Scientific Measurement QUALITATIVE VS QUANTITATIVE QUALITATIVE DESCRIPTIVE NONNUMERICAL

















- Slides: 31

Scientific Measurement: QUALITATIVE VS. QUANTITATIVE QUALITATIVE = DESCRIPTIVE, NONNUMERICAL. EX. : THE MORNINGS ARE GETTING VERY COLD. QUANTITATIVE = NUMERICAL, DEFINITE MEASUREMENT EX. : THE TEMPERATURE WAS 65°F THIS MORNING.

Scientific Measurement: Metric Know your metric relationships! kilo -hecta - deca -(base) - deci - centi – milli ( 103 - 102 -101 - (1) – 10 -1 - 10 -2 - 10 -3) King Henry Died By Drinking Chocolate Milk

Scientific Notation In science, we deal with some very LARGE numbers: 1 mole = 60200000000000 In science, we deal with some very SMALL numbers: Mass of an electron = 0. 000000000000000091 kg

Imagine the difficulty of calculating the mass of 1 mole of electrons! 0. 000000000000000091 kg x 60200000000000 ? ? ? ? ? ? ? ? ?

Scientific Notation: A method of representing very large or very small numbers in the form: M x 10 n ØM is a number between 1 and 10 Ø n is an integer

2 500 000 9 8 7 6 5 4 3 Step #1: Insert an understood decimal point Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n 2 . 1

2. 5 x 9 10 The exponent is the number of places we moved the decimal.

0. 0000579 1 2 3 4 5 Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n

5. 79 x -5 10 The exponent is negative because the number we started with was less than 1.

Scientific Notation Rules for using scientific notation Numbers are represented as a value between 1 <10 Exponents (+) (-) Ex: (+), (-) describe the decimal movement number grows larger number gets smaller 1. 2 x 10 3 = 1200 Math function and scientific notation: Be sure to use the [2 nd][EE] function on your calculator! Ex. 5. 03 x 1014 / 2. 9 x 107 = ?

Scientific Measurement: SI Units Standard Unit of measure Metric based measurements Length = meter (m) Mass = kilograms (kg) Time = seconds (s) Temperature = Kelvin (K) Amount of substance = mole (mol) Electric Current = ampere (A)

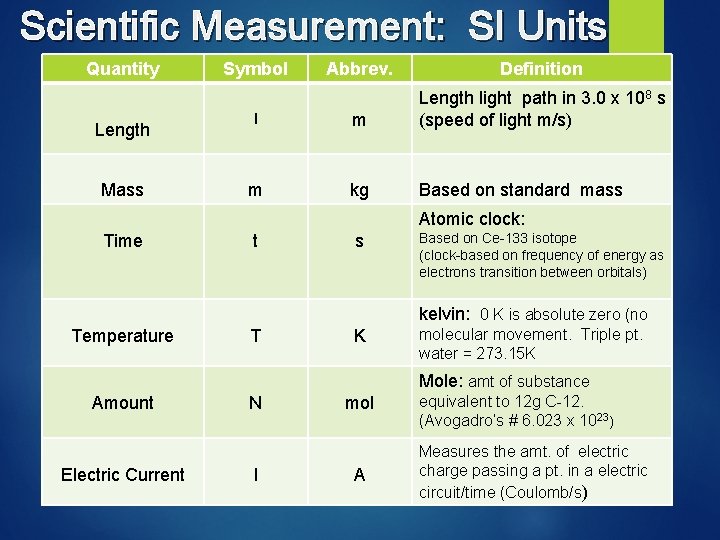
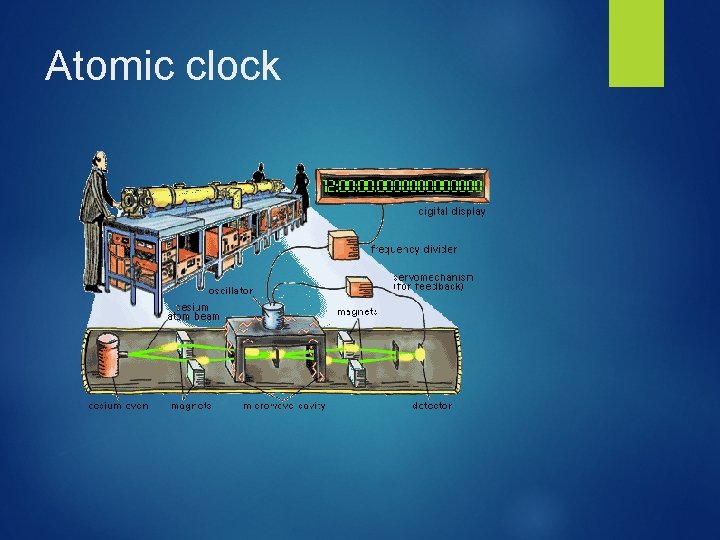
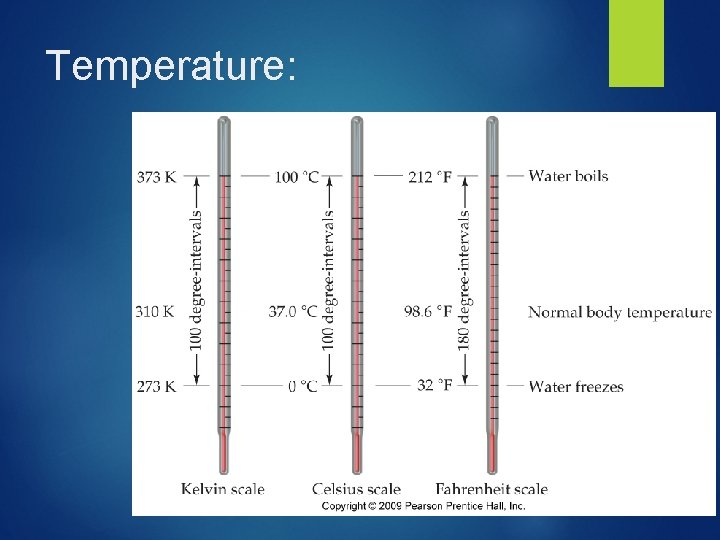
Scientific Measurement: SI Units Quantity Length Mass Symbol Abbrev. Definition l m Length light path in 3. 0 x 108 s (speed of light m/s) m kg Based on standard mass Atomic clock: Time t s Based on Ce-133 isotope (clock-based on frequency of energy as electrons transition between orbitals) kelvin: 0 K is absolute zero (no Temperature T K molecular movement. Triple pt. water = 273. 15 K Mole: amt of substance Amount Electric Current N l mol A equivalent to 12 g C-12. (Avogadro’s # 6. 023 x 1023) Measures the amt. of electric charge passing a pt. in a electric circuit/time (Coulomb/s)

Atomic clock

Temperature:

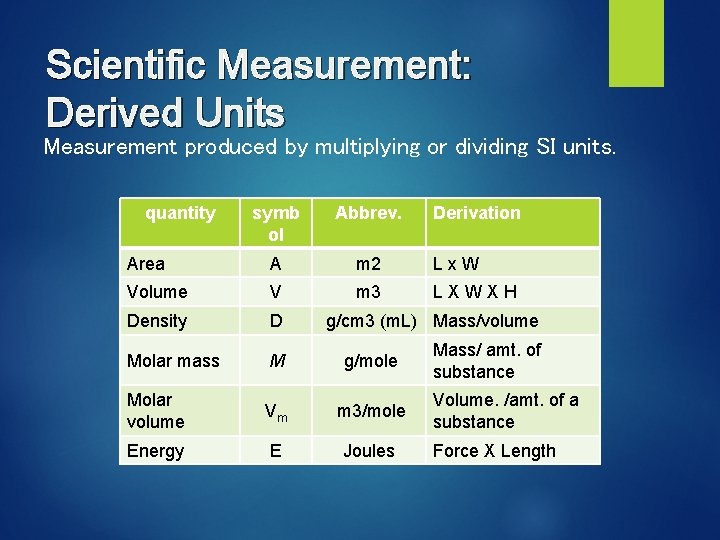
Scientific Measurement: Derived Units Measurement produced by multiplying or dividing SI units. quantity symb ol Abbrev. Derivation Area A m 2 Lx. W Volume V m 3 LXWXH Density D Molar mass M g/mole Molar volume Vm m 3/mole Energy E Joules g/cm 3 (m. L) Mass/volume Mass/ amt. of substance Volume. /amt. of a substance Force X Length

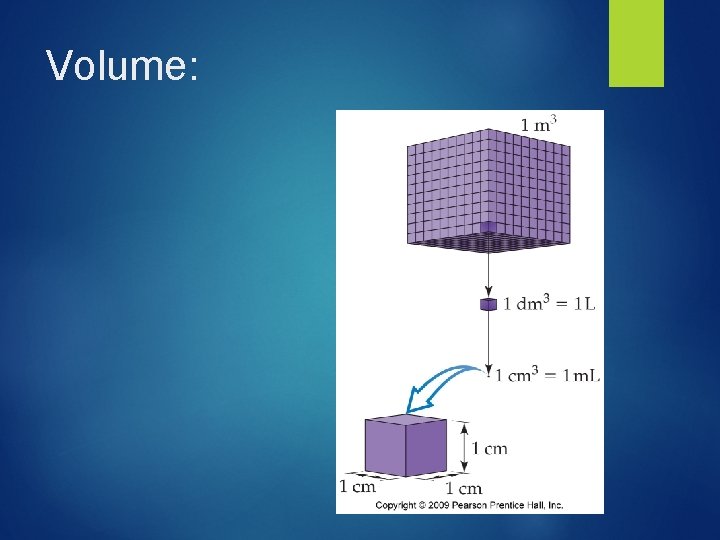
Volume Measurement The amount of space occupied by an object Some useful relationships: 1 Liter = 1 dm 3 = 103 cm 3 = 1000 m. L 1 cm 3 = 1 m. L

Volume:

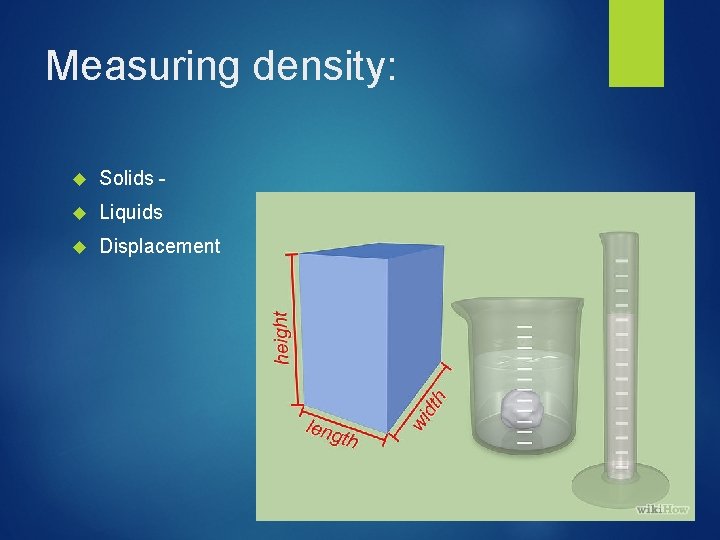
Density Measurement Describes the ratio of mass to volume Density is a physical property that is independent to amount (intensive property) D = Mass/Volume= (g/m. L or g/cm 3)

Measuring density: Solids - Liquids Displacement

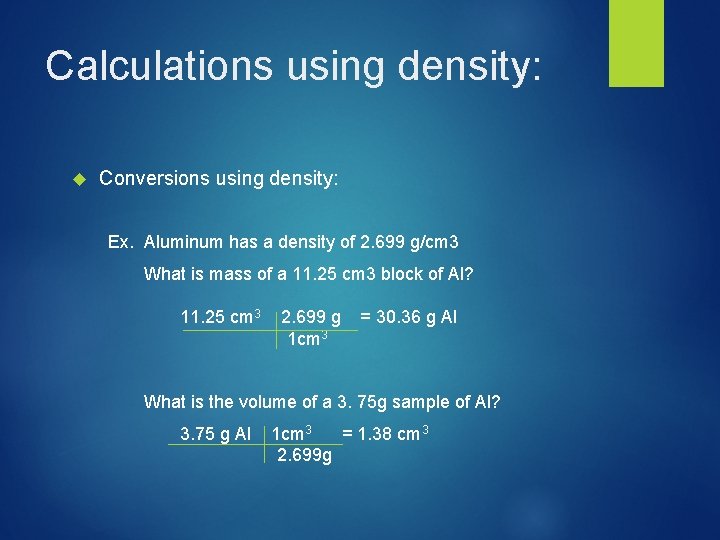
Calculations using density: Conversions using density: Ex. Aluminum has a density of 2. 699 g/cm 3 What is mass of a 11. 25 cm 3 block of Al? 11. 25 cm 3 2. 699 g 1 cm 3 = 30. 36 g Al What is the volume of a 3. 75 g sample of Al? 3. 75 g Al 1 cm 3 = 1. 38 cm 3 2. 699 g

Conversion Factors A relationship showing the equality between two different units that allows for conversion between these units. Examples: 1 week = 7 days 1 dozen eggs = 12 eggs ? ? ?

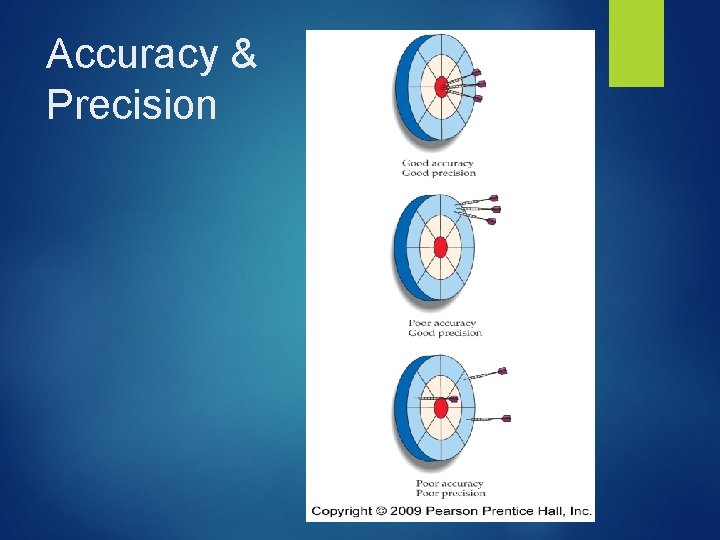
Scientific Measurement: Accuracy- close to the true value Precision- close within measurements % Error- describes how close your data is to the accepted value Accepted – Experimental x 100 Accepted Value

Accuracy & Precision

Scientific Measurement: Significant Figures Used to express the accuracy of a number. Used for “measured” numbers. Rules for determining Sig. Figs. 1. All non-zero numbers are significant 2. Captive zeros are significant 3. Leading zeros are not significant 4. Trailing zeros are significant if a decimal is present

Significant figures: Determine the number of sig figs 1. 3060 2. . 000789 3. 1. 40 x 10 6 4. 1000 5. 2030. 6. 45, 0060

Answers: Determine the number of sig figs 1. 3060 (3) 2. . 000789 (3) 3. 1. 40 x 10 6 (3) 4. 1000 (1) 5. 2030. 6. 45, 0060 (4) (5)

Calculations using Significant Figures Addition/Subtraction Limited to the placement of the least significant figure. ex. 12. 00 + 8. 3 + 14 = Multiplication/Division Limited to the lowest number of significant figures. Ex. 12. 0 x 8. 3 X 14 =

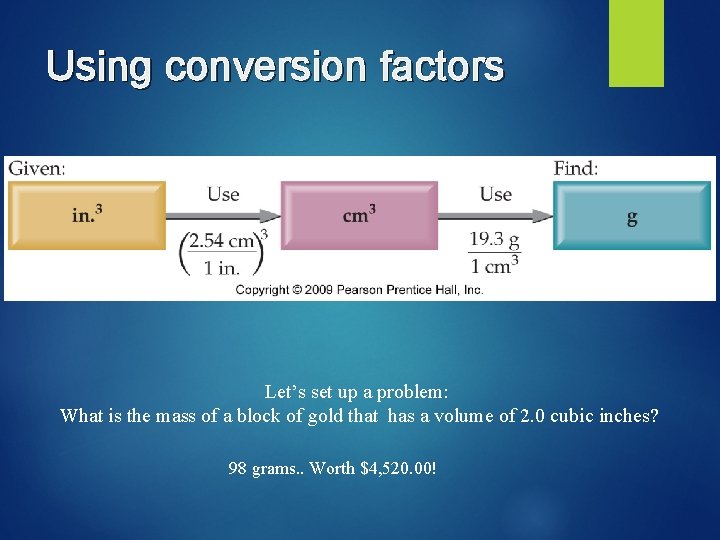
Using conversion factors Let’s set up a problem: What is the mass of a block of gold that has a volume of 2. 0 cubic inches? 98 grams. . Worth $4, 520. 00!

Problem Solving Steps to Problem Solving Approach: A. Analyze: determine starting point and plan steps required to get a solution (unknown). B. Plan: set up a strategy to solve the problem. C. Solve: conduct appropriate calculations based on plan. This may require multiple steps. D. Evaluate: Review answer to see if it seems reasonable.

Problem Solving a ratio of equivalent measurements ( 1 inch = 2. 54 cm) Conversion Factors- Dimensional Analysis- the technique for solving problems using unit conversions based on conversion factors Ex. 6. 42 inches = ? cm

Problem Solving Multi-step Problems – use more than one conversion factor: ex. 5 days = ? minutes Complex Problems- involves ratios of two units: ex. . 45 Km/hrs = ? m/s Golden Rule for conversions…always show your work!