Ch 3 NotesScientific Measurement Qualitative vs Quantitative Qualitative


- Slides: 22

Ch. 3 Notes---Scientific Measurement Qualitative vs. Quantitative • Qualitative measurements give results in a descriptive nonnumeric adjective describing form. (The result of a measurement is an _______ the object. ) short heavy cold *Examples: ___________, long, _____. . . • Quantitative measurements give results in numeric form. (The number results of a measurement contain a _______. ) 600 lbs. 5 ºC *Examples: 4’ 6”, _____, 22 meters, _____. . . Accuracy vs. Precision • single Accuracy is how close a ______ measurement is to the true _____ value ____ of whatever is being measured. • several measurements are to Precision is how close ______ each ______. other _____

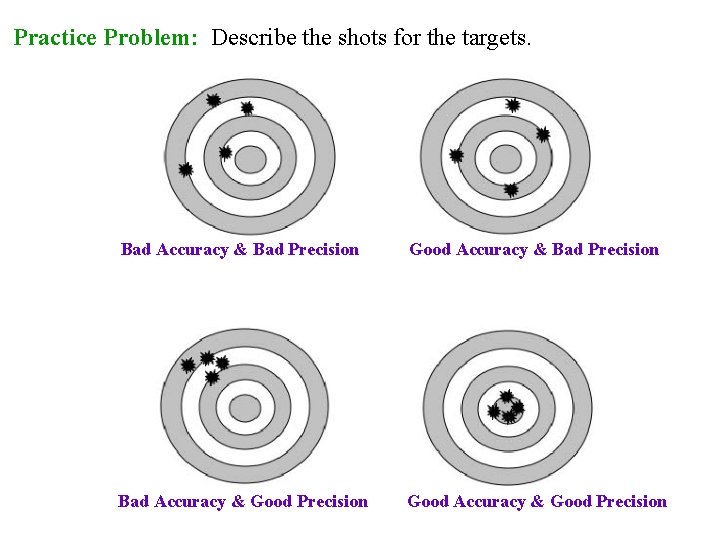
Practice Problem: Describe the shots for the targets. Bad Accuracy & Bad Precision Good Accuracy & Bad Precision Bad Accuracy & Good Precision Good Accuracy & Good Precision

Significant Figures • precision Significant figures are used to determine the _______ of a measurement. (It is a way of indicating how _____ precise a measurement is. ) *Example: A scale may read a person’s weight as 135 lbs. Another scale may read the person’s weight as 135. 13 lbs. The ______ second more significant figures in the scale is more precise. It also has ______ measurement. • • • Whenever you are measuring a value, (such as the length of an object with a ruler), it must be recorded with the correct number of sig. figs. Record ______ the numbers of the measurement known for sure. ALL Record one last digit for the measurement that is estimated. (This means that you will be ________________ of the device and _______ what the next number reading in between the is. ) marks estimating

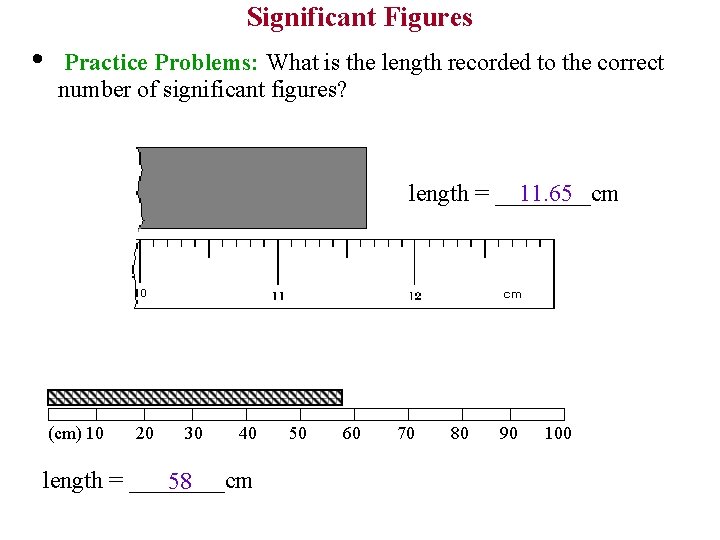
Significant Figures • Practice Problems: What is the length recorded to the correct number of significant figures? length = ____cm 11. 65 (cm) 10 20 30 40 length = ____cm 58 50 60 70 80 90 100

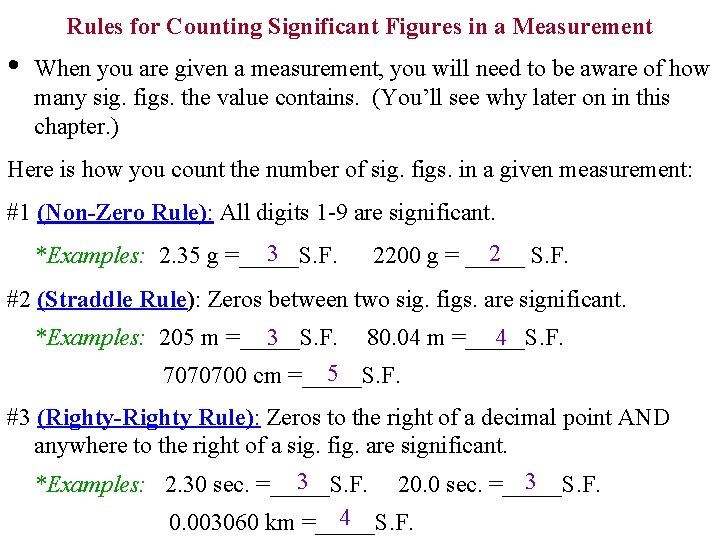
Rules for Counting Significant Figures in a Measurement • When you are given a measurement, you will need to be aware of how many sig. figs. the value contains. (You’ll see why later on in this chapter. ) Here is how you count the number of sig. figs. in a given measurement: #1 (Non-Zero Rule): All digits 1 -9 are significant. 3 *Examples: 2. 35 g =_____S. F. 2 S. F. 2200 g = _____ #2 (Straddle Rule): Zeros between two sig. figs. are significant. 3 4 *Examples: 205 m =_____S. F. 80. 04 m =_____S. F. 5 7070700 cm =_____S. F. #3 (Righty-Righty Rule): Zeros to the right of a decimal point AND anywhere to the right of a sig. fig. are significant. 3 3 *Examples: 2. 30 sec. =_____S. F. 20. 0 sec. =_____S. F. 4 0. 003060 km =_____S. F.

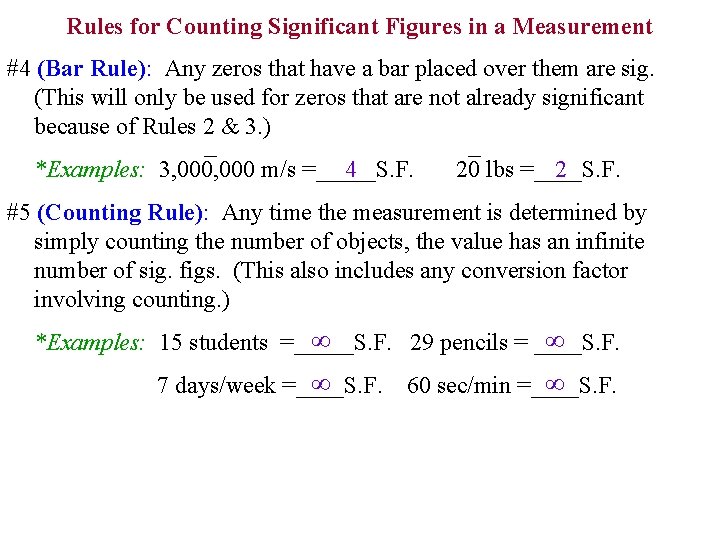
Rules for Counting Significant Figures in a Measurement #4 (Bar Rule): Any zeros that have a bar placed over them are sig. (This will only be used for zeros that are not already significant because of Rules 2 & 3. ) 4 *Examples: 3, 000 m/s =_____S. F. 2 20 lbs =____S. F. #5 (Counting Rule): Any time the measurement is determined by simply counting the number of objects, the value has an infinite number of sig. figs. (This also includes any conversion factor involving counting. ) ∞ ∞ *Examples: 15 students =_____S. F. 29 pencils = ____S. F. ∞ 7 days/week =____S. F. ∞ 60 sec/min =____S. F.

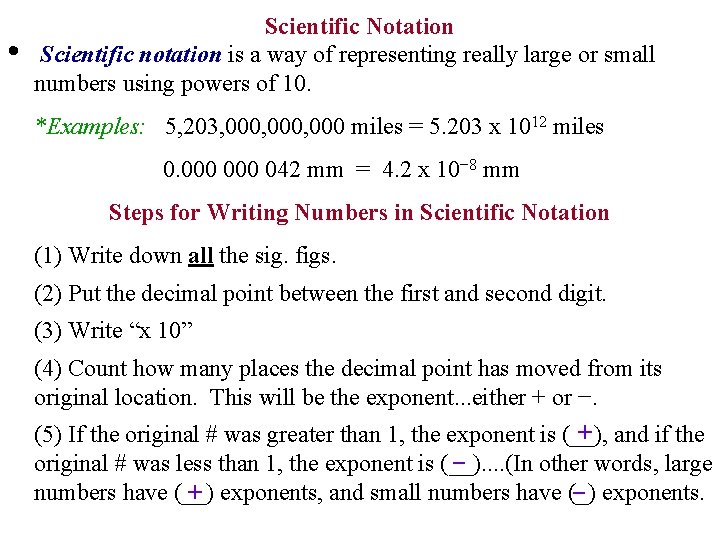
• Scientific Notation Scientific notation is a way of representing really large or small numbers using powers of 10. *Examples: 5, 203, 000, 000 miles = 5. 203 x 1012 miles 0. 000 042 mm = 4. 2 x 10− 8 mm Steps for Writing Numbers in Scientific Notation (1) Write down all the sig. figs. (2) Put the decimal point between the first and second digit. (3) Write “x 10” (4) Count how many places the decimal point has moved from its original location. This will be the exponent. . . either + or −. + and if the (5) If the original # was greater than 1, the exponent is (__), original # was less than 1, the exponent is (__). . (In other words, large − numbers have (__) + exponents, and small numbers have (_) − exponents.

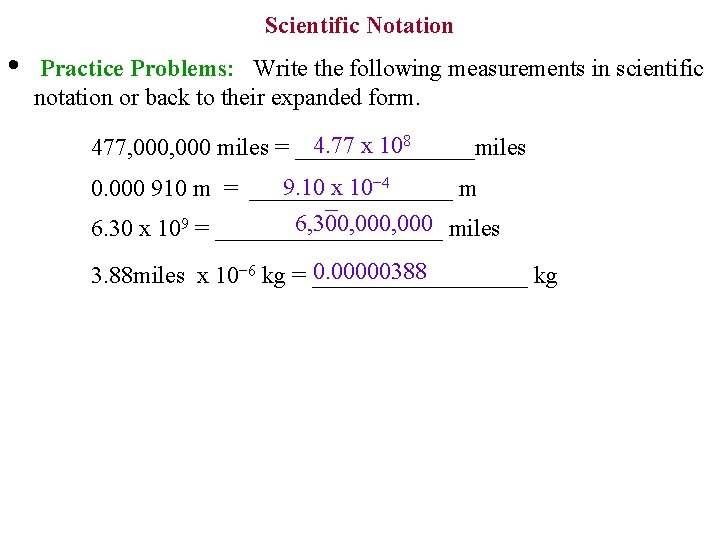
Scientific Notation • Practice Problems: Write the following measurements in scientific notation or back to their expanded form. 4. 77 x 108 477, 000 miles = ________miles 9. 10 x 10− 4 0. 000 910 m = _________ m − 9 6, 300, 000 6. 30 x 10 = __________ miles 0. 00000388 3. 88 miles x 10− 6 kg = _________ kg

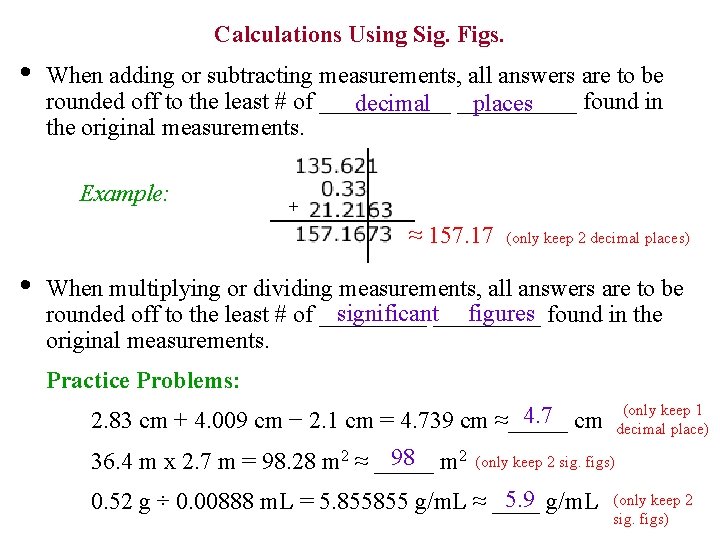
Calculations Using Sig. Figs. • When adding or subtracting measurements, all answers are to be rounded off to the least # of ______ found in decimal _____ places the original measurements. Example: + ≈ 157. 17 • (only keep 2 decimal places) When multiplying or dividing measurements, all answers are to be significant_____ figures found in the rounded off to the least # of _____ original measurements. Practice Problems: (only keep 1 decimal place) 4. 7 cm 2. 83 cm + 4. 009 cm − 2. 1 cm = 4. 739 cm ≈_____ 98 m 2 36. 4 m x 2. 7 m = 98. 28 m 2 ≈ _____ (only keep 2 sig. figs) 5. 9 g/m. L 0. 52 g ÷ 0. 00888 m. L = 5. 855855 g/m. L ≈ ____ (only keep 2 sig. figs)

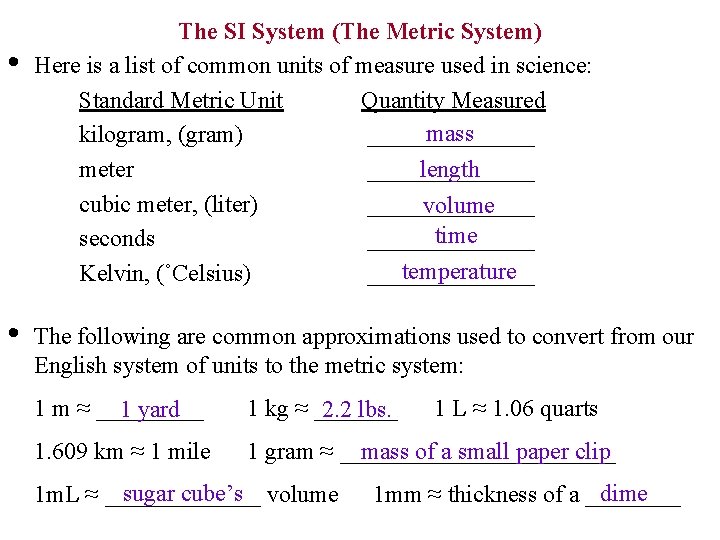
• The SI System (The Metric System) Here is a list of common units of measure used in science: Standard Metric Unit Quantity Measured mass kilogram, (gram) _______ meter cubic meter, (liter) seconds Kelvin, (˚Celsius) • length ______________ volume time _______ temperature _______ The following are common approximations used to convert from our English system of units to the metric system: 1 m ≈ _____ 1 yard 1 kg ≈ _______ 2. 2 lbs. 1. 609 km ≈ 1 mile mass of a small paper clip 1 gram ≈ ____________ sugar cube’s volume 1 m. L ≈ _______ 1 L ≈ 1. 06 quarts dime 1 mm ≈ thickness of a ____

The SI System (The Metric System)

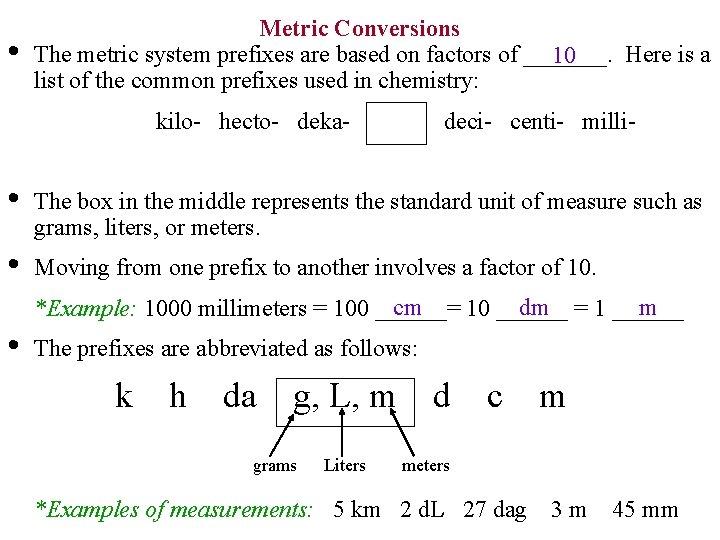
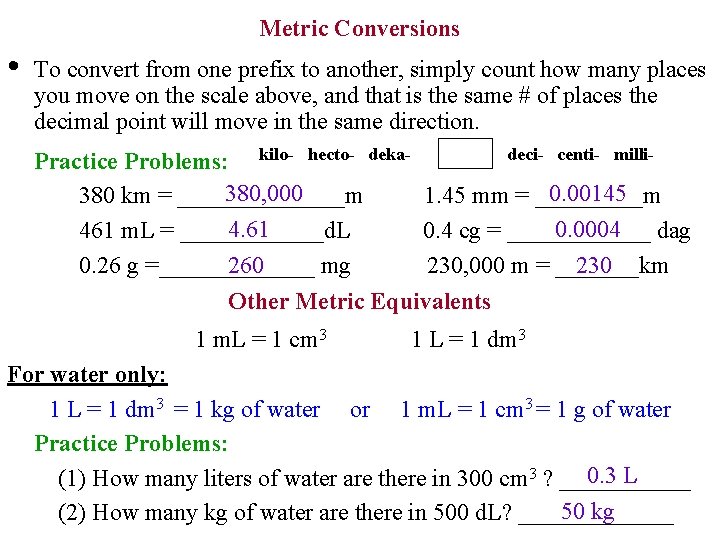
• Metric Conversions The metric system prefixes are based on factors of _______. Here is a 10 list of the common prefixes used in chemistry: kilo- hecto- deka- deci- centi- milli- • The box in the middle represents the standard unit of measure such as grams, liters, or meters. • Moving from one prefix to another involves a factor of 10. cm dm = 1 ______ m *Example: 1000 millimeters = 100 ______= 10 ______ • The prefixes are abbreviated as follows: k h da g, L, m grams Liters d c m meters *Examples of measurements: 5 km 2 d. L 27 dag 3 m 45 mm


Metric Conversions • To convert from one prefix to another, simply count how many places you move on the scale above, and that is the same # of places the decimal point will move in the same direction. deci- centi- milli. Practice Problems: kilo- hecto- deka 380, 000 0. 00145 380 km = _______m 1. 45 mm = _____m 4. 61 0. 0004 dag 461 m. L = ______d. L 0. 4 cg = ______ 0. 26 g =_______ mg 230, 000 m = _______km 260 230 Other Metric Equivalents 1 m. L = 1 cm 3 1 L = 1 dm 3 For water only: 1 L = 1 dm 3 = 1 kg of water or 1 m. L = 1 cm 3 = 1 g of water Practice Problems: 0. 3 L (1) How many liters of water are there in 300 cm 3 ? ______ 50 kg (2) How many kg of water are there in 500 d. L? _______

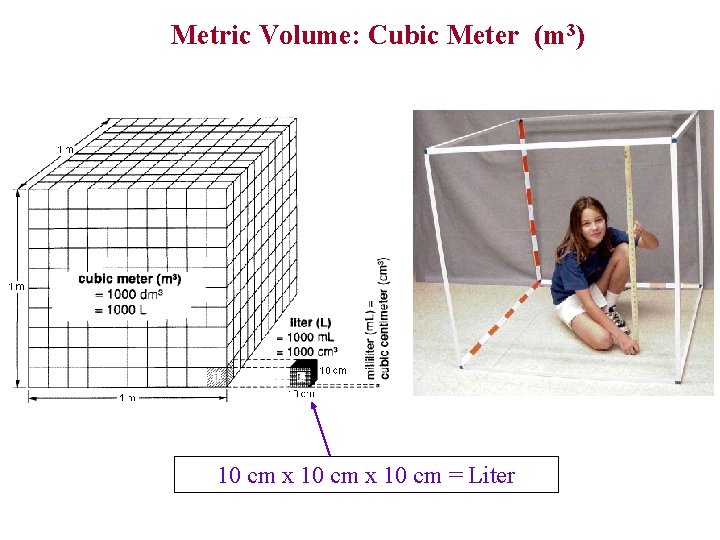
Metric Volume: Cubic Meter (m 3) 10 cm x 10 cm = Liter

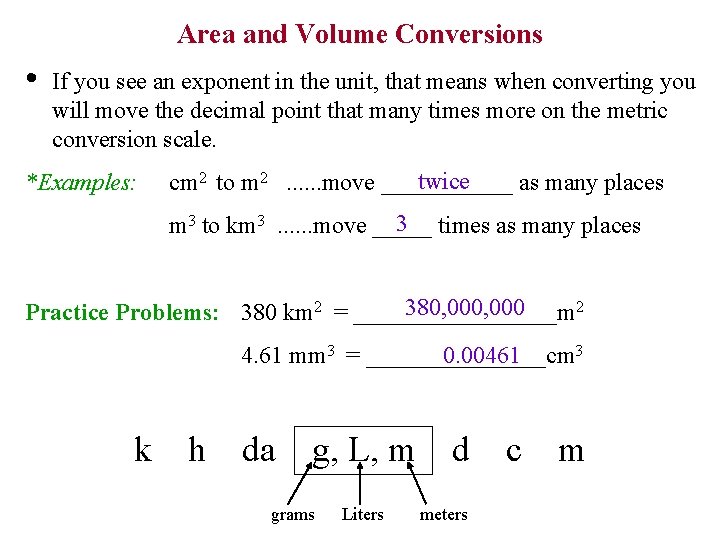
Area and Volume Conversions • If you see an exponent in the unit, that means when converting you will move the decimal point that many times more on the metric conversion scale. *Examples: twice cm 2 to m 2. . . move ______ as many places 3 times as many places m 3 to km 3. . . move _____ 2 380, 000 Practice Problems: 380 km 2 = _________m 3 4. 61 mm 3 = ________cm 0. 00461 k h da g, L, m grams Liters d meters c m

Mass vs. Weight • Mass depends on the amount of ______ in the object. matter • Weight depends on the force of ______ acting on the object. gravity • Weight _______ may change as you move from one location to another; mass ______ will not. • You have the same ______ on mass the moon as on the earth, but you weigh ______ less since there is less gravity on the moon. _____ Mass = 80 kg Weight = 176 lbs. Mass = 80 kg Weight = 29 lbs.

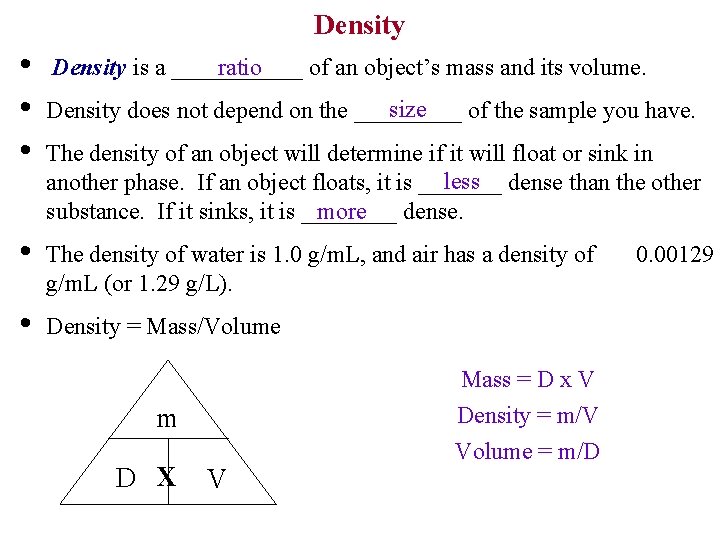
Density • • • ratio Density is a ______ of an object’s mass and its volume. size Density does not depend on the _____ of the sample you have. The density of an object will determine if it will float or sink in less dense than the other another phase. If an object floats, it is _______ more substance. If it sinks, it is ____ dense. • The density of water is 1. 0 g/m. L, and air has a density of g/m. L (or 1. 29 g/L). • Density = Mass/Volume m D X V Mass = D x V Density = m/V Volume = m/D 0. 00129

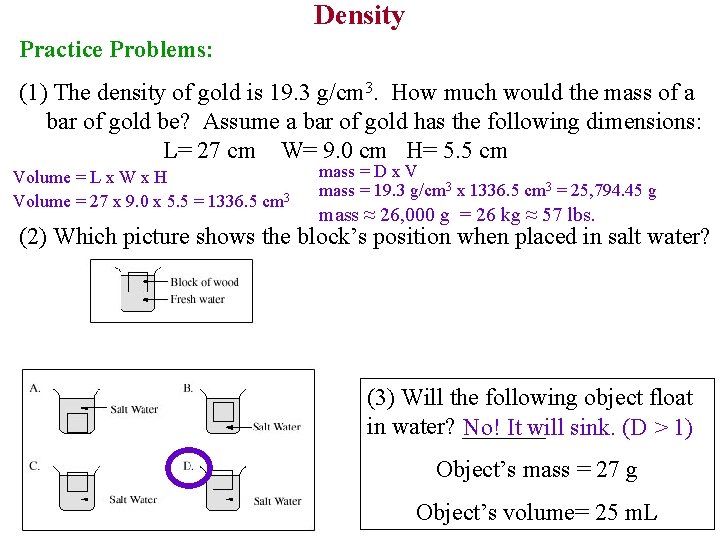
Density Practice Problems: (1) The density of gold is 19. 3 g/cm 3. How much would the mass of a bar of gold be? Assume a bar of gold has the following dimensions: L= 27 cm W= 9. 0 cm H= 5. 5 cm Volume = L x W x H Volume = 27 x 9. 0 x 5. 5 = 1336. 5 cm 3 mass = D x V mass = 19. 3 g/cm 3 x 1336. 5 cm 3 = 25, 794. 45 g mass ≈ 26, 000 g = 26 kg ≈ 57 lbs. (2) Which picture shows the block’s position when placed in salt water? (3) Will the following object float in water? _______ No! It will sink. (D > 1) Object’s mass = 27 g Object’s volume= 25 m. L

Measuring Temperature • • hotness coldness Temperature is the ______ or ______ of an object. The Celsius temperature scale is based on the freezing point and water boiling point of _____. F. P. = 0˚C B. P. = 100˚C The Kelvin temperature scale, sometimes called the “absolute temp. lowest scale”, is based on the ______ temperature possible, absolute stop zero. (All molecular motion would _____. ) Absolute Zero = 0˚ Kelvin = − 273˚ C To convert from one temp. scale to another: ˚C = Kelvin − 273 K= Celsius + 273 Practice Problems: Convert the following 298 K (25 + 273) 25˚C = _______ (473 – 273) 473 K = _______˚C 200

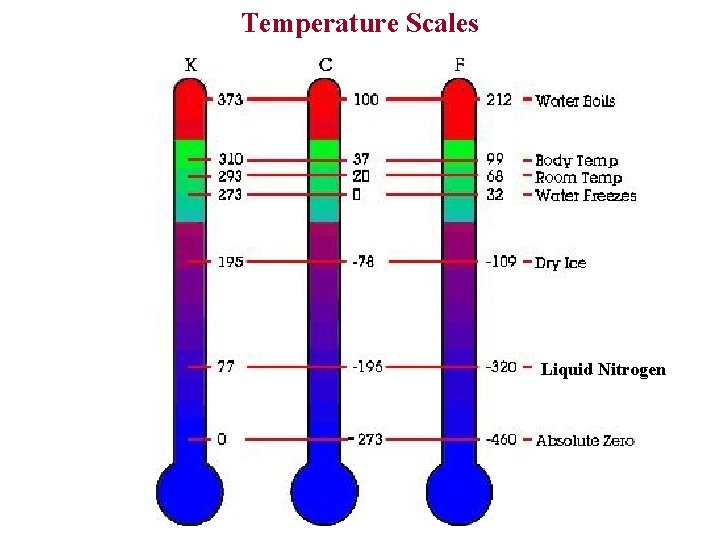
Temperature Scales Liquid Nitrogen

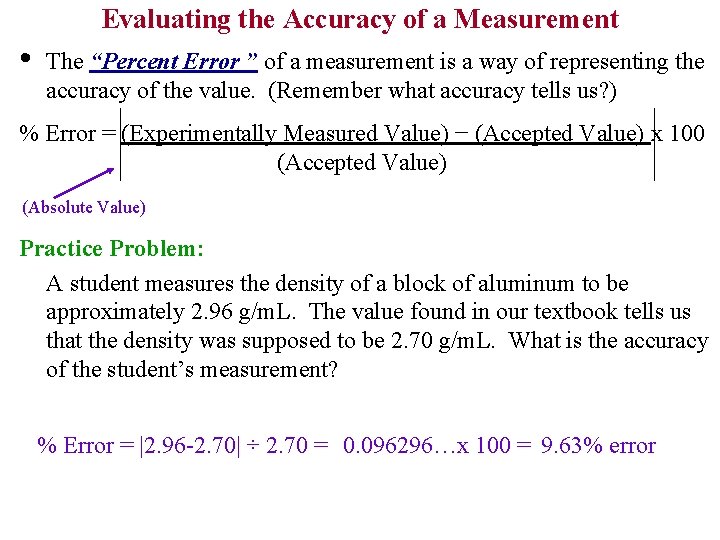
Evaluating the Accuracy of a Measurement • The “Percent Error ” of a measurement is a way of representing the accuracy of the value. (Remember what accuracy tells us? ) % Error = (Experimentally Measured Value) − (Accepted Value) x 100 (Accepted Value) (Absolute Value) Practice Problem: A student measures the density of a block of aluminum to be approximately 2. 96 g/m. L. The value found in our textbook tells us that the density was supposed to be 2. 70 g/m. L. What is the accuracy of the student’s measurement? % Error = |2. 96 -2. 70| ÷ 2. 70 = 0. 096296…x 100 = 9. 63% error