calorie to Joule conversion. • Calories are a stupid unit of measurement. – If I want you to do a stupid conversion, I’ll give you the conversion and the units.

3. 2 Quiz 1. What is the SI unit of length? 2. What is the name of the “before” measurement that is used to shorten SI measurements. – What is the “k” in “km” called (or the “c” in “cm”)? 3. What is the SI prefix for 1000? 4. What is larger m. L or mm 3? 5. Convert 95°C to K, using correct significant digits.

3. 2 Quiz 1. 2. 3. 4. 5. Meter (m) prefix Kilo m. L 368 K

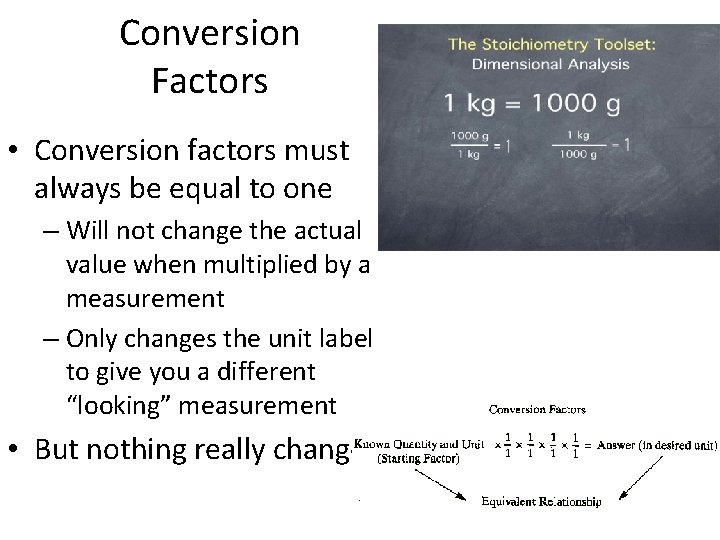
Dimensional Analysis • Also called “factor-label conversions” • Uses conversion factors to convert one measurement to an equal one using different units • Conversion factors are a ratio of two EQUAL measurements with different numbers – Ex) 1 mile = 1760 yards • Therefore 1 mi =1 1760 yards This is a stack of pennies. It has nothing to do with dimensional analysis, but when I Googled “dimensional analysis” this image came up, and it’s pretty awesome.

Conversion Factors • Conversion factors must always be equal to one – Will not change the actual value when multiplied by a measurement – Only changes the unit label to give you a different “looking” measurement • But nothing really changes

Another example problem. • Convert 1600 km/hr (the airspeed of a fighter jet) to m/s.

Another example problem. • Convert 1600 km/hr (the airspeed of a fighter jet) to m/s. – 444. 44… m/s – Rounds to 440 m/s with sig. figs.

• https: //youtu. be/h. Qp. Q 0 hx. VNTg? t=3 m 7 s

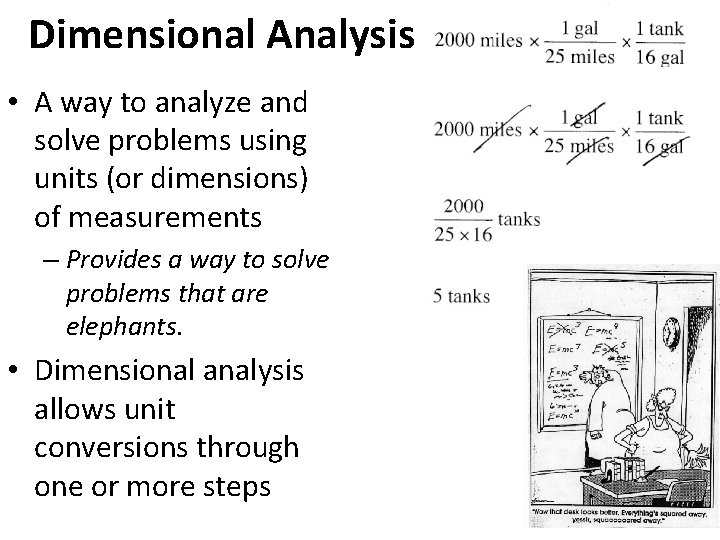
Dimensional Analysis • A way to analyze and solve problems using units (or dimensions) of measurements – Provides a way to solve problems that are elephants. • Dimensional analysis allows unit conversions through one or more steps

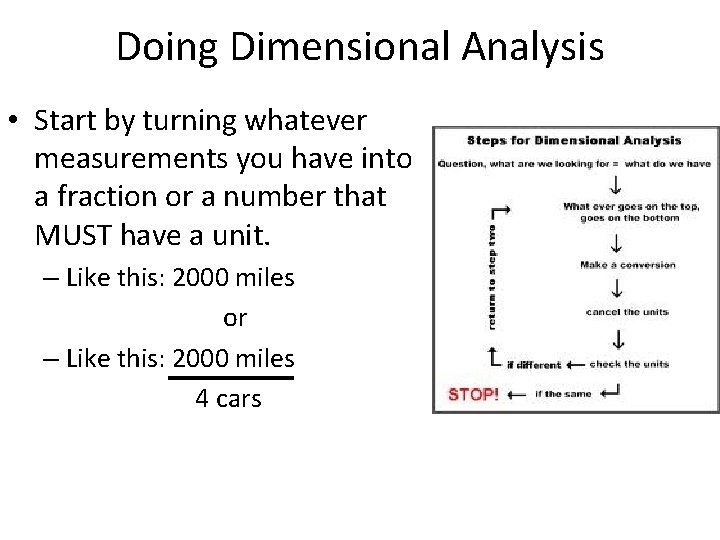
Doing Dimensional Analysis • Start by turning whatever measurements you have into a fraction or a number that MUST have a unit. – Like this: 2000 miles or – Like this: 2000 miles 4 cars

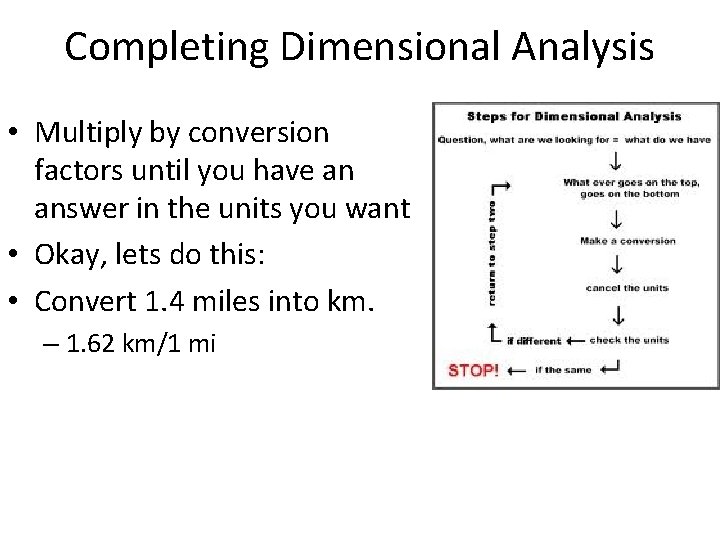
Completing Dimensional Analysis • Multiply by conversion factors until you have an answer in the units you want • Okay, lets do this: • Convert 1. 4 miles into km. – 1. 62 km/1 mi

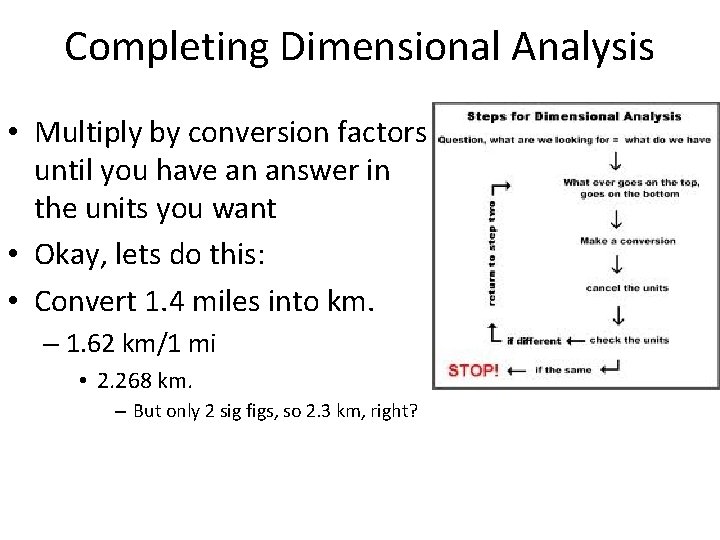
Completing Dimensional Analysis • Multiply by conversion factors until you have an answer in the units you want • Okay, lets do this: • Convert 1. 4 miles into km. – 1. 62 km/1 mi • 2. 268 km. – But only 2 sig figs, so 2. 3 km, right?

When sig digs matter. • Significant Digits only matter in MEASUREMENTS (which are estimates), not for conversions (which are definite). – This doesn’t count for converting from SI to non-SI, though.

Now the BIG problem. • The sink in the fume hood has been dripping since 1999. How much water in L has gone the drain since then?

On your own now… • I have 3. 12 x 103 papers to grade – I can grade 3 papers per hour – I can grade 8 hours per day – How many days will it take to grade them? – Present your answer in standard notation, with the correct number of significant digits.

Converting in SI • 2 methods of conversion – Dimensional Analysis method • Difficult, slow. – Lazy Mr. V method • Easy, quick. • DOES NOT WORK WITH NON-SI CONVERSIONS!!!

Practice Problems • Convert 1. 75 m into cm

Practice Problems • Convert 1. 75 m into cm – 0 – (-2) = 2 – Move decimal 2 places to the RIGHT.

Practice Problems • Convert 1. 75 m into cm – 0 – (-2) = 2 – Move decimal 2 places to the RIGHT. • Answer: 175 cm

On your own • Convert 678µg to kg.

On your own • Convert 678µg to kg. – µ = -6 to kg = 3 – -6 – 3 = -9 – 9 spots to the LEFT – 0. 000000678

Okay, how about this one? • 3 cm 3 to mm 3 – If you have cubic units, you must do the conversion 3 times. – (IF you have square units, you must do the conversion 2 times. )

Okay, how about this one? • 3 cm 3 to mm 3 – If you have cubic units, you must do the conversion 3 times. – (IF you have square units, you must do the conversion 2 times. ) – cm = -2 – mm= -3 – -2 – (-3) = 1 – 1 * 3 = 3, so 3 spots to the right – 3 cm 3 = 3000 mm 3

Try this one on for size: • Convert 3. 71 cm 3 to m 3. – Remember, it’s an elephant, but you can just eat it one bite at a time!

3. 3 Quiz 1. Convert 80. 0 k. L to m. L, and put in correct scientific notation with correct significant digits. 2. Convert 211 m/s into km/hr. – Answer in correct sig figs. 3. A conversion factor is always equal to (or the same as) what number? 4. T/F: A conversion factor’s significant digits matter when determining the final answer’s number of significant digits. – Assume that the conversion is an absolute, and not just a measured value. 5. Convert 30. 0 miles per gallon to km per liter if 1 mi=1. 61 km, and 3. 78541 L=1 gallon

3. 3 Quiz Answers 1. 8. 00 x 107 m. L 2. 760. km/hr 3. 1 4. F 5. 12. 8 km/L

Density • Density is an easily measured intensive property. – Does not matter how much matter you have. • Density is a ratio of mass divided by volume. • How much “stuff” fits in a certain space. • Density range: – Hydrogen (0. 000084 g/m. L) – Water (1. 000 g/m. L) – Osmium (22. 57 g/m. L)

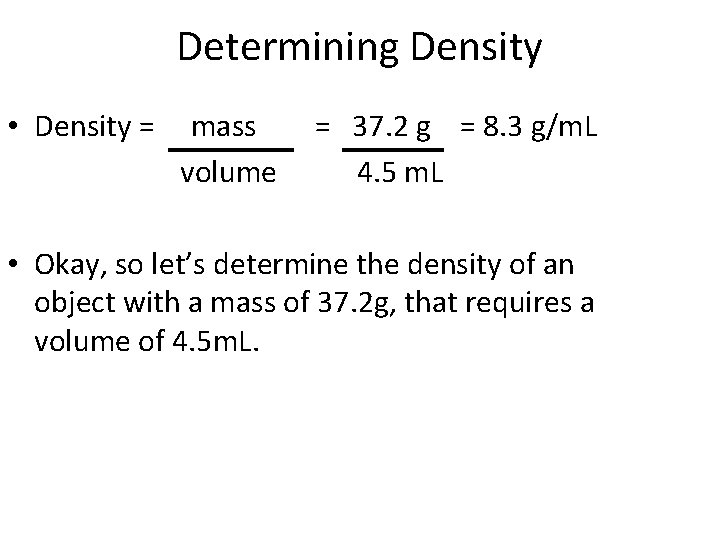
Determining Density • Density = mass volume • Okay, so let’s determine the density of an object with a mass of 37. 2 g, that requires a volume of 4. 5 m. L.

Determining Density • Density = mass volume = 37. 2 g = 8. 3 g/m. L 4. 5 m. L • Okay, so let’s determine the density of an object with a mass of 37. 2 g, that requires a volume of 4. 5 m. L.

So, what is it? • Density is 7. 9 g/m. L, rounded to 2 significant digits. Which metal is it? – Are you SURE? Density (g/m. L)

Density used to calculate mass or volume • If you KNOW the material you can determine the mass or volume. – If D = m/V – What is m? – What is V? • If lead has a density of 11. 34 g/m. L, and you need 100. 0 g of it, how much space will it occupy?

Determining Mass from Measurements • I have a fish aquarium with a height of 20. 0 cm, a width of 13. 0 cm, and a length of 16. 5 cm. If I fill it all the way up with water at 4°C (D = 1. 00 g/m. L) what will be the mass of the aquarium?

Density and Temperature • Usually density decreases as temperature increases – Solids are usually more dense than the liquids of the same compounds or elements • Water breaks this rule - ice is less dense than liquid water. • Matter “spreads out” when it gets warmer, so it has more volume, but the SAME MASS!

3. 4 Quiz 1. Write the equation for calculating density. 2. When temperature decreases, density usually _______. 3. Write an equation to determine the mass of an object based on its density and volume. 4. If an object has a mass of 36 g, and a volume of 6. 0 m. L, what is its density in g/m. L? 5. If an object has a mass of 3. 00 g, and a density of 39. 30 g/m 3, what is its volume?

3. 4 Quiz 1. D = m/V 2. increases 3. m=Dx. V 4. 6. 0 g/m. L 3 5. 0. 0763 m
 Antigentest åre
Antigentest åre 3 scientific measurement
3 scientific measurement Scientific notation
Scientific notation Scientific notation is a shorthand way of writing really
Scientific notation is a shorthand way of writing really Scientific measurements
Scientific measurements Unit 2 lesson 2 scientific notation
Unit 2 lesson 2 scientific notation Chapter 3 scientific measurement
Chapter 3 scientific measurement Measurements in chemistry
Measurements in chemistry Lesson 1 understanding science answer key
Lesson 1 understanding science answer key Lesson 2 measurement and scientific tools answer key
Lesson 2 measurement and scientific tools answer key Measurement and scientific tools lesson 2
Measurement and scientific tools lesson 2 Ib chemistry measurement and data processing worksheets
Ib chemistry measurement and data processing worksheets Information gathered during an experiment
Information gathered during an experiment How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Sign chapter 37
Sign chapter 37 Orthostatic vitals positive
Orthostatic vitals positive Percent error example
Percent error example Chapter 12 economic indicators and measurements
Chapter 12 economic indicators and measurements Scientific notation chemistry definition
Scientific notation chemistry definition Vcaa chemistry study design
Vcaa chemistry study design Introduction to chemistry section 3 scientific methods
Introduction to chemistry section 3 scientific methods Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Wishes about the past
Wishes about the past John p kotter what leaders really do
John p kotter what leaders really do What can you tell us about the film
What can you tell us about the film Simon armitage remains poem
Simon armitage remains poem Tongue twisters about spring
Tongue twisters about spring It really burned me up when you yelled at me.
It really burned me up when you yelled at me. A marketer can really satisfy everyone in the
A marketer can really satisfy everyone in the Heavenly father are you really there
Heavenly father are you really there Heavenly father prayer
Heavenly father prayer Propaganda definition
Propaganda definition Proofs that really count
Proofs that really count Personification
Personification Do ghosts really exist
Do ghosts really exist Really bad powerpoint
Really bad powerpoint Tell me whats really going on
Tell me whats really going on What did jesus look like
What did jesus look like What did elizabeth i really look like
What did elizabeth i really look like It's really fascinating
It's really fascinating Doesnt really matter
Doesnt really matter People really hate elephants on compact cars
People really hate elephants on compact cars Tell me whats really going on
Tell me whats really going on Contoh rss
Contoh rss Reported speech gramatika
Reported speech gramatika I enjoyed the experience
I enjoyed the experience 7 steps of the scientific method
7 steps of the scientific method What's the matter you look really tired
What's the matter you look really tired What is the program to fulfill the basic human aspirations
What is the program to fulfill the basic human aspirations What is the mood in the dining room at the start of act 2
What is the mood in the dining room at the start of act 2 What really happened on thanksgiving
What really happened on thanksgiving What british people really mean
What british people really mean This is really scary with long
This is really scary with long It's really fascinating
It's really fascinating On the cruelty of teaching computing science
On the cruelty of teaching computing science Black synoynm
Black synoynm Cups on a string
Cups on a string Attitude does matter
Attitude does matter Trojan labor
Trojan labor The animal i really dig
The animal i really dig She sings very beautifully
She sings very beautifully Transform in a sentence
Transform in a sentence Do subliminals really work
Do subliminals really work Bart simpson tin foil hat
Bart simpson tin foil hat Dr bhavesh patel
Dr bhavesh patel And the tree was happy
And the tree was happy If the books have been cataloged last week
If the books have been cataloged last week That was a really foolish thing to say
That was a really foolish thing to say Lucius verus gladiator
Lucius verus gladiator Time really
Time really Sustainable living meaning
Sustainable living meaning How did the writer feel about this experience she felt
How did the writer feel about this experience she felt Miniotec
Miniotec What my friends think i do meme
What my friends think i do meme Career exploration icebreakers
Career exploration icebreakers The lady and the spider by robert fulghum
The lady and the spider by robert fulghum We do really loud
We do really loud Were really needed
Were really needed A cart travels with a constant nonzero acceleration
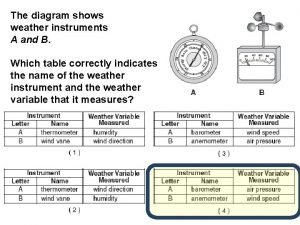
A cart travels with a constant nonzero acceleration The diagram below shows a weather instrument
The diagram below shows a weather instrument Genesis 6 15
Genesis 6 15 Significant figures definition
Significant figures definition