Chapter 3 Scientific Measurement Scientific Measurement Measurementis a








- Slides: 10

Chapter 3 Scientific Measurement

Scientific Measurement • Measurement-is a quantity that has both a number and a unit – Ex. 12 inches, 5 cm • Measurements are fundamental to the experimental sciences. For that reason it is important to be able to make measurements and decide whether those measurements are correct. • Scientific notation- a given number is written as the product of two numbers: a coefficient and 10 raised to a power. – Ex. 6. 9 x 105

Accuracy and Precision • Accuracy-is a measure of how close a measurement comes to the actual true value. • Precision- is a measure of how close a series of measurements are to one another. • To evaluate the accuracy of a measurement, the measured value must be compared to the correct value. • To evaluate precision of a measurement you must compare the values of two or more repeated measurements.

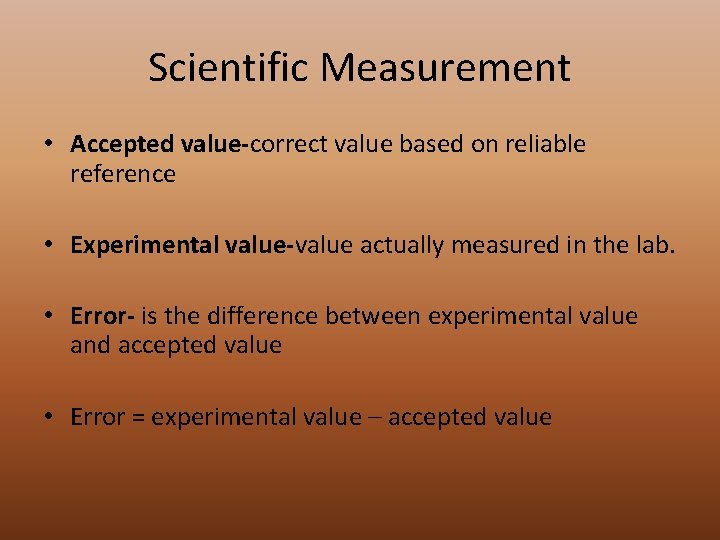
Scientific Measurement • Accepted value-correct value based on reliable reference • Experimental value-value actually measured in the lab. • Error- is the difference between experimental value and accepted value • Error = experimental value – accepted value

Percent Error • Percent error- is the absolute value of the error divided by the accepted value, multiplied by 100% • Percent error= error accepted value X 100%

International System of Units (SI) • The five SI base units used by chemists are the meter, the kilogram, the Kelvin, the second, and the mole. • Table 3. 1 in the book. • Common metric units of mass are the kilogram, milligram, and microgram.

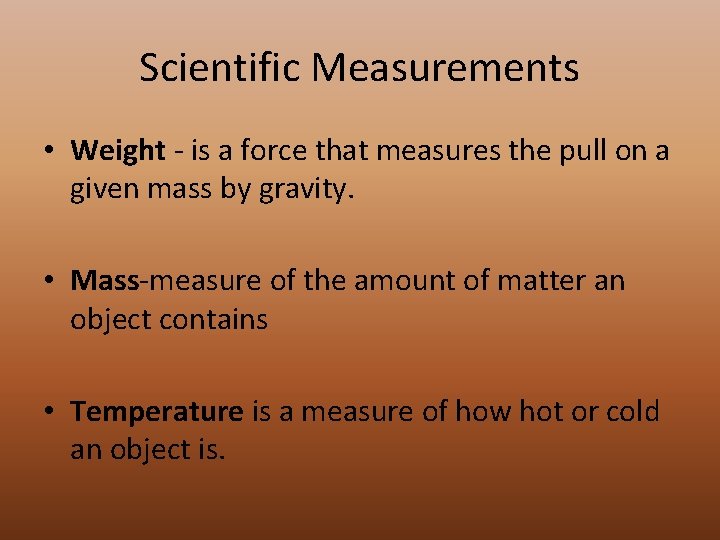
Scientific Measurements • Weight - is a force that measures the pull on a given mass by gravity. • Mass-measure of the amount of matter an object contains • Temperature is a measure of how hot or cold an object is.

Scientific Measurements • Scientist commonly use two equivalent units of temperature the degrees Celsius, or the degrees Kelvin • Celsius water freezes at 0 degrees and boils at 100 degrees • Kelvin- water freezes 273. 15 and boils 373. 15 • Conversions K=C + 273 C= K – 273 • Absolute zero – 0 point on Kelvin scale, the point at which all molecular movement stops.

Energy • Energy-is the capacity to do work or produce heat. • The joule and the calorie are common units of energy. 1 J=0. 2390 cal 1 cal = 4. 184 J

Density • Density-is the ratio of the mass of an object to it volume • Density= mass Volume • Density is an intensive property that depends only on the composition of a substance, not on the size of the sample. • The density of a substance generally decreases as temperature increases • Why? The volume usually increases as temperature increases. Therefore, making the denominator of the density formula larger and causing the density to be smaller.
 3 scientific measurement
3 scientific measurement Chapter 3 scientific measurement
Chapter 3 scientific measurement Understanding science lesson 1 answer key
Understanding science lesson 1 answer key Understanding science lesson 1 answer key
Understanding science lesson 1 answer key Lesson 2 measurement and scientific tools answer key
Lesson 2 measurement and scientific tools answer key Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Work measurement techniques
Work measurement techniques Chapter 5 measurement theory godfrey
Chapter 5 measurement theory godfrey Measurement elsevier
Measurement elsevier Normal pediatric vitals by age
Normal pediatric vitals by age


















