Scientific Measurement Chapter 3 Measurement Uncertainty Making measurements














- Slides: 31

Scientific Measurement Chapter 3

Measurement & Uncertainty • Making measurements and performing calculations with measurements is very important in science and many other fields • Any measurement has a number with a unit • How do you know if a measurement is true? • Are there limits to measurement?

Scientific Notation A convenient way of writing very large and very small numbers A way to indicate significant figures Standard (Decimal) notation 0. 00000106 m (radius of H atom) Scientific notation coefficient x 10 power first digit must be a non-zero (from 1 to 9) 0. 00000030 m = 1. 06 x 10 -10 m 1. 65 x 10 4 Correct format? 0. 053 x 10 -2 Correct format? 12. 63 x 10 15 Correct format?

Calculations with Scientific Notation • Review scientific notation in your text – Read pages R 56 -57, Appendix C • Your calculator uses a special key to enter scientific notation • EE, E, Exp, Sci • These keys mean “x 10 exp” to your calculator • Do not use 10^

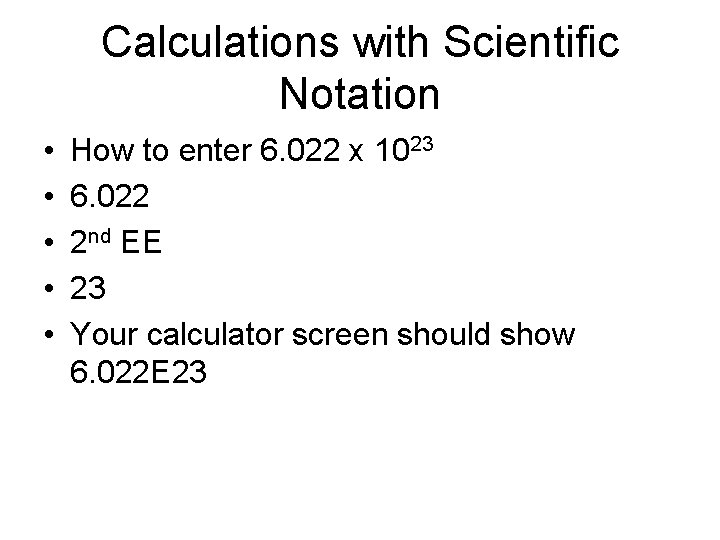
Calculations with Scientific Notation • • • How to enter 6. 022 x 1023 6. 022 2 nd EE 23 Your calculator screen should show 6. 022 E 23

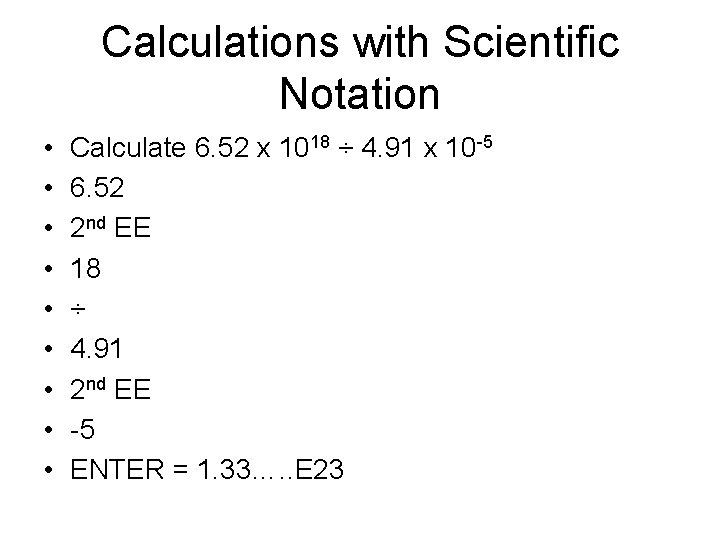
Calculations with Scientific Notation • • • Calculate 6. 52 x 1018 ÷ 4. 91 x 10 -5 6. 52 2 nd EE 18 ÷ 4. 91 2 nd EE -5 ENTER = 1. 33…. . E 23

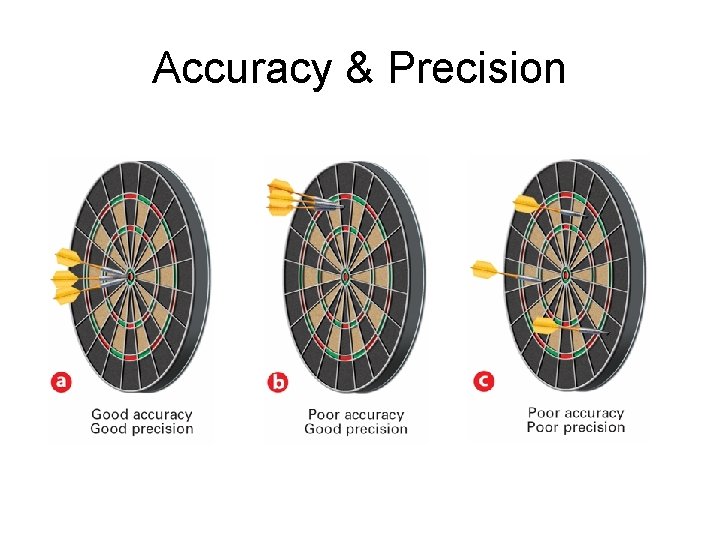
Accuracy, Precision, & Error Accuracy and precision are not the same thing Accuracy how close a measurement is to the true value (actual or accepted value) Precision how close measurements agree how exact a measurement is Example: a centigram balance (0. 01 g) is more precise than a decigram balance (0. 1 g) Error difference between actual and experimental value

Accuracy & Precision

Accuracy & Precision To evaluate accuracy of a measurement: compare measurement to true value To evaluate precision of a measurement: compare values of two or more repeated measurements

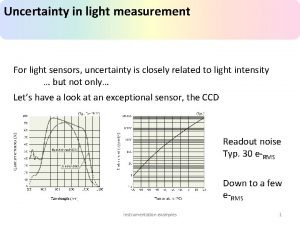
Uncertainty in Measurement • All measurements are approximations • All measurements contain error, so we can only report numbers that we know for sure (certain) • The certainty of a measurement is determined by the precision of the measurement • Significant figures are used to reflect certainty of measured value

Uncertainty in Measurement • Digital instruments (like our electronic scales) estimate the final digit • Example: 5. 67 g • In this measurement, the 7 is estimated by the scale • The uncertainty of the scale is the smallest division reported by the scale (0. 01 g) • Recording the measurement with its uncertainty: 5. 67 ± 0. 01 g

Significant Figures • All digits that are known, plus one last estimated digit • Represent certainty of a measurement • Must be handled properly in calculations to prevent overstating precision • Review rules to determine significant figures (p. 66 -67)

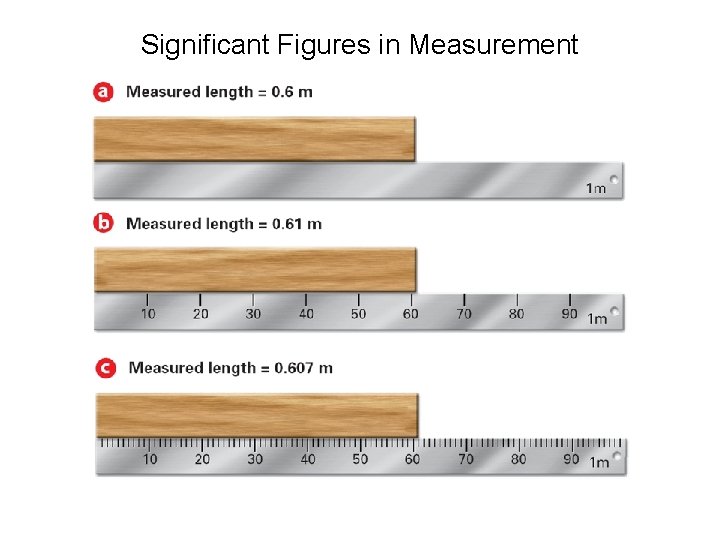
Significant Figures in Measurement

Rules for Determining Significant Figures • • • All non-zeros Zeros between non-zeros Zeros at the beginning of a # Zeros at the end, to right of “. ” Final zeros without “. ” Final zeros with “. ” YES NO YES

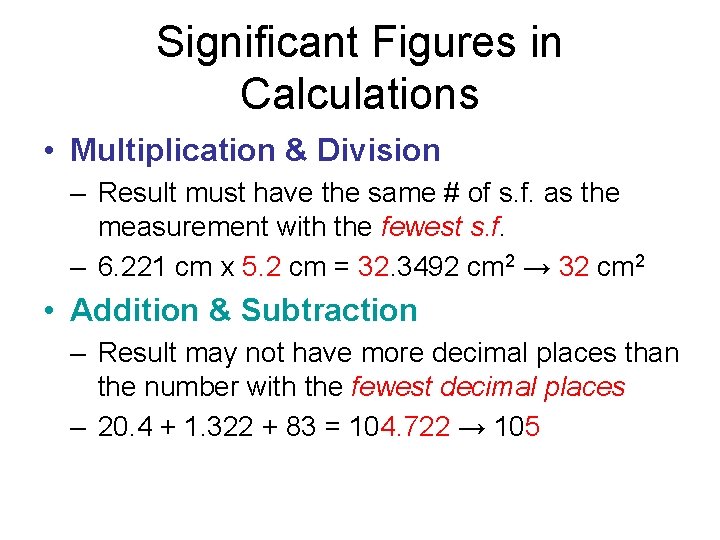
Significant Figures in Calculations • Multiplication & Division – Result must have the same # of s. f. as the measurement with the fewest s. f. – 6. 221 cm x 5. 2 cm = 32. 3492 cm 2 → 32 cm 2 • Addition & Subtraction – Result may not have more decimal places than the number with the fewest decimal places – 20. 4 + 1. 322 + 83 = 104. 722 → 105

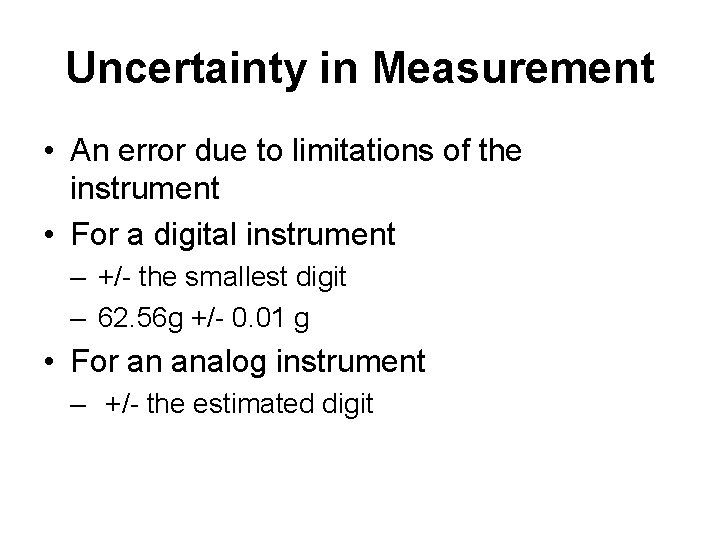
Uncertainty in Measurement • An error due to limitations of the instrument • For a digital instrument – +/- the smallest digit – 62. 56 g +/- 0. 01 g • For an analog instrument – +/- the estimated digit

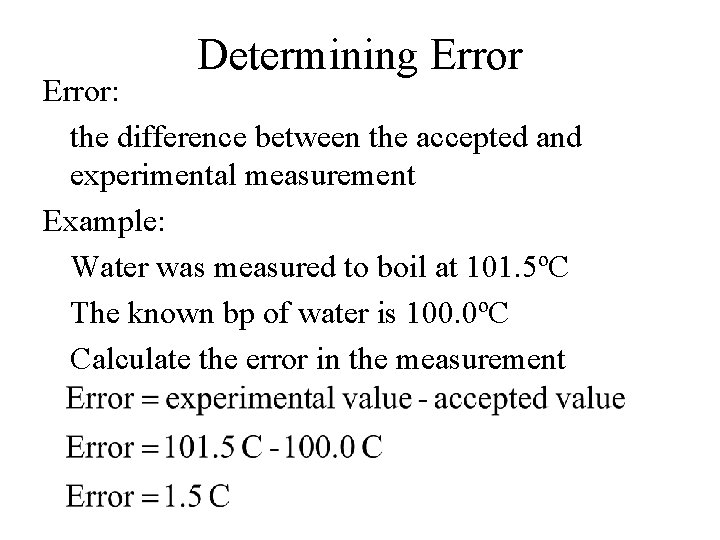
Determining Error: the difference between the accepted and experimental measurement Example: Water was measured to boil at 101. 5ºC The known bp of water is 100. 0ºC Calculate the error in the measurement

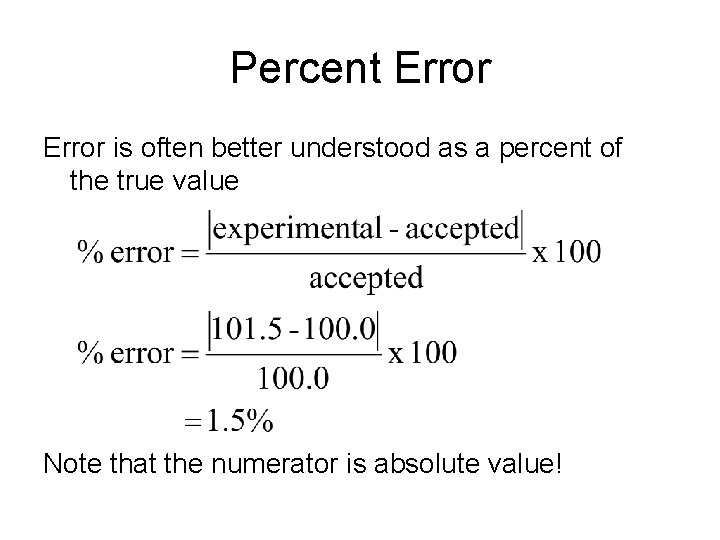
Percent Error is often better understood as a percent of the true value Note that the numerator is absolute value!

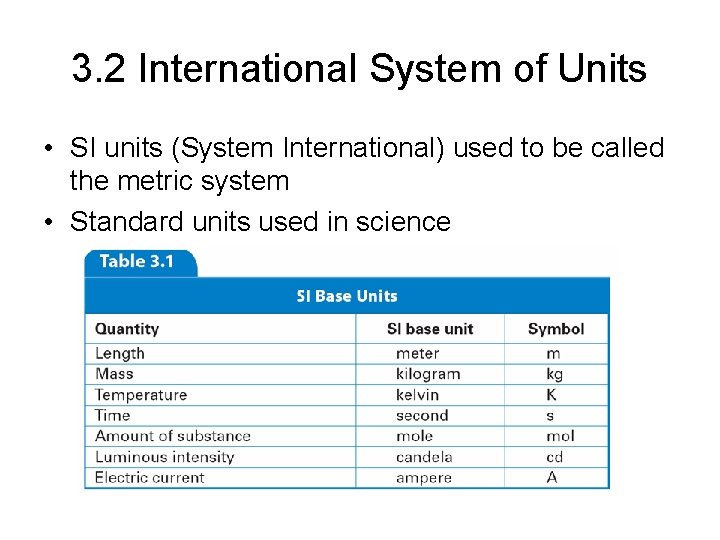
3. 2 International System of Units • SI units (System International) used to be called the metric system • Standard units used in science

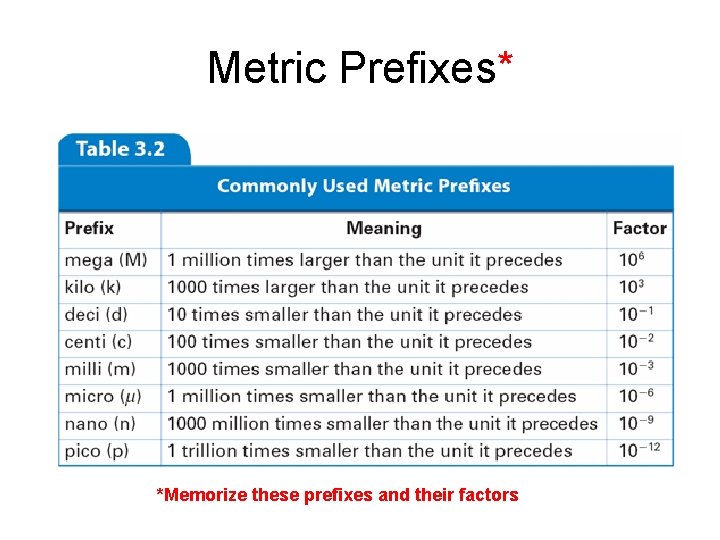
Metric Prefixes* *Memorize these prefixes and their factors

Common Units of Volume

Mass vs. Weight • • Mass and weight are different concepts Mass is a measure of matter (e. g kg) Anything that occupies space has mass Weight is a force – The force of gravity acting on a mass – The product of mass x gravitational constant

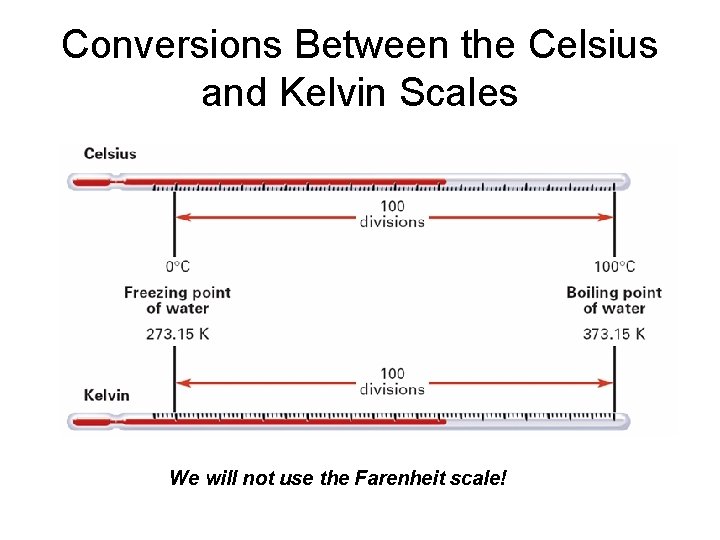
Temperature Scales Used in Science • Kelvin (Absolute Temperature) • Absolute zero • 0º K = -273. 15º C no negative temps in K • Celsius 0 C = +273. 15 K • A Kelvin degree and a Celsius degree have the same magnitude (size)

Conversions Between the Celsius and Kelvin Scales We will not use the Farenheit scale!


Energy Units of Energy • Energy is the capacity to do work or to produce heat. • The joule (J) is the SI unit of energy. • One calorie (cal) is the quantity of heat that raises the temperature of 1 g of pure water by 1°C.

• Energy can be converted into other forms, but the units are still joules (J) • This house is equipped with solar panels. The solar panels convert the radiant energy from the sun into electrical energy that can be used to heat water and power appliances.

3. 3 Conversion Problems • Conversion Factors • Ratio of two equivalent measurements • 1 dozen = 12 items

Dimensional Analysis • When solving problems, units must be consistent • Unit conversion are often necessary • Use conversion factors • Problem: Determine how many centimeters are in 1 yd. • 1 yd x 36. 0 in x 2. 54 cm = 91. 44 cm 1 yd 1 in

3. 4 Density • Density is the ratio of mass to volume • Density is an intensive property • Density of a pure substance is constant at a given temperature

Density • Depends on temperature temp density What if temp decreased? • Units g/cm 3 or g/m. L of kg/m 3 for solids & liquids g/L for gases
 Measurements and their uncertainty answer key
Measurements and their uncertainty answer key Unit 2 lesson 2 scientific notation
Unit 2 lesson 2 scientific notation Uncertainity in decision making
Uncertainity in decision making Decision-making under uncertainty
Decision-making under uncertainty Significant figures cartoon
Significant figures cartoon Guide to the expression of uncertainty in measurement
Guide to the expression of uncertainty in measurement Chemistry chapter 3 scientific measurement
Chemistry chapter 3 scientific measurement Chapter 3 scientific measurement
Chapter 3 scientific measurement Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Chapter 37 vital signs and measurements multiple choice
Chapter 37 vital signs and measurements multiple choice Normal range for vital signs
Normal range for vital signs How to calculate percent error in chemistry
How to calculate percent error in chemistry Chapter 12 economic indicators and measurements
Chapter 12 economic indicators and measurements Which form of art reflected postwar uncertainty?
Which form of art reflected postwar uncertainty? Chapter 15 section 1 postwar uncertainty
Chapter 15 section 1 postwar uncertainty What is inferring
What is inferring War making and state making as organized crime
War making and state making as organized crime Lesson outline lesson 1: understanding science answer key
Lesson outline lesson 1: understanding science answer key Understanding science lesson 1 answer key
Understanding science lesson 1 answer key Lesson 2 measurement and scientific tools answer key
Lesson 2 measurement and scientific tools answer key The two measurements necessary for calculating speed are
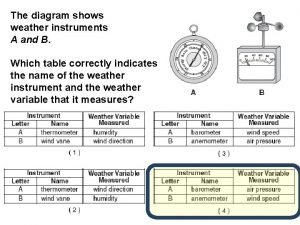
The two measurements necessary for calculating speed are Which diagram shows surface weather measurements
Which diagram shows surface weather measurements Genesis 6 16
Genesis 6 16 Significant figures in measurements
Significant figures in measurements 150 sig figs
150 sig figs Statistic vs parameter example
Statistic vs parameter example Qualitative vs quantitative measurements
Qualitative vs quantitative measurements Goniometer wrist
Goniometer wrist Qcpxxi5obqc -site:youtube.com
Qcpxxi5obqc -site:youtube.com Njrotc sdb uniform
Njrotc sdb uniform